Abstract
Acute administration of peptide YY(3-36) [PYY(3-36)] results in a reduction in food intake in several different vertebrates. However, long-term continuous administration of PYY(3-36) causes only a transient reduction in food intake, thus potentially limiting its therapeutic efficacy. We hypothesized that a fall in leptin levels associated with reduced food intake could contribute to the transient anorectic effects of continuous PYY(3-36) infusion and thus that leptin replacement might prolong the anorectic effects of PYY(3-36). Seven-day administration of 100 μg·kg body wt−1·day−1 PYY(3-36) using osmotic minipumps caused a significant reduction in food intake of ad libitum-fed rats, but only for the first 2 days postimplantation. Circulating levels of leptin were reduced 1 day following continuous infusion of PYY(3-36), and combined leptin infusion at a dose of leptin that had no anorectic effects on its own (100 μg·kg body wt−1·day−1) prolonged the anorectic actions of PYY(3-36) in ad libitum-fed rats for up to 6 days postimplantation and yielded reduced weight gain compared with either peptide alone. The inhibitory effects of 100 μg·kg body wt−1·day−1 PYY(3-36) on food intake were absent in rats refed after a 24-h fast and substantially reduced at a dose of 1,000 μg·kg body wt−1·day−1 PYY(3-36). Leptin replacement was unable to recover the anorectic effects of PYY(3-36) in fasted rats. Our results suggest that an acute fall in leptin levels is not solely responsible for limiting duration of action of chronic PYY(3-36) infusion, yet chronic coadministration of a subanorectic dose of leptin can extend the anorectic effects of PYY(3-36).
Keywords: obesity, food intake, osmotic minipumps, body weight, hormones
obesity is a complex metabolic disorder that is reaching pandemic proportions in developed countries. Several endocrine factors, mainly those produced from the brain and gut contribute to the maintenance of food intake (7, 37). Multiple appetite stimulatory (orexigenic) and inhibitory (anorexigenic) endocrine factors interact to regulate energy balance in vertebrates. Anorexigens are divided into short-term satiety signals (e.g., CCK) that act in the brain to convey the feeling of fullness immediately after a meal and long-term satiety signals (e.g., leptin) that signal the status of the body's energy stores to the brain (27, 36).
Peptide YY (PYY) is a gut hormone produced from the L-cells mainly present in the distal intestine (6). Within 15 min following a meal, circulating levels of PYY significantly increase and remain elevated for up to 6 h postmeal (6). Dipeptidyl peptidase IV cleaves the first two amino acids from the NH2-terminal region of the 1-36 form of PYY to generate PYY(3-36) (30, 37). Mice with knockout of the PYY gene are obese (12, 14), providing evidence for the important role of this gene in regulating energy balance. It has also been reported that PYY(3-36) administration causes taste aversion (22, 24) and malaise (22). The satiety actions of exogenous PYY(3-36) became controversial when a multicentre study failed to find any anorectic effects of acute injections of PYY(3-36) in several rodent models (14). However, several other studies have shown that both acute and chronic exogenous administration of PYY(3-36) inhibits food intake and weight gain of rodents (1–5, 17–21, 25, 26, 28, 33), primates (32), and humans (11, 23). Animal stress was suggested as one of the reasons why the multicenter study failed to see any food intake inhibitory actions of PYY(3-36) (3, 24). We (37) as well as others (4, 5) have shown that continuous administration of PYY(3-36) using osmotic minipumps causes a transient reduction in food intake. Apparent desensitization to long-term continuous administration of PYY(3-36) (37) may limit the antiobesity potential of PYY(3-36).
Receptor downregulation and changes in leptin action in the brain were put forward as some possible mechanisms that cause normalization of feeding in rats during continuous PYY(3-36) infusion (20). We hypothesized that reduced food intake during continuous infusion of PYY(3-36) causes a fall in the circulating levels of leptin that may trigger a drive to eat more, even in the presence of elevated PYY(3-36). Thus, the objectives of this study were to: 1) examine circulating leptin levels in rats during chronic administration of an anorectic dose of PYY(3-36), 2) study the effects of fasting on PYY(3-36)-induced satiety, and 3) investigate the appetite regulatory effects of leptin and PYY(3-36) coadministration in ad libitum-fed rats and rats fed after a 24-h fast. We found that: 1) continuous infusion of PYY(3-36) causes a transient reduction in food intake and a temporary decrease in plasma leptin levels; 2) fasting attenuates the satiety effects of PYY(3-36) and leptin; and 3) an acute reduction in leptin levels does not appear to be critical in limiting the anorectic actions of continuous PYY(3-36) administration, yet coadministration of leptin, at a dose that alone is ineffective in reducing food intake can extend the anorectic action of PYY(3-36).
MATERIALS AND METHODS
Animals.
Fischer 344 rats were purchased from Charles River Laboratories (Saint-Constant, Quebec, Canada). Age- and weight-matched (mean = 224.70 ± 3.77 g) male rats were used for all studies, and unless otherwise mentioned were individually housed in grid cages in a 12:12-h light (7 AM-7 PM)-dark (7 PM-7 AM) photoperiod at 23 ± 1°C and controlled humidity in the animal care facility of University of British Columbia or York University (for the study shown in Fig. 3). Unless otherwise mentioned, animals had ad libitum access to tap water and rat chow (Purina Mills, St. Louis, MO). Fresh rat chow was provided daily between 5 and 7 PM on the floor of grid cages, and this allowed us to collect residual food particles on a tray kept beneath cages to monitor food intake. For studies described in Fig. 3, food was provided in the feeder of regular rat cages. Research protocols used in this study adhered to the guidelines of the Canadian Council for Animal Care and were approved by the Animal Care Committee of the University of British Columbia or York University.
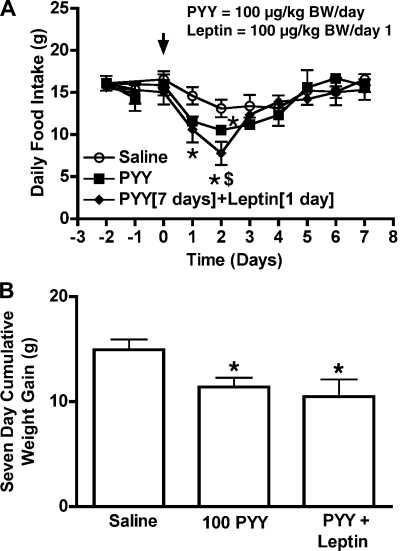
Fig. 3.
Effects of saline, PYY(3-36) (100 μg·kg body wt−1·day−1) or a combination of PYY(3-36) and leptin (100 μg·kg body wt−1·day−1) on 24-h cumulative food intake (A) and 7-day cumulative weight gain (B) of ad libitum-fed rats. Arrow indicates the day of pump implantation. In the coadministration group, PYY(3-36) was infused using a 7-day pump and leptin was administered using a separate 1-day pump. Data are presented as means ± SE. *Statistically significant difference in the food intake or body weight of the PYY(3-36) and PYY(3-36)+leptin groups compared with saline controls, $Statistically significant difference in the food intake of the PYY(3-36)+leptin group compared with the PYY(3-36) alone group, n = 4 rats/group.
We have previously shown that continuous administration of PYY(3-36) using osmotic minipumps inhibits food intake of acclimatized rats (37). Therefore, in this study, acclimatization of rats, subcutaneous implantation of pumps, and monitoring of food intake and body weight were conducted as described previously (37). Each study used a different cohort of acclimatized rats, and in each study a group of rats that received saline infusing pumps served as controls. Rats were housed in grid cages or regular cages for 3 days from the day of arrival and then were acclimatized for 7 days. Starting on day 1 of acclimatization, animals were transferred to the procedure room on a cart, anesthetized using 3% isoflurane using oxygen as gaseous carrier, and shaved in the area where the incision was to be made and weighed. All of the above acclimatization procedures except shaving were repeated for the next 6 days. On the surgery and implantation day (day 7 of acclimatization), rats were anesthetized, a small subclavicular incision was made, and osmotic minipumps were implanted subcutaneously. Following implantation, wounds were immediately sealed using wound clips, and antiseptics were administered using a cotton swab. Rats were then removed from the anesthetic machine, weighed, and returned to their cages and allowed to recover from anesthesia. Animals were returned to the animal care facility, preweighed quantities of food were given, and food intake was measured by deducting the quantity of food recovered after 24-h feeding from the initial amount of food given.
Materials.
Rat PYY(3-36) was purchased from Phoenix Pharmaceuticals (Belmont, CA). Human PYY(3-36) was synthesized at the Peptide Synthesis Unit, Biomedical Research Centre, University of British Columbia, Vancouver, Canada. Recombinant mouse leptin was purchased from Dr. A. F. Parlow (National Hormones and Peptides Program, Harbour-UCLA Medical Center, Los Angeles, CA). All peptides were HPLC purified to ≥95% purity. Peptides were lot matched when multiple vials were required in a study and were freshly prepared in 0.9% saline for each study. We found that both rat PYY(3-36) and human PYY(3-36) are equipotent in reducing food intake and weight gain of rats (S. Unniappan and T. J. Kieffer, unpublished results), and hence we used rat or human PYY(3-36) in our studies as indicated. One-day (model 2001D), 7-day (model 2ML1), and 14-day (model 2ML2) Alzet osmotic minipumps were purchased from Durect (Cupertino, CA). Rat/mouse leptin ELISA kit (cat. no. 022-LEP-E06) was purchased from Linco Research (Windham, NH).
Effects of continuous infusion of PYY(3-36) on food intake of ad libitum-fed rats.
Fourteen-day pumps infusing saline or rat PYY(3-36) (100 μg·kg body wt−1·day−1 = 25 nmol·kg body wt−1/day−1) were implanted into acclimatized rats, and daily food intake was monitored for 7 days. Our previous results (37) indicated that continuous infusion of PYY(3-36) for 7 days yields only a transient reduction in food intake during the treatment period. To eliminate the possibility that the transient effects were due to pump failure or loss of activity of the PYY(3-36), in this study we removed both saline and PYY(3-36) pumps on day 7 and reimplanted them into two new groups of acclimatized rats. These new recipients were then monitored for daily food intake for 7 days (to day 14 of the pump).
Effects of continuous infusion of PYY(3-36) on plasma leptin levels of ad libitum-fed rats.
We conducted this experiment to test the hypothesis that administration of PYY(3-36) results in a reduction in circulating levels of leptin and that this fall in leptin may attenuate the effects of continuous PYY(3-36) administration on daily food intake of rats. Since the primary aim of this study was to collect plasma samples, ad libitum-fed rats were housed three rats per cage. Seven-day pumps infusing saline or rat PYY(3-36) (100 μg·kg body wt−1·day−1) were implanted into acclimatized ad libitum-fed rats, blood samples (250 μl) were collected daily by tail bleeding from all animals at 3:30–4:15 PM, and plasma was separated by centrifugation at 10,000 g for 9 min and stored at −20 C until assay for leptin was conducted following the manufacturer's protocol.
Determination of a subanorectic dose of leptin in ad libitum-fed rats.
To find a subanorectic dose of leptin in rats, saline or leptin at 100, 200, 400, or 800 μg·kg body wt−1·day−1 was infused for 24 h using 1-day osmotic minipumps. Twenty-four-hour cumulative food intake and body weight were monitored. In a second study, leptin levels were determined in plasma samples from saline and leptin (100 μg·kg body wt−1·day−1) infused ad libitum-fed animals at 0 (9 AM), 12 (9 PM), and 24 h (9 AM next day) postpump implantation.
Effects of coadministration of leptin and PYY(3-36) on food intake and body weight of ad libitum-fed rats.
To test whether coadministration of leptin can prolong the ability of PYY(3-36) to inhibit food intake of ad libitum-fed rats, saline, leptin (100 or 200 μg·kg body wt−1·day−1), human PYY(3-36) (100 or 1,000 μg·kg body wt−1·day−1), or a combination of both leptin and human PYY(3-36) [100 μg·kg body wt−1·day−1 PYY(3-36) + 100 μg·kg body wt−1·day−1 leptin, 100 μg·kg body wt−1·day−1 PYY(3-36) + 200 μg·kg body wt−1·day−1 leptin, or 1,000 μg·kg body wt−1·day−1 PYY(3-36) + 100 μg·kg body wt−1·day−1 leptin] was infused. In one study, the leptin administration was for 1 day (n = 4 per group); in other studies it was for 7 days (n = 6 per group). Daily food intake and body weight were monitored for 7 days. In the coadministration studies, a single pump was used to deliver both PYY(3-36) and leptin when both peptides were administered for 7 days, while separate pumps were used when leptin was delivered only 1 day.
Effects of leptin replacement on satiety actions of PYY(3-36) in rats refed after a 24-h fast.
We hypothesized that the anorectic actions of PYY(3-36) may be attenuated in fasting rats as a result of a fasting-induced fall in leptin levels that might override the actions of PYY(3-36). We tested this via leptin replacement during PYY(3-36) treatment in fasted rats. Rats were fasted for 24 h, and osmotic minipumps infusing saline, leptin (100, 200, 400 or 800 μg·kg body wt−1·day−1), human PYY(3-36) (100 or 1,000 μg·kg body wt−1·day−1), or a combination of leptin and human PYY(3-36) [100 μg·kg body wt−1·day−1 PYY(3-36) + 400 μg·kg body wt−1·day−1 leptin or 1,000 μg·kg body wt−1·day−1 PYY(3-36) + 400 μg·kg body wt−1·day−1 leptin] were implanted at the end of fasting. Twenty-four-hour cumulative food intake and body weight were monitored in all studies. In the coadministration study, a single pump was used to deliver both PYY(3-36) and leptin.
Statistical analyses.
All data are presented as means ± SE. Data were analyzed using t-test or ANOVA followed by Student-Newman-Keuls or Tukeys multiple comparison post hoc test as indicated. P < 0.05 was considered statistically significant. Graphing and statistical analyses were conducted using GraphPad Prism version 4 (GraphPad Software, San Diego, CA).
RESULTS
Effects of continuous infusion of PYY(3-36) on food intake of ad libitum-fed rats.
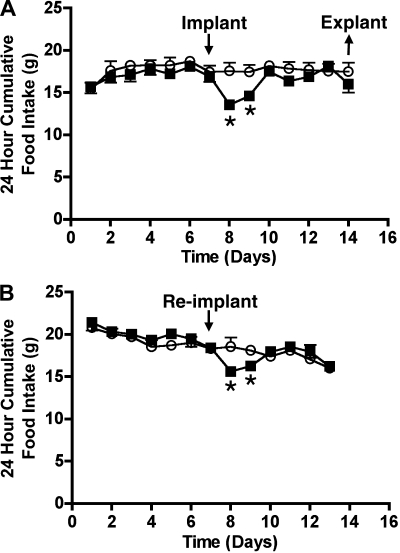
A significant reduction in food intake was seen in 100 μg·kg body wt−1·day−1 PYY(3-36)-treated rats on day 1 (control, 17.58 ± 0.99 g vs. treatment, 13.58 ± 0.27 g; P < 0.01) and day 2 (control, 17.44 ± 0.87 g vs. treatment, 14.62 ± 0.49 g; P < 0.01) postimplantation of osmotic minipumps; but food intake reached control levels by day 3 postimplantation (Fig. 1A). However, reimplantation of PYY(3-36) pumps recovered from these rats into a new group of rats again resulted in a significant reduction in food intake for 2 days postimplantation of the pumps (day 1 = control, 18.58 ± 0.62 g vs. treatment, 15.64 ± 0.50 g; day 2 = control, 18.25 ± 0.62 g vs. treatment, 15.94 ± 0.21 g; P < 0.05; Fig. 1B), with food intake returning to control levels on day 3 postimplantation.
Fig. 1.
Effects of continuous infusion of saline (○) or 100 μg·kg body wt−1·day−1 peptide YY(3-36) [PYY(3-36)] (▪) on daily food intake (A) of male Fischer 344 rats when infused using 14-day osmotic minipumps. Pumps were recovered from the animals after 7 days and reimplanted into another group of rats, and their daily food intake was measured (B). Data are presented as means ± SE. *Statistically significant difference compared with saline controls; n = 6 rats/group.
Effects of continuous infusion of PYY(3-36) on plasma leptin levels of ad libitum-fed rats.
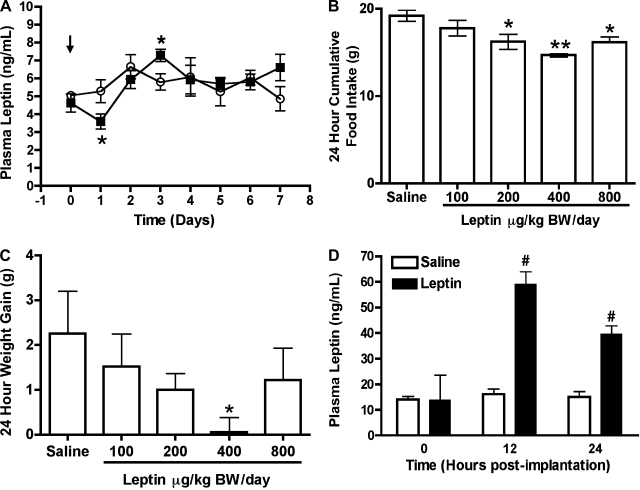
A significant reduction in plasma leptin levels was found in 100 μg·kg body wt−1·day−1 PYY(3-36)-treated rats on day 1 postimplantation (control, 5.28 ± 0.6 vs. PYY, 3.9 ± 0.42 ng/ml, P < 0.05, Fig. 2A), but leptin levels were normal on day 2 postimplantation and were significantly increased in PYY(3-36)-treated rats on day 3 postimplantation (control, 5.79 ± 0.45 vs. PYY, 7.28 ± 0.3 ng/ml, P < 0.05, Fig. 2A).
Fig. 2.
Effects of saline (○) or PYY(3-36) (100 μg·kg body wt−1·day−1; ▪) on daily plasma levels of leptin during continuous infusion using 7-day osmotic minipumps (A). Effects of leptin on 24-h cumulative food intake (B) and weight gain (C) when infused using 1-day osmotic minipumps. Circulating levels of leptin at 12 h and 24 h during the continuous leptin infusion of 100 μg·kg body wt−1·day−1 using osmotic minipumps for 1 day are shown in D. Arrow in A indicates day of pump implantation. Data are presented as means ± SE. *Statistically different (P < 0.05) from saline; **Statistically different (P < 0.01) from saline; #Statistically different (P = 0.0001) from saline; n = 6 rats/group.
Determination of a subanorectic dose of leptin in ad libitum-fed rats.
Compared with food intake of saline-treated rats (19.22 ± 0.63 g), leptin (200, 400, and 800 μg·kg body wt−1·day−1) significantly reduced food intake (16.22 ± 0.86 g, P < 0.05; 14.70 ± 0.18 g, P < 0.01; 16.18 ± 0.63 g, P < 0.05, respectively, Fig. 2B), while the leptin dose of 100 μg·kg body wt−1·day−1 did not. Among the doses tested, only 400 μg·kg body wt−1·day−1 leptin caused a significant reduction in body weight (control, 2.26 ± 0.94 g vs. treatment, 0.06 ± 0.32 g; P < 0.05, Fig. 2C). Circulating levels of leptin in 100 μg·kg body wt−1·day−1 leptin-treated rats were significantly higher than control rats at 12 h (control, 16.13 ± 2.03 ng/ml vs. treatment, 59.23 ± 4.81 ng/ml, P < 0.0001) and 24 h (15.06 ± 2.03 ng/ml vs. 39.67 ± 3.19 ng/ml, P < 0.0001) postimplantation of pumps (Fig. 2D).
Effects of coadministration of both leptin and PYY(3-36) on food intake and body weight of ad libitum-fed rats.
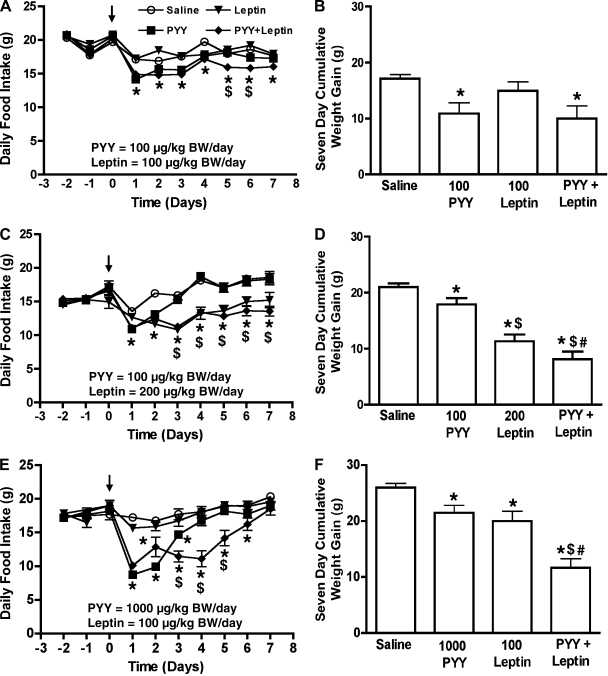
PYY(3-36) alone at 100 μg·kg body wt−1·day−1 (Figs. 3A and 4, A and C) and 1,000 μg·kg body wt−1·day−1 (Fig. 4E) caused a transient reduction in food intake of rats, compared with saline controls. Leptin alone at 100 μg·kg body wt−1·day−1 did not cause any effects on food intake (Fig. 4A), while 200 μg·kg body wt−1·day−1 leptin caused a significant reduction in food intake compared with saline-treated controls during the study period (Fig. 4C). Coadministration of 100 μg·kg body wt−1·day−1 leptin on day 1 during the 7-day infusion of 100 μg·kg body wt−1·day−1 PYY(3-36) did not prolong the anorectic effects of PYY(3-36) compared with saline-treated rats or rats that received PYY(3-36) alone (Fig. 3A), although food intake was significantly (P < 0.05) reduced on day 2 by the coadministration with leptin compared with PYY(3-36) alone. However, coadministration of this same dose of leptin for the full 7 days with either 100 μg·kg body wt−1·day−1 PYY(3-36) (Fig. 4A) or 1,000 μg·kg body wt−1·day−1 PYY(3-36) (Fig. 4E) prolonged the anorectic effects of PYY(3-36) compared with saline-treated rats or rats that received either peptide alone.
Fig. 4.
Effects of saline, PYY(3-36), leptin. or a combination of leptin and PYY(3-36) on daily food intake (A, C, and E), and 7-day cumulative weight gain (B, D, and F) of ad libitum-fed rats. Doses of peptides used are as indicated in each panel. In the body weight (BW) graphs, doses of peptides (μg·kg body wt−1·day−1) are provided in the X-axis together with the name of the peptide. PYY+leptin group received a combination of both PYY(3-36) and leptin at the individual doses tested in that study. Arrows indicate the implantation of pumps. In the coadministration study, both peptides were administered using the same pump. Data are presented as means ± SE. A and E: *statistically significant difference when the PYY(3-36)+leptin or PYY(3-36)-alone group was compared with saline (A and E); $statistically significant difference when the PYY(3-36)+leptin group was compared with PYY(3-36) alone-treated group. C: *statistically significant difference of both PYY(3-36)+leptin and leptin-alone groups compared with saline-treated controls; $statistically significant difference of these groups compared with PYY(3-36)-alone group. B, D, and F: *statistically significant difference of a group compared with saline controls; $statistically significant difference compared with PYY(3-36) alone-treated rats; #statistically significant difference compared with leptin alone-treated rats; n = 6 rats/group.
Relative to saline, administration of 100 μg·kg body wt−1·day−1 PYY(3-36) significantly reduced 7-day cumulative weight gain (17.15 ± 0.76 g vs. 10.88 ± 1.96 g, P < 0.01, Fig. 4B; 21.16 ± 0.63 g vs. 17.6 ± 1.02 g, P < 0.05, Fig. 4D and 26 ± 0.77 g vs. 21.48 ± 1.32 g, P < 0.05, Fig. 4F) as did leptin alone at 100 μg·kg body wt−1·day−1 (26 ± 0.77 g vs. 20 ± 1.79 g, P < 0.05, Fig. 4F) and 200 μg·kg body wt−1·day−1 (21.16 ± 0.63 g vs. 11.31 ± 1.25 g, P < 0.001, Fig. 4D). A combination of 100 μg·kg body wt−1·day−1 leptin and PYY caused a significant reduction in body weight (10.0 ± 2.28 g; P < 0.01; Fig. 4B) compared with saline controls (17.15 ± 0.76 g; Fig. 4B), but this reduction was not greater than that caused by PYY or leptin alone. Weight gain of rats was further diminished relative to controls when either the leptin or PYY doses was increased [100 μg·kg body wt−1·day−1 PYY(3-36) + 200 μg·kg body wt−1·day−1 leptin; 8.11 ± 1.37 g vs. 21.16 ± 0.63 g, P < 0.001, Fig. 4D] and 1,000 μg·kg body wt−1·day−1 PYY(3-36) + 100 μg·kg body wt−1·day−1 leptin (11.7 ± 1.61 g vs. 26 ± 0.77 g, P < 0.001, Fig. 4F). These decreases caused by the coadministration of leptin and PYY(3-36) were also greater compared with the weight gain of rats that received leptin or PYY(3-36) alone (Fig. 4, D and F).
Effects of leptin replacement on satiety actions of PYY(3-36) in rats fed after a 24-h fast.
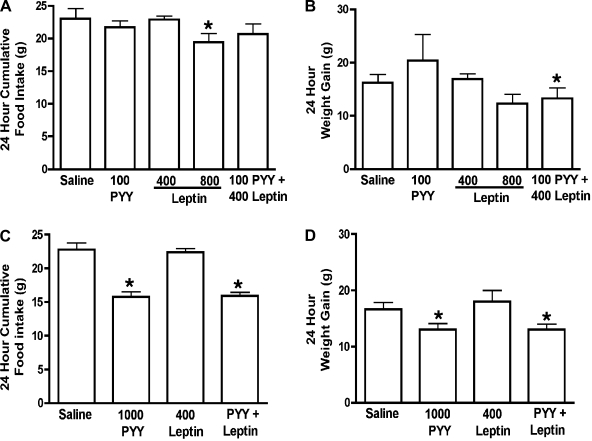
Infusion of 100 μg·kg body wt−1·day−1 PYY(3-36), an anorectic dose of PYY(3-36) in ad libitum-fed rats, was ineffective in reducing food intake in fasted rats (control, 23 ± 1.61 g vs. treatment, 21.7 ± 1 g, Fig. 5A). Administration of 1,000 μg·kg body wt−1·day−1 PYY(3-36) only caused ∼30% reduction in food intake in fasted rats (control, 22.78 ± 1.01 g vs. treatment, 15.76 ± 0.77 g, P < 0.01, Fig. 5C), whereas the same dose reduced day 1 food intake ∼42% reduction in fed rats (control, 17.26 ± 0.59 vs. treatment, 10.11 ± 0.46 g, P < 0.001, Fig. 4E). Leptin alone reduced food intake in the fasted rats when used at a dose of 800 μg·kg body wt−1·day−1 (control, 23 ± 1.61 g vs. leptin, 19.44 ± 1.34 g, P < 0.05, Fig. 5A) but not at 400 μg·kg body wt−1·day−1. Moreover, in contrast to the results obtained with fed rats (Fig. 4, D and F), coadministration of this relatively high dose of leptin (400 μg·kg body wt−1·day−1) and 1,000 μg·kg body wt−1·day−1 PYY(3-36) did not cause a greater reduction in weight gain compared with fasted rats that received PYY(3-36) alone (Fig. 5D). Leptin administration did not restore the anorectic effects of 100 μg·kg body wt−1·day−1 PYY(3-36) in fasted rats (Fig. 5A).
Fig. 5.
Effects of saline, PYY(3-36), leptin, or a combination of PYY(3-36) and leptin on 24-h cumulative food intake (A and C) and weight gain (B and D) of rats fed after a 24-h fast. Doses of peptides (μg·kg body wt−1·day−1) are provided in the X-axis together with the name of the peptide. The PYY+leptin group received a combination of both PYY(3-36) and leptin at the individual doses tested in that study. In the coadministration study, both peptides were administered using the same pump. Data are presented as means ± SE. *Statistically significant difference compared with saline controls; n = 6 rats/group.
DISCUSSION
Our results provide further evidence for the anorectic effects of PYY(3-36) in lean rats. The magnitude of reduction in food intake we found during PYY(3-36) administration was similar to that seen in other studies that delivered PYY(3-36) by osmotic pumps (4, 5). Despite the transient reduction in food intake we observed with chronic administration of PYY(3-36), the explant and reimplant studies indicate that PYY(3-36) remained biologically active while in the pumps for at least 9 days. The results of the present study are in agreement with our own previously published data (37) and results of others (19), which indicate that chronic administration of PYY(3-36) causes a transient reduction in food intake in rats. Commensurate with reduced food intake, 7-day continuous infusion of PYY(3-36) resulted in a significant reduction in circulating leptin levels of ∼35%. Unexpectedly, the reduction in plasma leptin levels was exclusive to 1-day postimplantation, reaching control levels on day 2. Interestingly, plasma leptin levels were significantly higher in the PYY(3-36)-treated rats compared with saline-treated controls on day 3 postimplantation of the pumps. This unanticipated increase might be a delayed compensatory response to the initial fall in leptin or caused by other physiological processes. Further studies are required to explore the possibility that PYY(3-36) modulates leptin release.
Chelikani et al. (21) found that daily intermittent infusion of PYY(3-36) caused a more prolonged decrease in food intake and body weight of lean rats than our findings with continuous PYY(3-36) delivery. These findings suggests that receptor desensitization or downregulation are two possible reasons why chronic administration of PYY(3-36) fails to cause a sustained reduction in food intake and body weight. However, it is also possible that the transient reduction in leptin levels during PYY(3-36) administration may have contributed to the lack of sustained anorectic effects of PYY(3-36) during chronic infusion. We hypothesized that the anorectic effects of PYY(3-36) could be prolonged by preventing this transient fall in leptin. First, we found a dose of leptin (100 μg·kg body wt−1·day−1) that on its own does not reduce food intake, despite yielding vastly increased circulating levels. Then, we coinfused this subanorectic dose of leptin for 1 day during a 7-day PYY(3-36) administration and found that preventing the transient fall in leptin by exogenous administration was unable to extend the anorectic effects of PYY(3-36). However, in a series of experiments shown in Fig. 4, we found that the coinfusion of this subanorectic dose of leptin for the entire duration of the PYY(3-36) infusion caused a prolonged reduction in food intake in ad libitum-fed rats. The reduction in body weight was greater in rats coinfused with certain doses of leptin and PYY(3-36) compared with rats treated with saline, leptin, or PYY(3-36) alone. While the prolonged reduction in food intake during coadministration of PYY(3-36) and leptin undoubtedly contributed to a greater reduction in body weight, effects of this peptide combination on other aspects of metabolism including energy expenditure, energy conversion, and physical activity might also have contributed.
The anorectic effects of PYY(3-36) are believed to be mediated through the distinct regions of the brain (1, 2). PYY(3-36) stimulates c-fos mRNA expression in a subset of neurons in the nucleus tractus solitarius of mice (25), and PYY(3-36) administration to humans modulates neural activity within both corticolimbic and higher cortical areas as well as homeostatic brain regions including the hypothalamus (13). It is possible that leptin modulates the actions of PYY(3-36) in one or more of these locations. Leptin action in the forebrain has been demonstrated to regulate the hindbrain responses to the satiety effects of CCK (32). This finding led the authors to propose that forebrain signaling by leptin limits food intake on a meal-to-meal basis by regulating the hindbrain response to short-acting satiety signals. Further investigations are required to unravel the precise mechanisms by which leptin and PYY(3-36) interact.
Fasting results in a reduction in plasma leptin levels (16, 26, 29). We found the satiety effects of PYY(3-36) were attenuated in fasted rats during a 24-h refeeding period. Previously it was demonstrated that the fasting-induced fall in leptin results in attenuation of the satiety effects of acute administration of CCK (29). However, unlike the response to CCK in that study, we were unable to recover the effects of PYY(3-36) on food intake in fasted rats after leptin replacement. This suggests that in fasted rats, factors in addition to leptin are involved in regulating the satiety effects of PYY(3-36). The fasting-induced fall in plasma leptin levels also does not appear to alter PYY levels (18). Fasting causes several other neuroendocrine changes, including changes in the expression of several appetite regulatory peptides and their receptors in the brain (26). Our results indicate that anorectic effects of both PYY(3-36) and leptin are blunted during fasting and exogenous leptin fails to reinstate the satiety effects of PYY(3-36) in fasted rats.
Perspectives and Significance
Our studies demonstrate the enhanced anorectic and weight-reducing effects of coadministration of leptin and PYY(3-36) and suggest this hormone combination could represent a promising approach to promote weight loss. However, the appetite regulatory effects of several peptides are species specific, and it has been shown that even PYY(3-36) itself displays varying degrees of anorectic potency among rodents (33). Therefore, further studies will be required to examine the effects of coadministration of PYY(3-36) and leptin in other animals. The weight loss effects of this therapy are predicted to be less in obese subjects who are leptin resistant. However, as recently demonstrated by Shapiro et al. (35), even a modest amount of physical activity may be able to reverse leptin resistance and thus would be anticipated to enhance the efficacy of a leptin-PYY combination therapy. Future studies are warranted to elucidate the mechanisms by which leptin prolongs the anorectic effects of PYY(3-36) along with the neuroendocrine factors responsible for attenuating the satiety effects of PYY(3-36) and leptin during fasting.
GRANTS
T. J. Kieffer is a Michael Smith Foundation for Health Research (MSFHR) scholar and received grant funding from the Canadian Institutes of Health Research (CIHR) and the MSFHR. S. Unniappan is a recipient of postdoctoral fellowships from the CIHR and the MSFHR. A part of this study was aided by York University start-up funds.
Acknowledgments
Recombinant murine leptin (lot no. AFP565) was obtained through the National Hormones and Peptides Program, National Institute of Diabetes and Digestive and Kidney Diseases, and Dr. A. F. Parlow.
We thank Sima Mortazavi for assistance in studies described in Fig. 3 of this article.
Present address of Suraj Unniappan: Laboratory of Integrative Neuroendocrinology, 221 Lumbers Bldg., Dept. of Biology, York University, 4700 Keele St., Toronto, ON M3J 1P3, Canada.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3-36) on food intake. Brain Res 1043: 139–144, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Abbott CR, Small CJ, Sajedi A, Smith KL, Parkinson JRC, Broadhead LL, Ghatei MA, Bloom SR. The importance of acclimatization and habituation to experimental conditions when investigating the anorectic effects of gastrointestinal hormones in the rat. Int J Obes 30: 288–292, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Adams SH, Won WB, Schonhoff SE, Lieter AB, Paterniti JR Jr. Effects of peptide YY[3-36] on short-term food intake in mice are not affected by prevailing plasma ghrelin levels. Endocrinology 145: 4967–4975, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Adams SH, Lei C, Jodka CM, Nikoulina SE, Hoyt JA, Gedulin B, Mack CM, Kendall ES. PYY(3-36) administration decreases the respiratory quotient and reduces adiposity in diet-induced obese mice. J Nutr 136: 195–201, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89: 1070–1077, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science 307: 1909–1914, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L, Guarnieri G. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology 124: 1532–1535, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Batterham RL, Cowley MA, Small CJ, Herzog HH, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418: 650–654, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 349: 941–948, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Batterham RL, Heffron H, Kapoor A, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4: 223–233, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 450: 106–109, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, Couzens M, Slack K, Dallmann R, Sainsbury A, Herzog H. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia 49: 1360–1370, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Boggiano MM, Chandler PC, Oswald KD, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Schindler M, Arndt K, Rudolf K, Mark M, Schoelch C, Joost HG, Klaus S, Thone-Reineke C, Benoit SC, Seeley RJ, Beck-Sickinger AG, Koglin N, Raun K, Madsen K, Wulff BS, Stidsen CE, Birringer M, Kreuzer OJ, Deng XY, Whitcomb DC, Halem H, Taylor J, Dong J, Datta R, Culler M, Ortmann S, Castaneda TR, Tschop M. Does gut hormone PYY(3-36) decrease food intake in rodents? Nature 430: 165–166, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Chacon F, Esquifino AI, Perello M, Cardinali DP, Spinedi E, Alvarez MP. 24-hour changes in ACTH, corticosterone, growth hormone, and leptin levels in young male rats subjected to calorie restriction. Chronobiol Int 22: 253–265, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O'Rahilly S. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc Natl Acad Sci USA 101: 4695–4700, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan JL, Stoyneva V, Kelesidis T, Raciti P, Mantzoros CS. Peptide YY levels are decreased by fasting and elevated following caloric intake but are not regulated by leptin. Diabetologia 49: 169–173, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Chelikani PK, Haver AC, Reidelberger RD. Comparison of the inhibitory effects of PYY(3-36) and PYY(1–36) on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 287: R1064–R1070, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3-36) potently inhibits food intake in rats. Endocrinology 146: 879–888, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Chelikani PK, Haver AC, Reeve JR Jr, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3-36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol 290: R298–R305, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3-36) on conditioned taste aversion in rats. Peptides 27: 3193–3201, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology 29: 1430–1436, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Halatchev IG, Cone RD. Peripheral administration of PYY(3-36) produces conditioned taste aversion in mice. Cell Metab 1: 159–168, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology 145: 2585–2590, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kappeler L, Zizzari P, Grouselle D, Epelbaum J, Bluet-Pajot MT. Plasma and hypothalamic peptide-hormone levels regulating somatotroph function and energy balance in fed and fasted states: a comparative study in four strains of rats. J Neuroendocrinol 16: 980–988, 2004. [DOI] [PubMed] [Google Scholar]
- 27.King PJ The hypothalamus and obesity. Curr Drug Targets 6: 225–240, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Koda S, Date Y, Murakami N. The role of the vagal nerve in peripheral PYY(3-36)-induced feeding reduction in rats. Endocrinology 146: 2369–2375, 2005. [DOI] [PubMed] [Google Scholar]
- 29.McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology 141: 4442–4448, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Mederois MD, Turner AJ. Processing and metabolism of peptide-YY: pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology 134: 2088–2094, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE. Peptide YY(3-36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 288: R384–R388, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittner RA, Moore CX, Bhavsar SP, Gedulin BR, Jodka CM, Parkes DG, Paterniti JR, Srivastava VP, Young AA. Effects of PYY(3-36) in rodent models of diabetes and obesity. Int J Obes Relat Metab Disord 28: 963–971, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 36: 199–211, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro A, Matheny M, Zhang Y, Tümer N, Cheng KY, Rogrigues E, Zolotukhin S, Scarpace PJ. Synergy between leptin therapy and a seemingly negligible amount of voluntary wheel running prevents progression of dietary obesity in leptin-resistant rats. Diabetes 57:614–622, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology 128: 175–191, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Unniappan S, McIntosh CHS, Demuth HU, Heiser U, Wolf R, Kieffer TJ. Effects of dipeptidyl peptidase IV on the satiety actions of peptide YY. Diabetologia. [DOI] [PubMed]