Abstract
Systemic viral infections produce a highly regulated set of responses in sickness behavior, such as fever, anorexia, and adipsia. Toll-like receptor (TLR)7, activated by viral RNA during infection, potently stimulates the innate and adaptive immune responses that aid in viral clearance. However, the physiological consequences of TLR7 activation have not been thoroughly studied. In these experiments, we used a potent synthetic TLR7 ligand, 9-benzyl-8-hydroxy-2-(2-methoxyethoxy)adenine (SM360320; 1V136), to investigate the consequences of TLR7 activation in genetically defined strains of mice. Administration of the drug by the nasal, intragastric, or intraperitoneal routes caused transient hypophagia, hypodypsia, and hypothermia. Analyses of mutant mouse strains indicated that these effects were dependent on the expression of TLR7, its adaptor protein MyD88, and TNF-α, and independent of IL-1β, IL-6 and cyclo-oxygenase-1 (COX1). Partial roles were also implied for mast cells and COX2. Although plasma TNF-α levels were significantly higher after systemic drug delivery, the behavioral effects were maximal when the agent was administered to the mucosa. Tissue and mucosal mast cells are known to express high levels of TLR7 and to rapidly release TNF-α upon TLR7 ligation. Mice deficient in tissue mast cells, W/W(v), had significantly less anorexia after TLR7 activation, and this response was restored with mast cell reconstitution. Our results thus suggest that tissue mast cells may play a role in the anorexia induced by mucosal activation of TLR7.
Keywords: anorexia, hypothermia
bacterial or viral infections are associated with inflammatory responses in the host that result in cytokine release from innate immune cells. As an essential part of the innate immune system, panels of Toll-like receptors (TLRs) have been identified as receptors that recognize pathogen-associated molecular patterns (28, 55, 56) and trigger cytokine release as part of the first line immune defense (52). Several of these cytokines, including IL-6, TNF-α, and IL-1β have been implicated in sickness syndrome, including fever, anorexia, adipsia, and depressed mobility. Studies examining the potential contribution of TLRs to a systemic sickness response have largely focused on the TLR4 ligand LPS (11, 26, 57, 58). Administration of LPS can mimic systemic inflammatory signals, which can result in anorexia, adipsia, and changes in temperature (7, 23, 57). The potential contributions of other TLR ligands have not yet been fully established.
Natural agonists for other TLRs have been identified as microbial or viral components, including peptidoglycans (TLR2) (49), viral RNA (TLR3) (1), flagellin (TLR5) (18), and bacterial DNA (TLR9) (5, 21). A natural ligand for TLR7 was recently identified as guanine and uridine-rich single-stranded (ss) RNA (13), which is found in several known ssRNA viruses, including vesicular stomatitis virus, measles virus, Sendai virus, mumps virus, parainfluenza virus-III, and coxsackie virus B1. In addition, several low-molecular-weight activators of TLR7 have been discovered, including imidazoquinolines, and purinelike molecules (20, 36, 37). Among the latter, 9-benzyl-8-hydroxy-2-(2-methoxyethoxy) adenine (SM360320; designated here as 1V136), has been shown to be a potent and specific TLR7 agonist (33). This compound induces high levels of type 1 IFN production by human peripheral blood leukocytes and inhibits hepatitis C viral replication in human TLR7-expressing hepatocytes (16, 22, 37).
Although administration of 1V136 might have beneficial antiviral effects, the potential for inducing a sickness syndrome also exists. Except for TLR3, all of the TLRs and IL-1β utilize the intracellular adaptor protein MyD88 to transmit proinflammatory signals (29, 59). This common intermediary suggests that TLR7 signaling will result in similar physiological responses, as LPS and IL-1β stimulation. The signaling cascades that stem from TLR ligation result in the nuclear translocation of transcription factors that induce the production of type I IFN, IL-6, TNF-α, and cyclooxygenase-2 (COX2). All of these factors have been described in the sickness response (4, 10).
In this study, we demonstrate that the mucosal administration of the TLR7 agonist, 1V136, transiently reduces food intake, and induces weight loss in mice, accompanied by hypothermia. Mice that lack lymphocytes have an enhanced anorexic response, suggesting that the innate immune cells are essential for this response. As part of the host's innate defense mechanisms, tissue mast cells release cytokines into the immediate area upon TLR stimulation (42). Analysis of W/W(v) mast cell-deficient TLR7 mice suggests that the mucosal mast cells are involved in the anorexic behavior induced by intranasally administered 1V136. Investigations of potential mediators indicate that TNF-α and COX-2 contribute to the anorexia, whereas IL-1β, IL-6, and COX-1 are not essential.
MATERIAL AND METHODS
Mice.
C57BL/6 (B6) mice, Lepob/ob, RAG-1−/−, IL-6−/−, IL-1 receptor (IL-1R)−/−, TNF-α−/−, WBB6F1/J-KitW/KitW−v [W/W(v)], and their counterpart control (W/W+/+) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). Type 1 IFN receptor-deficient (IFNAR−/−) and control 129SvEv mice were purchased from B&K Universal (East Yorkshire, UK). COX-1 and COX-2-null mice were purchased from Taconic Farms and fully backcrossed onto the C57BL/6 background. TLR4−/−, TLR7−/−, TLR9−/−, and MyD88−/− mice were a gift from Dr. S. Akira, (Osaka University, Osaka, Japan) and were backcrossed 10 generations onto the C57BL/6 background. Animals were bred and maintained at University of California, San Diego, in rooms at 22 ± 0.5°C on a 12:12-h light-dark cycle from 7 AM to 7 PM. Mice had ad libitum access to water and rodent chow with 10 kcal% fat (Research Diets, New Brunswick, NJ). All procedures and protocols were approved by the Institutional Animal Care and Use Committee.
Reagents.
SM360320 (1V136) was synthesized as previously described (33, 37), dissolved in DMSO (100 mM), and stored at −80°C until use. LPS (Escherichia coli, serotype 026:B6, Sigma, St. Louis, MO) was dissolved in isotonic pyrogen-free saline. Dexamethasone (DEX), chlorpheniramine maleate (CM, H1 receptor antagonist) (12), cimetidine (CIM, H1/H2 receptor antagonist), and cromolyn sodium (CROM) were purchased from Sigma. All reagents were suspended in saline before administration to mice.
In vivo experimental procedures.
Mice were separated into individual cages 1 day before treatment, and the bedding was not changed during the experiment. Individually housed mice were weighed and lightly anesthetized with isoflurane before drug administration. The mice received drug or vehicle intragastrically in 200 μl, intraperitoneally in 200 μl, or intranasally in 30–50 μl saline between 8 and 9 AM and were placed in cages with preweighed chow. LPS was dissolved in saline and 2 μg per mouse was administered intraperitoneally (27). For pharmacologic inhibition studies, mice were injected intraperitoneally with DEX (3 mg/kg), histamine receptor antagonists (CM or CIM) (20 mg/kg) (12), or CROM (400 mg/kg) (32) in 200 μl saline 1 h before administration of 1V136. Food intake was recorded by weighing the food before treatment and 24 h after 1V136 administration using a scale, model VIC511 (Acculab, Edgewood, NY). Percent food intake is expressed relative to mean food intake for a contemporaneous control group of the same strain (change in weight of food for treated mouse )/( mean change in weight of food from DMSO control mice of the same strain) × 100. In some experiments, water intake was measured using metabolic cages (Braintree Scientific, Braintree, MA). Blood was collected via retroorbital bleed using heparinized Natelson capillary tubes (Fischer Scientific, Pittsburgh, PA). Serum was separated by centrifugation and kept at −20°C.
Telemetric measurement of body temperature and locomotor activity.
Ten-week-old female C57BL/6 mice were anesthetized by isoflurane inhalation (1.2–2.0%). An implantable radio frequency transmitter [Data Sciences International (DSI) St. Paul, MN; TA 10ETA-F20] was surgically placed in the peritoneal cavity. Buprenorphine (0.1 mg/kg) was given 15–30 min before the anticipated recovery time of animals after surgery. The mice were allowed at least 6 days to recover before experimentation. The ambient temperature was 24 ± 2°C. Signals were calibrated to degrees Celsius (for temperature) or to counts per minute (for locomotor activity), as recommended by the manufacturer using the software Dataquest A.R.T. (DSI, St. Paul, MN). The transmitter signals were recorded intermittently for at least 6 days before the actual recording in an experiment. The body temperature and activity patterns were stabilized after this period of acclimation. Mice with transmitters were lightly anesthetized with isoflurane. Groups of eight mice were treated with 1V136 intranasally in 50 μl saline, intranasal saline; or LPS 2 μg ip starting at 9 AM. Data for temperature and activity were measured at 10-min intervals.
Conditioned taste aversion.
A modified method to the previously described protocol was used (14). First, mice were individually housed and habituated to water restriction. Mice were conditioned for 2 wk to scheduled, daily, 2-h water access during the light cycle in the test cage. Consumption was measured by the difference between the pre- and postconsumption weight of the total water bottle. Trained mice were given a 0.125% saccharin (Sigma) solution to drink for the first 30 min of fluid access. They were randomly divided into a vehicle treatment group, an intranasally administered 500 1V136 nmol treatment group, and a subcutaneous lithium chloride (LiCl, 20 mg/kg sc, Sigma) treatment group. Lithium chloride has been described to produce taste aversion and was used as a control. Each animal was given three training sessions in test cages, where they were presented with tap water and saccharin solution (0.125%) in two separate bottles. During the third training session the total intake of tap water and saccharin solution was measured 24 h after presentation as a baseline. The next day, the mice were given 1 h access to saccharin solution only in the test cages and received the treatments. Following treatment, they were presented with a two-bottle choice of water and saccharin solutions in the test cages, and their water and saccharin intake was measured for 24 h after presentation. The data were expressed as % saccharin-preference {100 × [saccharin intake/ (saccharin intake + water intake)]}.
Mast cell reconstitution.
Bone marrow cells were harvested from the femurs and tibia of donors and cultured in RPMI 1640 with 10% FCS (Omega Scientific, Tarzana, CA), 1% penicillin/streptomycin, 0.1 mM nonessential amino acids, 5 × 10−5M 2-mercaptoethanol and 3 ng/ml IL-3 (BD Biosciences Pharmingen, San Diego CA) for 6 wk serially monitoring the cultures until they were >95% pure by toluidine blue staining. The recipient W/W(v) mice were injected with 107 cells in 100 μl RPMI 1640 intravenously. After 6 wk of reconstitution the mice were tested for their anorexic response to 1V136.
Cytokine measurements.
Serum cytokine levels were measured by multiplex bead assay (Linco Research, St. Charles, MI) per the manufacturer's recommendations. The lowest limit of detection was 10 pg/ml for IL-6, 20 pg/ml for TNF-α, and 15 pg/ml for IL-1β. Serum leptin was measured using a commercial ELISA kit from Antigenix America (Huntington Station, NY), and the lower limit of detection was 70 pg/ml.
RNA expression in the hypothalamus.
After euthanasia, the brain was removed from the skull, leaving the pituitary under the dura and the ventral surface on top. To remove the hypothalamus, we used the optic chiasm and the rostral edge of the mamillary bodies as rostral and caudal limits, respectively. The hypothalamic sulci were used as lateral limits. The samples were immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was isolated using RNeasy Lipid Tissue Kit (Qiagen, Valencia, CA). Quantity and purity were determined by absorbance using a NanoDrop spectrophotometer. cDNA was prepared from total RNA (1 μg) using an iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA), according to the manufacturer's instructions. All genes of interest were assayed by real-time PCR in a 25-μl reaction volume using TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) using an iCycler IQ (Bio-Rad). All samples were normalized to the housekeeping reference gene 18s RNA (ΔCt). The comparative ΔΔCt method was employed to measure fold changes in expression of RNA transcript levels between untreated (t = 0 h) and drug-treated mice for each strain.
Statistical analysis.
Data are expressed as means ± SE. The analysis used a paired or unpaired Students' t-test for comparing two groups and ANOVA for multiple-group comparisons. Dunnett's post hoc tests were used for multiple comparisons to a control group, and Bonferonni post hoc tests for multiple pairwise comparisons. Telemetric temperature and activity data were analyzed using repeated-measures ANOVA under compound symmetry. The complete time course of the data is presented graphically, and we indicate statistically significant (Bonferroni corrected) differences for each 10-min interval on the corresponding graphs. In addition, the 9 h of the study period were grouped into 3-h periods, and average differences over each period were studied by an ANOVA with main effects for period, treatment, and a period × treatment interaction. If the period × treatment interaction was significant, then treatment effects and 95% confidence intervals are presented within each time period. Statistical significance was assessed at the 5% level.
RESULTS
Intranasal administration of 1V136 suppressed oral intake, enhanced weight loss, and induced hypothermia but did not diminish activity.
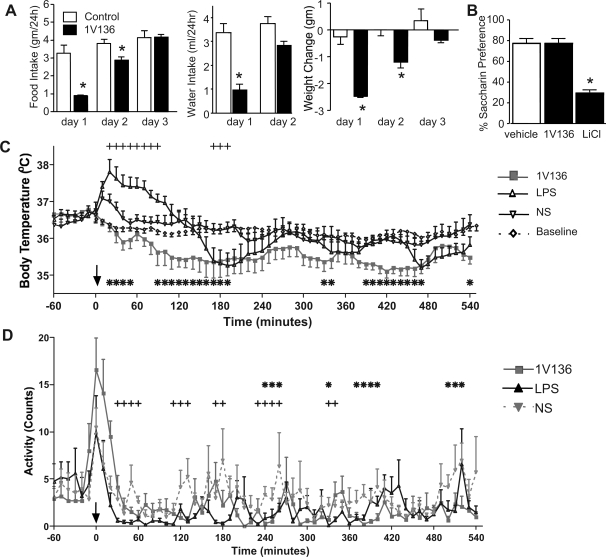
LPS, a TLR4 agonist, has been well documented to induce behavioral responses known as “sickness behavior” (10). These include decreased locomotor activity, oral intake, and dose-dependent hypothermia or hyperthermia (30, 31, 45). To assess the physiological effects of the TLR7 agonist, 1V136, mice were treated intranasally with 500 nmol of 1V136 or with vehicle (10% DMSO) alone within the second hour of the light cycle. In preliminary experiments, the food intake was measured at 2 h, 4 h, 6 h, 8 h, and 24 h. The control mice consumed less than 10% of their daily intake within the first 8 h (data not shown), and only the 24-h time point was assessed in subsequent experiments. During 24 h, the mice that received 1V136 reduced their food and water intake and lost weight (Fig. 1A). The mice recovered their water intake after 1 day, and their food intake and body weight after 2 days (Fig. 1A). Intranasal 1V136 did not induce a conditioned taste aversion (Fig. 1B). There were no significant gender differences in the induction of anorexia by 1V136 (data not shown).
Fig. 1.
Intranasal administration of 1V136 decreased food and water intake, induced weight loss, and reduced body temperature. A: six- to eight-week-old female C57BL/6 mice (n = 6) received 500 nmol intranasally. 1V136 or saline intranasally containing 10% DMSO (vehicle). Food, water intake, and body weight were monitored as indicated. Data shown are given as means ± se. *P < 0.03 compared with the vehicle control group by unpaired Student's t-test. B: conditioned taste aversion was assessed following treatment with vehicle intranasally (n = 6), IV136 500 nmol intranasally (n = 9), or subcutaneous lithium chloride (LiCl) (20 mg/kg) (n = 4). After treatment, the mice were presented with a 2-bottle choice of water and 0.125% saccharin solution, and their intake was measured for 24 h. The data are expressed as mean% saccharin preference. *P < 0.01 using Dunnet's post hoc test of multiple comparisons to the vehicle control group. C: mice were implanted with radiofrequency transmitters and allowed at least 6 days to reestablish their baseline in food intake and body weight. The average of the baseline temperature tracings for the day prior to treatment is shown. At time 0, mice (n = 8/group) received normal saline intranasally, 1V136 (500 nmol) intranasally, or LPS (2 μg) intraperitoneally. In a two-way repeated-measures ANOVA, the 9 h of the study period were grouped into 3-h periods, and temperature was compared between treatments averaged over these 3-h periods: for treatment (P = 0.03), period (P < 0.001), and treatment × period (P < 0.001). *, +Significant time points for 1V136- and LPS-treated mice, respectively, by Bonferroni post hoc comparisons. D: activity level of the same mice was also monitored over this period. In a two-way repeated measures ANOVA, the 9 h of the study period were grouped into 3-h periods, and temperature was compared between treatments averaged over these 3-h periods: treatment (P = 0.03), period (P = 0.005), and treatment × period (P = 0.002) were significant. *, +Significant time points for 1V136- and LPS-treated mice, respectively, by Bonferroni post hoc comparisons.
Sickness behavior during inflammation or infection is accompanied by changes in core temperature, as well as anorexia (38). To study the effects of mucosal administration of 1V136 on body temperature, we monitored saline and 1V136 intranasally treated mice for temperature fluctuations over 9 h using implanted radio frequency transducers (Fig. 1C). In the analysis, we first grouped the 9 h of the study period into three 3-h periods and compared temperature between treatments averaged over these 3-h periods. In a two-way repeated-measures ANOVA, treatment (P = 0.03), period (P < 0.001), and treatment × period (P < 0.001) were significant, indicating that temperature differences between treatments were not the same for the three time periods studied. In a more detailed analysis, we examined differences in each 10-min period using the same repeated-measures ANOVA with a Bonferroni correction. Estimating treatment differences averaged over each 3-h period showed a statistically significant decrease in temperature for the 1V136-treated mice compared with saline during the first 3-h period [mean difference −0.80; 95% confidence interval (CI) −1.57, −.04], while temperature in the LPS mice was increased, although not significantly so, compared with controls (mean difference 0.33; 95% CI −0.46, 1.11). Detailed analysis showed temperature in the LPS mice to be significantly elevated during the beginning of the 3-h period and significantly decreased at the end of the 3-h period (Fig. 1C). From 3 h to 6 h postinjection, average temperature was significantly decreased in the 1V136 mice [mean difference from controls −1.36 (95% CI −2.13, −0.60)] and slightly decreased, although not significantly so, in the LPS mice [mean difference from controls −0.34 (95% CI −1.13, 0.45)]. From 6 to 9 h, average temperature was decreased, but not significantly so, for both the 1V136 and LPS mice [mean difference from controls 1V136 −0.70 (95% CI −1.46, 0.06); LPS −0.28 (95% CI −1.06, 0.51)]. Detailed analysis showed the temperature for the IV136 mice was significantly decreased in the middle of this period, around 420 min. A comparison of mean activity between 1V136 and LPS-treated mice showed that temperature was significantly depressed for 1V136 during the first (P = 0.0012) and second (P < 0.0001) 3-h period but did not differ significantly by the third 3-h period (P = 0.35).
The activity of the mice was also recorded (Fig. 1D). In a similar two-way repeated-measures ANOVA, treatment (P = 0.03), period (P = 0.005), and treatment × period (P = 0.002) were significant, indicating that treatment differences were not the same across the three time periods studied. Estimating treatment differences within each period showed a small and nonsignificant increase in activity for the 1V136-treated mice compared with saline during the first 3-h period [mean difference 1V136 − saline 0.42 (95% CI −0.83, 1.67)], while activity in the LPS mice was significantly decreased compared with controls [mean difference −1.73 (95% CI −3.02,−0.43)]. From 3 h to 6 h postinjection, activity was significantly decreased in both the 1V136 and the LPS mice [mean difference from controls 1V136 −1.33 (95% CI −2.59, −0.08); LPS −1.6 (95% CI −2.89, −0.31)]. From 6 to 9 h, activity was significantly decreased in the 1V136 mice [mean difference from controls −1.33 (95% CI −2.75, −0.24)] and decreased, although not significantly so, in the LPS mice [mean difference from controls −0.82 (95% CI −2.11, 0.47)]. A comparison of mean activity between 1V136- and LPS-treated mice showed that activity was significantly elevated for 1V136 during the first 3 h (P < 0.002) but did not differ significantly in either of the other two periods (P value for each comparison >0.15).
Mucosal administration more potently induced anorexia than systemic injection.
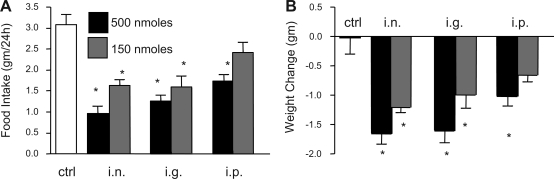
Initially, the biologically effective doses of intranasal 1V136 were identified by dose titration. A lower dose of 50 nmol, however, did not produce anorexic response (data not shown). Doses of 150 and 500 nmol administered to mice intranasally resulted in anorexic behavior and weight loss over a 24-h period (Fig. 2, A and B). A lower dose of 50 nmol in, however, did not elicit an anorexic response or weight loss (data not shown). We then tested the effect of different routes of exposure to the TLR7 agonist by administering 1V136 intraperitoneally, intragastrically, or intranasally. All routes differed significantly from control at the 500-nmol dose, and all except the 150-nmol ip dose (Dunnet's comparsons vs. control, P < 0.01) differed. In a two-way ANOVA, dose and treatment were significant (P < 0.01), but the interaction term was not (P > 0.50). Mucosal delivery by either intranasal or intragastric routes resulted in weight loss relative to intraperitoneal administration (P < 0.001), but the two mucosal routes, intranasal and intragastric did not differ significantly from each other (P = 0.64) (Fig. 2A). Results for 24-h weight loss were similar (Fig. 2B).
Fig. 2.
Mucosal administration of 1V136 is more potent than systemic administration. C57BL/6 mice were treated intranasally, intragastrically, or intraperitoneally with 1V136 (500 or 150 nmol). Group sizes included 500 nmol ig, n = 8; 150 nmol ig, n = 11; 500 nmol in, n = 6; 150 nmol in, n = 12; 500 nmol ip, n = 12; 150 nmol ip, n = 12; and control, n = 12. Food intake (A) and body weight (B) were monitored for 24 h. Data shown are given as means ± SE. *P < 0.01 using Dunnet's post hoc test of multiple comparisons to the vehicle control group.
Proinflammatory cytokines, such as IL-6 and TNF-α, are induced by TLR ligation and are known to cause anorexia (11). In mice treated with 150 nmol 1V136 ip or in, the peripheral serum levels peaked 2 h after either route of administration (Table 1). Although anorexic behavior induced by 150 nmol of 1V136 was greater in the intranasally treated group than in the intraperitoneally injected group, the peak levels of serum cytokines were higher in the intraperitoneally treated group (P < 0.05). For comparison, intraperitoneal LPS also resulted in a significant rise in systemic IL-6 and TNF-α, also peaking at 2 h. An increase in the appetite suppressant leptin has been implicated in LPS-induced anorexia (15, 48). The level of leptin roughly doubled in the serum of intranasally treated 1V136 mice peaking by 6 h, similar to the LPS-treated mice.
Table 1.
Serum cytokine levels following 1V136 administration
| Route |
IL-6 (pg/ml) |
TNF-α, pg/ml
|
Leptin, ng/ml
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Vehicle |
1V136, 150 nmol
|
LPS, 2 μg
|
Vehicle
|
1V136, 150 nmol
|
LPS, 2 μg
|
1V136, 150 nmol
|
LPS, 2 μg
|
|||||
| IN | IP | IN | IP | IP | IN | IP | IN | IP | IP | IN | IP | |
| Time* | ||||||||||||
| 0 h | <10 | <10 | <10 | <10 | <10 | < 20 | < 20 | < 20 | < 20 | < 20 | 1.34±0.24 | 1.32±0.18 |
| 2 h | <10 | <10 | 1350±475‡ | 4576±338‡§ | 6304±530‡§ | 48±47 | < 20 | 265±69 | 510±84‡§ | 415±93‡ | 1.43±0.44 | 1.21±0.41 |
| 6 h | <10 | <10 | 368±82‡ | 566±75‡ | 1172±327‡ | < 20 | < 20 | 23±4 | 33±6 | 39±8 | 2.57±0.50 | 2.47±0.58 |
| 24 h | 22±20 | <10 | 115±56 | 74±29 | 372±118 | < 20 | < 20 | < 20 | < 20 | < 20 | 1.10±0.30 | 1.32±0.22 |
Values are expressed as means ± SE. Mice were treated with vehicle intranasally (n = 6), 150 nmol in 1V136 (n = 8) or intraperitoneally (n = 8), or 2 μg ip LPS (from Escherichia coli 026:B6) (n = 8). Sera were collected 0, 2, 6, and 24 h after the treatment.
Hours after treatment.
† P < 0.001 compared to 0 h by paired Student's t-test.
P < 0.05 compared to intranasal administration by unpaired Student's t-test.
1V136-induced anorexia is mediated by the TLR7 and MyD88 pathway.
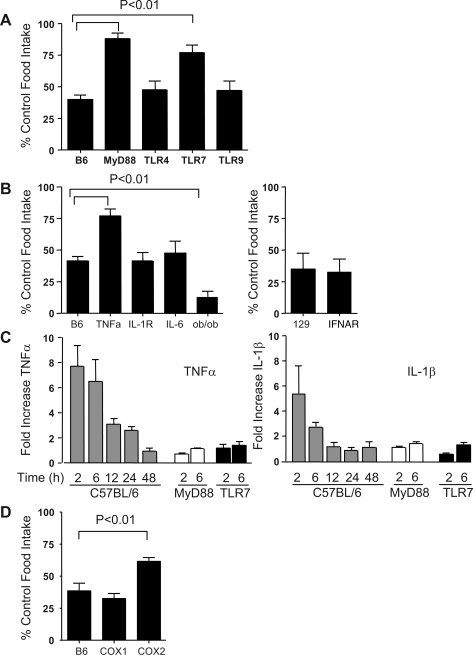
We previously demonstrated the TLR7 specificity of 1V136 in antiviral activity (37). To examine whether the 1V136-induced anorexic response was also induced via the TLR7 pathway, we administered 1V136 to various TLR-deficient mice. All mice received 500 nmol 1V136 in, and food intake and body weight were monitored for 24 h. TLR7−/− and MyD88−/− showed significantly less anorexia, whereas the TLR4−/− and TLR9−/− mice reduced their food intake and lost weight (not shown), similar to wild-type mice (Fig. 3A).
Fig. 3.
Reduction of food intake by 1V136 is mediated by the TLR7/MyD88 pathway and is TNF-α and COX-2 dependent. Mice from each strain were randomly divided into two age-matched groups and treated intranasally with vehicle or 1V136 (500 nmol). Food intake was measured 24 h after treatment and is shown as a mean percentage of control ± SE. A: data from three repeated experiments for the indicated mouse strain, in which the number of mice (n) that received 1V136, mean initial weight (MIW) ± SE, and the mean control food intake (MCFI) ± SE for a similar number of mice from that strain treated with vehicle alone are shown. Mutant mice tested include strains MyD88−/− (n = 15, MIW 28.1 ± 1.7 g, MCFI 4.0 ± 0.2 g), TLR4−/− (n = 6, MIW 30.2 ± 0.5 g, MCFI 3.7 ± 0.2 g), TLR7−/− (n = 9, MIW 23.1 ± 0.5 g, MCFI 3.9 ± 0.2 g), TLR9 −/− (n = 8, MIW 23.5 ± 1.3 g, MCFI 3.4 ± 0.2 g), and C57BL/6 mice (n = 7, MIW 22.3 ± 0.6 g, MCFI 3.7 ± 0.2 g). B: pooled data for two repeated experiments with C57BL/6 (n = 16, MIW 23.4 ± 0.4 g, MCFI 3.9 ± 0.2 g), TNF-α−/− (n = 10, MIW 16.4 ± 0.3 g, MCFI 3.7 ± 0.2 g), IL-1R−/− (n = 15, MIW 22.4 ± 0.9 g, MCFI 3.8 ± 0.2 g), IL-6−/−(n = 13, MIW 20.6 ± 0.4 g, MCFI 3.2 ± 0.2 g), and Lepob/ob (n = 5, MIW 32.0 ± 0.7 g, MCFI 5.0 ± 0.6 g). On a separate axis is shown pooled data from two experiments using wild-type 129SvEv (n = 4, MIW 23.1 ± 0.7 g, MCFI 3.4 ± 0.2 g), and IFNAR−/−-deficient mice (n = 13, MIW 22.8 ± 0.5 g, MCFI 2.9 ± 0.3 g). C: C57BL/6 (n = 4/group), MyD88 (n = 3/group), or TLR7−/ mice were untreated (t = 0) or treated intranasally with 500 nmol of 1V136 and killed 2 h, 6 h, 12 h, 24 h, or 48 h later. The hypothalami were removed, and the relative levels of mRNA expression levels were assessed by quantitative PCR for TNF-α and IL-1β. D: pooled data from four experiments with C57BL/6 (n = 7, MIW 20.8 ± 0.8 g, MCFI 3.3 ± 0.3 g), COX-1−/− (n = 12, MIW 22.9 ± 0.6 g, MCFI 3.2 ± 0.2 g), and COX-2−/− (n = 18, MIW 22.0 ± 0.5 g, MCFI 3.3 ± 0.2 g) mice are shown. Significant differences were determined by ANOVA with Dunnett's multiple-comparison post test compared with the wild-type group.
Mediators of the anorexic effects of 1V136.
Several proinflammatory cytokines and endocrine hormones have been suggested as mediators in anorexia induced by inflammation or infections (11, 58). Specifically, leptin, IL-1β, IL-6, and TNF-α have been described to mediate TLR4-induced anorexia (15, 26, 48, 58, 60). In addition, type I IFN exerts direct effects on the endocrine system by activating the neurosecretory hypothalamic neurons, regulating the hypothalamic-pituitary-adrenocortical axis, and thus modulating food intake (8). We, therefore, used various gene-deficient mice to study the mechanism of 1V136-induced anorexia. 1V136-induced anorexic behavior in IFNAR−/−, IL-1R−/−, and IL-6−/− was not significantly different from the wild-type controls (Fig. 3B). Leptin deficient (ob/ob) mice demonstrated an enhanced anorexic response to 1V136 (Fig. 3B). In contrast, TNF-α-deficient mice were relatively refractory to the compound (Fig. 3B).
To further examine the central response of intranasal 1V136, the hypothalami of treated mice were removed at 0 h, 2 h, 6 h, 12 h, 24 h, and 48 h posttreatment (Fig. 3C). The mRNA expression levels of key inflammatory cytokines were measured by quantitative PCR, and the relative increases were compared with the baseline levels (Fig. 3C). There was an increase in the level of TNF-α mRNA that diminished with time but remained greater than twofold up to 24 h in the hypothalami of 1V136-treated mice. In contrast, there was a more modest fivefold increase in IL-1β mRNA that rapidly declined to approximately twofold after 6 h. The levels of TNF-α and IL-1β mRNA in 1V136-treated MyD88 and TLR7 mice remained less than twofold that of untreated mice. There was no measurable increase in IL-6 mRNA levels (data not shown).
Another potential central nervous system mediator of 1V136-associated hypophagia and anorexia is prostaglandin E2 (PGE2) and other prostanoids (4). The cyclooxygenases (COX) are essential in the central and peripheral production of PGE2. In mutant mice deficient in the inducible form of cyclooxygenase, COX-2, the effect of TLR7 stimulation was diminished, but not in COX-1-deficient mice (Fig. 3D).
Anorexic behavior induced by 1V136 is attenuated in W/W(v) mice.
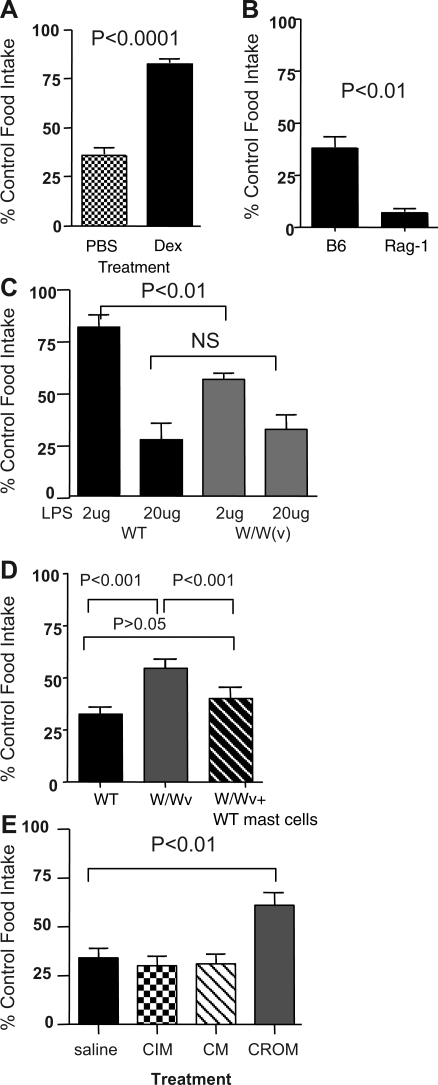
Dexamethasone treatment suppressed the anorexic response to 1V136, implicating an immune cell-mediated mechanism subsequent to TLR7 ligation (Fig. 4A). TLRs are expressed on a variety of immune cells, including T and B cells, macrophages, dendritic cells, and mast cells (2). To separate the innate immune components from the adaptive immune system, T/B cell-deficient mice (RAG-1−/−) were tested for their response to intranasal 1V136. The RAG-1-deficient mice became severely anorexic (Fig. 4B). The profound response in the RAG-1-deficient mice suggested that innate immune cells may be predominantly involved and that these effects were modulated by cells of the adaptive immune compartment.
Fig. 4.
W/W(v) mice are resistant to 1V136-induced anorexia, and 1V136 mast cell reconstitution restores the response. A: age-matched female C57BL/6 mice (n = 10/group) were injected intraperitoneally with saline, or dexamethasone (3 mg/kg) 60 min prior to intranasal treatment of 1V136, and food intake was monitored for 24 h. These responses were normalized to control groups that received PBS or dexamethasone intraperitoneally and then vehicle intranasally. Mean percent control food intake ± SE is shown. Dexamethasone-treated mice were relatively refractory to 1V136-induced anorexia (P = 0.01 by Student's t-test). B: C57BL/6 (n = 6, MIW 22.3 ± 0.8 g, MCFI 3.9 ± 0.8 g), and RAG-1−/− (n = 6, MIW 24.3 ± 0.3 g, MCFI 3.2 ± 0.1 g), mice were treated intranasally with vehicle or 500 nmol 1V136, and food intake was monitored for 24 h. Lymphocyte deficiency enhances the response to 1V136 (P < 0.01 by unpaired Student's t-test). C: W/W(v) and wild-type mice (W/W+/+) (n = 7 per group) received vehicle alone, 2 μg or 20 μg LPS intraperitoneally, and food intake for 24 h was measured. W/W(v) or wild-type (WT) mice treated with vehicle alone served as the controls. D: W/W(v) mice, their counterpart WT controls (W/W+/+), and W/W(v) mice that were reconstituted for 6 wk with WT mast cells were divided into two groups and treated intranasally with vehicle or 500 nmol 1V136, and food intake was monitored for 24 h. WT (n = 20, MIW 18.5 ± 0.3 g, MCFI 2.9 ± 0.1 g), W/W(v) (n = 19, MIW 16.8 ± 0.6 g, MCFI 2.4 ± 0.1 g), and W/W(v) reconstituted with WT mast cells (n = 12, MIW 20.8 ± 0.5 g, MCFI 3.5 ± 0.2 g) were tested in two experiments. Mean percent control food intake ± SE is shown. Reported P values are from ANOVA with Bonferroni multiple comparison post hoc analysis. E: saline or histamine receptor antagonists cimetidine (CIM) or chlorpheniramine maleate (CM), or cromolyn (CROM), were intraperitoneally administered 1 h prior to intranasal administration of 1V136, and food intake was measured at 24 h. The control group received saline intraperitoneally and then vehicle intranasally. Data are pooled from two experiments for a total of 8 mice/group of age-matched C57BL/6 female mice. Mean percent control food intake ± SE is shown. Significant differences between groups were determined by ANOVA with Dunnett's post hoc test compared with the control group.
Recently, connective tissue mast cells have been reported to release proinflammatory cytokines and chemokines upon TLR7 ligation (42). As mucosal administration of 1V136 was more potent than systemic administration (Fig. 2, A and B), we hypothesized that connective tissue mast cells located near the mucosal surface might be involved in TLR7-induced anorexia. We, therefore, tested the anorexic response of W/W(v) mice, which are tissue mast cell deficient or control mice (W/W+/+) to two doses of intraperitoneal LPS (Fig. 4C). The W/W(v) mice had a diminished response to the lower dose of LPS (2 μg). They reduced their food intake to a comparable level to the wild-type mice when receiving the higher dose (20 μg), but they started with lower food intake overall.
We then compared the responses of the W/W(v) mice and the control mice (W/W+/+) to intranasal 1V136. The W/W(v) mice showed ∼25% resistance to this compound compared with the controls (Fig. 4D). Reconstitution of the mast cell-deficient mice with wild-type mast cells recapitulated the wild-type response to 1V136 (Fig. 4D). The effects in the mast cell-deficient mice could partially be attributed to changes in peripheral serum TNF-α or IL-6, as there was a trend for these cytokines to be lower in the 1V136 treated W/W(v) mice compared with controls (Table 2). Serum IL-1β levels were below the reliable detection limit.
Table 2.
Serum cytokine release after 1V136 administration
| Strain | Treatment | IL-6, pg/ml | TNF-α, pg/ml |
|---|---|---|---|
| W/W+/+ | DMSO | <10 | <20 |
| 1V136 treated | 460±86* | 146±23 | |
| W/W(v) | DMSO | <10 | <20 |
| 1V136 treated | 190±14 | 112±28 |
Values are expressed as means ± SE. WBB6F1/J-KitW/KitW−v [W/W(v)] or wild-type controls (W/W+/+) mice were treated (n = 10) intranasally with 500 nmol 1V136 or DMSO, and sera were sampled 2 h later.
* P < 0.01 compared to the 1V136 treated W/W(v) group by Student's t-test.
In addition to cytokines, histamine is a signature effector molecule released during mast cell degranulation, and some of the histamine receptors contribute to behavioral changes in animals (43, 53). We therefore, injected histamine receptor antagonists, chlorpheniramine maleate (CM, H1 receptor antagonist), cimetidine (CIM, H1/H2 receptor antagonist), and a mast cell stabilizer, cromolyn sodium (CROM), prior to 1V136 administration (Fig. 4E). The mast cell membrane stabilizing agent reduced the anorexic effects of the TLR7 agonist, while the histamine receptor antagonists were ineffective.
DISCUSSION
Microbial infection is often accompanied by sickness behavior that includes fever, anorexia, and lethargy. These reactions are considered as active and adaptive responses initiated by the immune system that produces temporary suspension of normal homeostasis to facilitate clearance of pathogens and return to normal status (4). During the first stage of a microbial infection, mammalian TLRs play a major role as sensors for invasion of infectious agents and activate the innate immune system (55). TLR-induced proinflammatory cytokines secreted by the activated immune cells are known to reduce animals' motivation for food intake (10, 26, 57). Here, we showed that the mucosal administration of a low-molecular-weight synthetic TLR7 ligand, 1V136, induced temporary anorexia via the TLR7/MyD88 pathway. The hypophagia and hypodipsia were accompanied by hypothermia and a reduction in locomotor activity (Fig. 1). Mucosal delivery of 1V136 did not induce a conditioned taste aversion as 1V136-treated mice preferred water with 0.125% saccharin at similar levels to vehicle-treated mice (Fig. 1B). This was a relative assessment as the 1V136-treated mice developed hypodipsia and had an overall reduced drive for fluid intake. Alternatively, the hypophagia and weight loss induced by 1V136 might have been primarily due to the hypodipsia. Another consideration is the ambient temperature. All values measured may have been altered if the animals had been tested within their thermoneutral zone, as mice are expected to be metabolically stimulated at relatively low housing temperatures.
Of the known TLR ligands, LPS, a component in the cell walls of gram-negative bacteria, is the best studied in rodent models of pathogen-induced sickness behavior (11, 26, 58). LPS is a potent TLR4 agonist. Although the intracellular signals activated by TLR4 ligation can be transmitted through MyD88, TLR4 can alternatively utilize the adaptor TRIF, whereas TLR7 obligately signals through the adapter protein MyD88. MyD88 has also been shown to be a key mediator of LPS-induced anorexia, and similarly, the anorexia induced by the TLR7 agonist 1V136 was strictly dependent on the adapter protein MyD88 (44). The alternatives in signaling cascades between TLR4 and the other TLRs may result in different responses.
In our experiments, intranasal 1V136 resulted in a hypothermia response that lasted ∼8 h, and the pyrexia induced by intraperitoneal LPS peaked ∼30 min after administration. The hyperthermia reported for intraperitoneal TLR2 and TLR4 ligands is dose dependent, and particularly high doses of LPS cause hypothermia (23, 47). Hence, we tested mice treated with a lower dose of 150 nmol of intranasal 1V136 and found a hypothermia response using a rectal probe to the lower dose as well (data not shown). It has yet to be determined whether the hypothermic effects of LPS might be due to the relative levels of the lipid A component, which can alter the signaling ratio between the TRIF and MyD88 pathways (17).
Anorexia is a complex response controlled by a number of signals from the immune, neural, metabolic, and endocrine systems (4, 34). Because proinflammatory cytokines and endocrine hormones are known to regulate LPS-induced anorexia, we tested the involvement of type 1 IFN, IL-1β, IL-6, TNF-α, leptin, and cyclooxygenase in 1V136-induced anorexia (11, 26, 58)]. The TLR7-initiated anorexia was dependent on TNF-α (Fig. 3B), which is partially responsible for the full effect of LPS-induced anorexia (46). Although IL-1β production is partially responsible for the MyD88-dependent anorexic response to LPS (35, 44), IL-1R-deficient mice demonstrated anorexic behavior comparable to wild-type mice when treated with the TLR7 agonist (Fig. 3B). Similarly, IL-1R-null mice are not protected from LPS-induced anorexia, as they are more sensitive to TNF-α (6). Intranasal administration of 1V136 also resulted in a sustained increase in hypothalamic TNF-α mRNA and a shorter-lived increase in IL-1β mRNA transcription. The effect of the TLR7 agonist may have been from both circulating and centrally produced cytokines. However, administering 150 nmol ip of the drug resulted in higher peripheral serum cytokine levels than intranasal administration, yet had a lesser anorexic response (Table 1). This difference suggests that cytokines in the central nervous system might have played a predominant role in producing anorexia following mucosal TLR7 stimulation.
1V136 is an orally available compound, and gastric administration resulted in significant anorexia at 150 nmol (Fig. 2). The anorexia at this dose was greater for oral than for intraperitoneal administration. Both routes of mucosal administration (intranasal and intragastric) of 1V136 were found to exert more potent behavioral effects than systemic administration of the same drug (Fig. 2). These data suggest that resident tissue cells rather than circulating leukocytes regulated the behavioral changes. In barrier tissues, mast cells that express TLRs facilitate first-line responses by secreting chemokines that recruit immune cells to the sites of infection (42). Our experiments showed that c-kit mutant mice [W/W(v)], which lack mast cells, displayed significantly less anorexia, than wild-type animals after mucosal TLR7 activators. Moreover, the anorexic response was restored in W/W(v) mice by reconstitution with wild-type mast cells.
Mast cells are a major source of biogenic amines, which are known to influence food intake, sleeping patterns, and thermoregulation (40, 54). We found that cromolyn, which stabilizes cell membranes, significantly dampened the anorexic response to TLR7 stimulation, reinforcing the potential importance of a released factor. Mast cells have large granules rich in histamine and heparin. However, peripheral administration of histamine receptor H1 and H2 receptor antagonists did not impact 1V136-induced anorexia (24, 43, 53), suggesting that these major components of mast cell granules are not mediating this effect.
TLR7 stimulation of mast cells initiates the rapid release of IL-1β and TNF-α but does not result in degranulation (42). The release of IL-1β and TNF-α might have induced cyclooxygenases (3, 41). Our data indicate that COX-2 was also involved in TLR7-induced anorexia. However, COX-1 did not significantly impact the overall anorexic response. In LPS and IL-1β-induced hypophagia, COX-1 might effect behavior immediately after stimulation, whereas COX-2 plays the predominant role later in the response (25, 39, 50, 51). In our studies, we are not able to discern whether these effects were centrally or peripherally mediated, as we used mutant mice that do not express any of the targeted mediators.
Perspectives and Significance
Innate immune stimulation by TLR7 agonists has been proposed as a systemic treatment for cancer, as well as atopic and infectious diseases (9, 19). As shown here, however, pharmacologic activation of TLR7 can also produce transient anorexia and hypothermia, mediated, in part, by proinflammatory cytokines that are released by tissue mast cells, and that act centrally as well as peripherally. Pharmacologic stabilization of mast cells may diminish the anorexia and temperature fluxes that are potentially induced by TLR7 activation, while permitting activation of other components of the immune system.
GRANTS
This work was funded in part by National Institutes of Health Grants AI40682, 5 U01 AI056453, and CA119335.
Acknowledgments
We appreciate the genotyping services provided by the Animal Genetics Core, and the monitoring services by Dr. Yusu Gu of the Physiology Core. We are thankful to Dr. Laura Dugan, who took the time to teach us how to remove the hypothalami of mice.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413: 732–738, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol 14: 1065–1074, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Arias-Negrete S, Keller K, Chadee K. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem Biophys Res Commun 208: 582–589, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Asarian L, Langhans W. Current perspectives on behavioural and cellular mechanisms of illness anorexia. Int Rev Psychiatry 17: 451–459, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bird AP Functions for DNA methylation in vertebrates. Cold Spring Harb Symp Quant Biol 58: 281–285, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci 12: 4447–4456, 2000. [PubMed] [Google Scholar]
- 7.Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C). Brain Behav Immun 21: 490–502, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Dafny N, Yang PB. Interferon and the central nervous system. Eur J Pharmacol 523: 1–15, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Dalpke A, Zimmermann S, Heeg K. CpG DNA in the prevention and treatment of infections. BioDrugs 16: 419–431, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dantzer R Cytokine-induced sickness behavior: mechanisms and implications. Ann NY Acad Sci 933: 222–234, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dantzer R Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 500: 399–411, 2004. [DOI] [PubMed] [Google Scholar]
- 12.De Bie JJ, Henricks PA, Cruikshank WW, Hofman G, Jonker EH, Nijkamp FP, Van Oosterhout AJ. Modulation of airway hyperresponsiveness and eosinophilia by selective histamine and 5-HT receptor antagonists in a mouse model of allergic asthma. Br J Pharmacol 124: 857–864, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303: 1529–1531, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Ebenezer IS, Houston AJ, Crook TJ. Systemic administration of baclofen inhibits water intake in rats. Gen Pharmacol 23: 375–379, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Faggioni R, Fuller J, Moser A, Feingold KR, Grunfeld C. LPS-induced anorexia in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice. Am J Physiol Regul Integr Comp Physiol 273: R181–R186, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 174: 1259–1268, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Greer GG, Rietschel ET. Lipid A-induced tolerance and hyperreactivity to hypothermia in mice. Infect Immun 19: 357–368, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, Rao SP, Takabayashi K, Van Uden JH, Kornbluth RS, Baird SM, Taylor MW, Carson DA, Catanzaro A, Raz E. Enhancement of innate immunity against Mycobacterium avium infection by immunostimulatory DNA is mediated by indoleamine 2,3-dioxygenase. Infect Immun 69: 6156–6164, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3: 196–200, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745., 2000. [DOI] [PubMed] [Google Scholar]
- 22.Hirota K, Kazaoka K, Niimoto I, Kumihara H, Sajiki H, Isobe Y, Takaku H, Tobe M, Ogita H, Ogino T, Ichii S, Kurimoto A, Kawakami H. Discovery of 8-hydroxyadenines as a novel type of interferon inducer. J Med Chem 45: 5419–5422, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hubschle T, Mutze J, Muhlradt PF, Korte S, Gerstberger R, Roth J. Pyrexia, anorexia, adipsia, and depressed motor activity in rats during systemic inflammation induced by the Toll-like receptors-2 and −6 agonists MALP-2 and FSL-1. Am J Physiol Regul Integr Comp Physiol 290: R180–R187, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Izumizaki M, Iwase M, Kimura H, Kuriyama T, Homma I. Central histamine contributed to temperature-induced polypnea in mice. J Appl Physiol 89: 770–776, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PM, Vogt SK, Burney MW, Muglia LJ. COX-2 inhibition attenuates anorexia during systemic inflammation without impairing cytokine production. Am J Physiol Endocrinol Metab 282: E650–E656, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RW Immune and endocrine regulation of food intake in sick animals. Domest Anim Endocrinol 15: 309–319, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Joppa MA, Ling N, Chen C, Gogas KR, Foster AC, Markison S. Central administration of peptide and small molecule MC4 receptor antagonists induce hyperphagia in mice and attenuate cytokine-induced anorexia. Peptides 26: 2294–2301, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol 117: 979–987, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11: 115–122, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci 13: 24–28, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Kent S, Kelley KW, Dantzer R. Effects of lipopolysaccharide on food-motivated behavior in the rat are not blocked by an interleukin-1 receptor antagonist. Neurosci Lett 145: 83–86, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Kolaczkowska E, Seljelid R, Plytycz B. Role of mast cells in zymosan-induced peritoneal inflammation in Balb/c and mast cell-deficient WBB6F1 mice. J Leukoc Biol 69: 33–42, 2001. [PubMed] [Google Scholar]
- 33.Kurimoto A, Ogino T, Ichii S, Isobe Y, Tobe M, Ogita H, Takaku H, Sajiki H, Hirota K, Kawakami H. Synthesis and evaluation of 2-substituted 8-hydroxyadenines as potent interferon inducers with improved oral bioavailabilities. Bioorg Med Chem 12: 1091–1099, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Larson SJ, Dunn AJ. Behavioral effects of cytokines. Brain Behav Immun 15: 371–387, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol 279: R93–R98, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci USA 100: 6646–6651, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Wu CC, Lee KJ, Chuang TH, Katakura K, Liu YT, Chan M, Tawatao R, Chung M, Shen C, Cottam HB, Lai MM, Raz E, Carson DA. Activation of anti-hepatitis C virus responses via Toll-like receptor 7. Proc Natl Acad Sci USA 103: 1828–1833, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leon LR Hypothermia in systemic inflammation: role of cytokines. Front Biosci 9: 1877–1888, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Lugarini F, Hrupka BJ, Schwartz GJ, Plata-Salaman CR, Langhans W. A role for cyclooxygenase-2 in lipopolysaccharide-induced anorexia in rats. Am J Physiol Regul Integr Comp Physiol 283: R862–R868, 2002. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol 51: 2–9, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Mark KS, Trickler WJ, Miller DW. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J Pharmacol Exp Ther 297: 1051–1058, 2001. [PubMed] [Google Scholar]
- 42.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol 173: 531–541, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Meade S, Denbow DM. Feeding, drinking, and temperature responses of chickens to intracerebroventricular histamine. Physiol Behav 73: 65–73, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Ogimoto K, Harris MK Jr, Wisse BE. MyD88 is a key mediator of anorexia, but not weight loss, induced by lipopolysaccharide and interleukin-1β. Endocrinology 147: 4445–4453, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Otterness IG, Seymour PA, Golden HW, Reynolds JA, Daumy GO. The effects of continuous administration of murine interleukin-1α in the rat. Physiol Behav 43: 797–804, 1988. [DOI] [PubMed] [Google Scholar]
- 46.Porter MH, Hrupka BJ, Altreuther G, Arnold M, Langhans W. Inhibition of TNF-α production contributes to the attenuation of LPS-induced hypophagia by pentoxifylline. Am J Physiol Regul Integr Comp Physiol 279: R2113–R2120, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. Endotoxin shock: thermoregulatory mechanisms. Am J Physiol Regul Integr Comp Physiol 270: R693–R703, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Sachot C, Poole S, Luheshi GN. Circulating leptin mediates lipopolysaccharide-induced anorexia and fever in rats. J Physiol 561: 263–272, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 274: 17406–17409, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Swiergiel AH, Dunn AJ. Cyclooxygenase 1 is not essential for hypophagic responses to interleukin-1 and endotoxin in mice. Pharmacol Biochem Behav 69: 659–663, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Swiergiel AH, Dunn AJ. Distinct roles for cyclooxygenases 1 and 2 in interleukin-1-induced behavioral changes. J Pharmacol Exp Ther 302: 1031–1036, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi O, Akira S. Signaling pathways activated by microorganisms. Curr Opin Cell Biol 19: 185–191, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, Wu Y, Lee DH, Yanai K, Sakurai E, Watanabe T, Liu C, Chen J, Barbier AJ, Turek FW, Fung-Leung WP, Lovenberg TW. Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol 62: 389–397, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Tuomisto L, Eriksson L. Antidiuresis induced by infusions of histamine into the brain ventricles of conscious hydrated goats. Eur J Pharmacol 54: 191–201, 1979. [DOI] [PubMed] [Google Scholar]
- 55.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med 84: 712–725, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem 282: 15319–15323, 2007. [DOI] [PubMed] [Google Scholar]
- 57.von Meyenburg C, Hrupka BH, Arsenijevic D, Schwartz GJ, Landmann R, Langhans W. Role for CD14, TLR2, and TLR4 in bacterial product-induced anorexia. Am J Physiol Regul Integr Comp Physiol 287: R298–R305, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Waelput W, Brouckaert P, Broekaert D, Tavernier J. A role for leptin in the systemic inflammatory response syndrome (SIRS) and in immune response. Curr Drug Targets Inflamm Allergy 1: 277–289, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J Immunol 169: 6668–6672, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Yao JH, Ye SM, Burgess W, Zachary JF, Kelley KW, Johnson RW. Mice deficient in interleukin-1β converting enzyme resist anorexia induced by central lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol 277: R1435–R1443, 1999. [DOI] [PubMed] [Google Scholar]