Abstract
The circadian clock in the suprachiasmatic nucleus (SCN) maintains phase synchrony among circadian oscillators throughout the organism. Environmental light signals entrain the SCN, but timed, limited meal access acts as an overriding time cue for several peripheral tissues. We present data from a peripheral oscillator, the submaxillary salivary gland, in which temporal restriction of meals fails to entrain gene expression. In day-fed rats, submaxillary gland rhythms in expression of the clock gene Period1 (Per1) stay entrained to the light cycle (peaking at night) or become arrhythmic. This result suggests that feeding cues compete weakly with light cycle cues to set the phase of clock genes in this tissue. Since the submaxillary glands receive sympathetic innervation originating in the SCN, which relays light cycle cues to other oscillators, we attempted to assess the role of this neural input in phase control of submaxillary Per1 expression. We sympathetically denervated the submaxillary glands before subjecting rats to daytime-restricted feeding. After denervation, Per1 rhythms in all submaxillary glands shifted phase 180° and entrained to daytime feeding. These results support the hypothesis that peripheral oscillators may receive multiple signals contributing to their phase of entrainment. Sympathetic efferents from the SCN can relay light cycle information, while other external cues may reach tissues through other efferents or nonneural pathways. In an abnormal, disruptive regimen such as daytime-restricted feeding, these different signals compete. Arrhythmicity may result if one signal is not clearly dominant. Elimination of the dominant signal (e.g., surgical sympathectomy) may allow a secondary signal to control phase.
Keywords: submaxillary gland, Period1, sympathectomy, restricted feeding
circadian rhythms are daily variations of behavior and physiology that are generated independently of external stimuli. Networks of circadian oscillators form hierarchical systems within organisms and are responsible for producing rhythmic outputs (18, 38, 42). Individual oscillators in this system generate rhythms intrinsically, but the phase of these rhythms is entrained by cues from other oscillators and, ultimately, from environmental cycles (9). Light cycles act as a particularly strong external synchronizer of endogenous rhythms. In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus, widely regarded as the oscillator at the top of the hierarchy, receives information about the light cycle from specialized retinal photoreceptors via the retinohypothalamic tract (15). The SCN then relays this phase information to oscillators throughout the organism (32, 50, 51) through endocrine signals and/or neural circuits (25, 36).
Schedules of food availability constitute another critical environmental cue, sometimes acting as an even more salient entrainment cue than the light cycle (44). Although feeding schedules often maintain a fixed relationship to the light cycle under natural conditions, a dramatic change in this phase relationship can uncouple oscillators that normally act in synchrony (10, 26). When nocturnal animals are restricted to a daytime meal, rhythms in food anticipatory activity (45), physiology, energy metabolism, and gene expression in various tissues (10, 11) preferentially entrain to the feeding schedule, while gene expression rhythms in the SCN remain entrained to the light cycle (19, 41, 46). Little is known about the mechanisms by which restricted feeding entrains peripheral oscillators, and less still is known about what determines a given oscillator's phase in the face of contradictory light and food cycles. Previous research has suggested that, in mammals, feeding is the dominant entrainment cue for peripheral oscillators and, in contrast to light entrainment, does not necessarily depend on the SCN (41). In the present study, we report a peripheral oscillator, the submaxillary salivary gland, which, in contrast to previously investigated tissues, fails to entrain to daytime-restricted feeding.
If not to feeding, then to what entrainment cues are these peripheral oscillators responding? Moreover, how might these cues reach peripheral oscillators? Previous research suggests several pathways by which the SCN might act to set the phases of peripheral tissues. The SCN controls the phases of many endocrine cycles neurally via synapses onto magnocellular neurons and parvocellular hypophysiotropic neurons (36). SCN transplants and parabiosis experiments have demonstrated that nonneural signaling from the SCN is sufficient to synchronize diurnal patterns of locomotor activity (16, 43) but not to coordinate phases of neuroendocrine rhythms (27, 28) or of rhythms of clock gene expression in all peripheral oscillators (16, 17). Transsynaptic tract tracing has revealed that the SCN is also neurally connected to many peripheral tissues via the sympathetic and parasympathetic branches of the autonomic nervous system (3, 47). Sympathetic projections, in particular, appear critical to maintaining physiological rhythms of various peripheral tissue functions, including pineal melatonin production and secretion (33–35), liver control of plasma glucose (6, 24), and adrenal corticosteroid release (14, 20, 30). Furthermore, pulses of norepinephrine (mimicking adrenergic signals from sympathetic nerves) can phase shift rhythms of clock gene expression in blood vessel epithelium (37) and in the liver (48). These results suggest that SCN-controlled sympathetic outflow is a strong candidate for mediating phase of peripheral oscillator clock gene rhythms.
Submaxillary salivary glands receive ipsilateral innervation from sympathetic chain fibers originating in the superior cervical ganglion (SCG), which in turn receives input from the SCN via neurons in the paraventricular nucleus (PVN) of the hypothalamus, brain stem, and intermediolateral spinal cord (49). To assess the extent to which this neural input from the SCN may contribute to the light- and/or food-entrained rhythms in these glands, we used the Per1-luc transgenic rat model. This model expresses a luciferase reporter under the control of the promoter for the clock gene Period1 (Per1), providing a bioluminescent output proportional to levels of Per1 transcription at any given time. We measured the submaxillary glands' response to daytime-restricted feeding in the presence of a light cycle with and without transection of the sympathetic nerve supply.
METHODS
Animals and Maintenance
All animals were 1- to 6-mo-old transgenic Kyoto-Wistar rats bearing a construct in which the Per1 gene promoter is linked to a luciferase reporter (50). Since luciferase expression in these rats is under the control of the same transcriptional regulatory elements as expression of the clock gene Per1, the peak phase of luciferase expression (and, thus, bioluminescent output from the tissues of these animals) coincides with times of peak levels of Per1 protein. Rats were bred and raised at the University of Virginia Life Sciences Vivarium. After they were weaned, rats were housed one to a cage in a 12:12-h light-dark cycle. With the exception of restricted-feeding experiments, they were allowed ad libitum access to Purina Rat Chow and water. All experimental procedures were approved by the University of Virginia Animal Care and Use Committee.
Organ Culture
Except where noted, cultures were performed between zeitgeber time (ZT) 9 and ZT 12 (ZT 0 = lights on). Animals were killed by decapitation under CO2 anesthesia, and portions of the liver and submaxillary glands were rapidly dissected and placed in cold Hanks' basic salt solution (Invitrogen) supplemented with HEPES buffer and antibiotics. Under a light microscope, a pair of scalpels was used to cut the tissues into small sections (∼0.5-mm-thick squares, ∼1.5 mm across). They were cultured on Millicell culture membranes (PIMC ORG 50, Millipore) in a 35-mm culture dish with 1.2 ml of culture medium containing Dulbecco's modified Eagle's medium (GIBCO, Carlsbad, CA) supplemented with 10 mM HEPES (pH 7.2), B27 (2% GIBCO), antibiotics (25 U/ml penicillin and 25 μg/ml streptomycin), and 0.1 mM luciferin (Promega, Madison, WI). Culture dishes were sealed with vacuum grease and a glass coverslip and moved to a dark 36°C incubator for recordings.
Bioluminescence Recordings and Data Analysis
Per1 gene expression in tissues from these transgenic rats was measured indirectly by recording bioluminescence output from tissue explants using photomultiplier detectors (Hamamatsu, Bridgewater, NJ). Luminescence counts were plotted against time, starting with ZT 0 on the day of culture. Before the data were plotted, they were detrended (subtraction of the 24-h running average from the raw data) and smoothed (using a 2-h running average on the detrended data). The criterion for rhythmicity was the presence of three peaks and troughs in the first 72 h of recordings, each ∼24 h apart, assessed visually by a reviewer blind to the experimental condition of the animal. The peak phase (ZT of peak Per1 expression) was determined qualitatively as the first peak after a complete 24 h in culture and quantified using OriginPro software to identify the local maximum in the smoothed data between 24 and 48 h in culture.
Surgery
Transection of the sympathetic chain.
Animals were weighed and anesthetized via intramuscular injection of ketamine-xylazine-acepromazine anesthetic solution at 1.1 ml/kg. The surgical site was shaved and scrubbed with two rounds of povidone-iodine (Betadine) antiseptic solution followed by alcohol. The sympathetic chain projection from the SCG was approached via a ventral incision. The vagosympathetic trunk was localized under a dissecting microscope. In 17 rats the whole vagosympathetic trunk was cut on the left side; in 5 others the sympathetic chain projection from the SCG was isolated from the vagus nerve before transection (only on the left side). Immediately after the surgery, as well as 24 h postoperatively, an analgesic (ketoprofen, 2–5 mg/kg) was administered subcutaneously. Animals were also given an antibiotic (enrofloxacin, Baytril) in their drinking water for 1 wk postoperatively. Success of the sympathectomy was verified when ptosis (drooping eyelid) of the eye ipsilateral to the transected sympathetic chain was observed. Animals failing to show this symptom postoperatively were not used in experiments.
Sham transection.
Sham-transected animals underwent every part of the sympathetic denervation procedure described above, except the sympathetic chain was not cut.
RESULTS
Phase of Circadian Oscillations of Per1 Expression in Submaxillary Glands Is Not Affected by Explantation and Culture Protocol
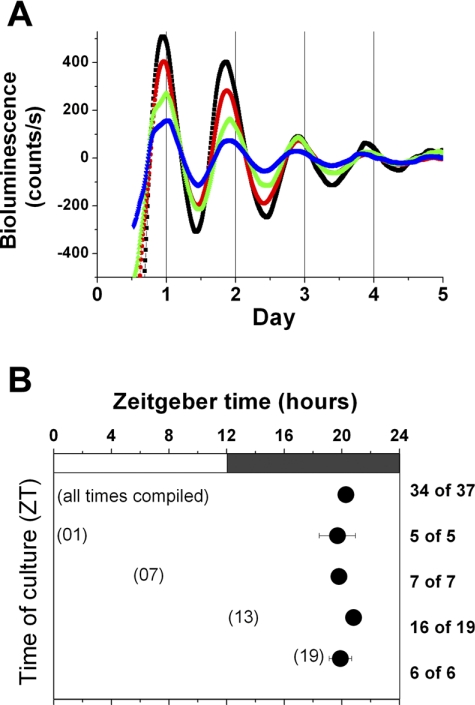
Per1 expression in submaxillary glands in vitro is robustly rhythmic and persists for ≥3 days (Fig. 1A). The average peak phase of submaxillary gland explants in vitro was ZT 20.27 ± 0.31. To confirm that phase of in vitro rhythmicity is not set or affected by explantation and culture of these tissues, cultures were performed at four ZTs (±2 h): ZT 1 (n = 5), ZT 7 (n = 7), ZT 13 (n = 19), and ZT 19 (n = 6; Fig. 1B). ANOVA for time of culture vs. peak phase showed no significant interaction between peak phase of the submaxillary glands in vitro and their ZT of culture [F(3,30) = 0.85, P = 0.478].
Fig. 1.
A: representative bioluminescence recordings from submaxillary glands in vitro. B: peak phases for submaxillary glands cultured at different zeitgeber times (ZT). Peak phase is the local maximum between 24 and 48 h in culture. Numbers at right denote proportion of submaxillary gland cultures in each group that were rhythmic. Values are means ± SE.
Per1 Expression in Submaxillary Glands of Day-Fed Intact Rats Does Not Entrain to a Feeding Schedule
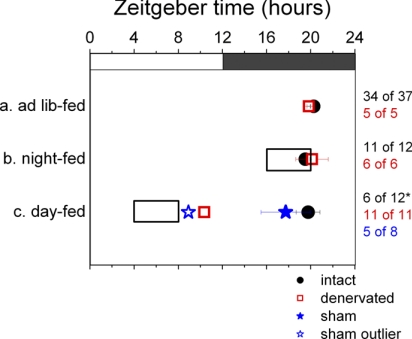
Intact rats on a 12:12-h light-dark cycle were placed on a daytime-restricted feeding regimen for 10 days, long enough to achieve food entrainment (29). This group (n = 12) had access to food exclusively from ZT 4 to ZT 8, in the middle of the light period. On day 10 of this feeding regimen, the liver and submaxillary glands of these animals were cultured, and their peak phases of Per1 expression were measured. All livers cultured from rats in this day-fed group showed a phase advance peaking at ZT 9.48 ± 0.41 (as opposed to livers from ad libitum-fed rats, which peaked at ZT 17.93 ± 0.78; Fig. 2). This result matches previous findings, confirms entrainment of the liver to the feeding schedule, and serves as a positive control demonstrating that food entrainment was actually achieved (13, 46). However, submaxillary glands of day-fed rats did not entrain to the feeding schedule; they either peaked at ZT 19.76 ± 1.08 (6 of 12) or were completely arrhythmic (6 of 12; Fig. 3). In the submaxillary cultures from day-fed rats that were rhythmic, peak phases were not significantly different from those of ad libitum-fed rats (P = 0.79, by t-test).
Fig. 2.
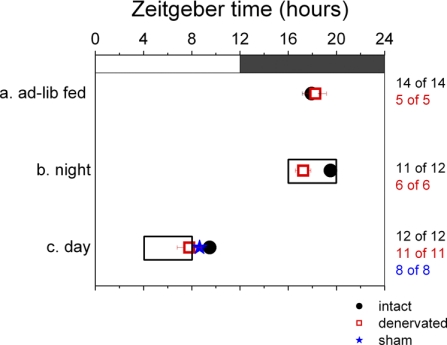
Peak phases for liver tissue cultured from animals in different experimental groups: intact animals (black circles) and animals with autonomically denervated left submaxillary glands (red squares) on ad libitum feeding regimen (a), intact animals (black circles) and animals with autonomically denervated left submaxillary glands (red squares) on nighttime-feeding regimen (b), and intact animals (black circles), animals with autonomically denervated left submaxillary glands (red squares), and sham-operated animals (blue stars) on daytime-feeding regimen (c). Clear box indicates times of restricted feeding. Numbers at right denote proportion of cultures in each group that were rhythmic. Values are means ± SE.
Fig. 3.
Peak phases for submaxillary glands cultured from animals in different experimental groups: intact animals (black circles) and animals with autonomically denervated left submaxillary glands (red squares) on ad libitum feeding regimen (a), intact animals (black circles) and animals with autonomically denervated left submaxillary glands (red squares) on nighttime-feeding regimen (b), and intact animals (black circles), animals with autonomically denervated left submaxillary glands (red squares), and sham-operated animals (blue stars) on daytime-feeding regimen (c). Clear box indicates times of restricted feeding. Values are means ± SE. Numbers at right denote proportion of submaxillary gland cultures in each group that were rhythmic. *Experimental group in which a significantly higher proportion of the cultures was arrhythmic than was the case under baseline conditions. Of the 5 rhythmic cultures from the sham-operated group, 1 is phase-advanced and is shown as a hollow blue star; remaining 4 cultures are grouped in the solid blue star with error bars. Where left and right submaxillary glands from 1 animal were cultured, the point used to make this plot is the average of peak phases from left and right.
To ensure that arrhythmicity produced in 50% of submaxillary glands was in fact due to feeding at a disruptive time, rather than caloric restriction or confinement of eating to a 4-h period, another group of rats was placed on a nighttime-restricted feeding regimen. This group (n = 12) received food exclusively for 4 h during the dark period, from ZT 16 to ZT 20, when ad libitum-fed rats would normally eat. Liver and submaxillary glands from night-fed rats showed Per1 expression patterns and peak phases similar to ad libitum-fed rats (P = 0.12 for liver and P = 0.197 for submaxillary glands, by t-test), peaking at ZT 19.50 ± 0.43 and ZT 19.64 ± 0.66, respectively (Figs. 2 and 3, respectively). No disruption or phase shift in Per1 rhythms was observed in response to caloric restriction alone.
Per1 Expression in Submaxillary Glands of Day-Fed Sympathectomized Rats Does Entrain to the Feeding Schedule
Seventeen rats underwent surgery to transect their left vagosympathetic trunk at the cervical level, interrupting sympathetic afferents to the submaxillary gland. After surgery, the rats were allowed ad libitum access to food (n = 5), put on a 10-day night-feeding regimen (n = 6), or put on a 10-day day-feeding regimen (n = 6). Then the liver and submaxillary glands from each of these animals were cultured, and the peak phase of in vitro Per1 expression was measured. Liver and submaxillary glands from ad libitum- and night-fed animals showed clear circadian rhythms of Per1 expression, with ad libitum average peak phases at ZT 19.84 ± 0.19 (submaxillary glands) and ZT 18.25 ± 0.92 (liver) and night-fed average peak phases at ZT 20.12 ± 1.47 (submaxillary glands) and ZT 17.24 ± 0.64 (liver), similar to the phases of explants from intact animals (Figs. 3 and 2, respectively). In contrast, day-fed animals showed a 10-h phase shift, with average peak phases at ZT 9.87 ± 0.63 (submaxillary glands) and ZT 7.79 ± 1.00 (liver). This result in liver explants serves as a positive control indicating that food entrainment took place.
Left and right submaxillary glands (ipsilateral and contralateral to the sympathetic chain transected) exhibited this phase shift in Per1 rhythms, although the nerve tract that was cut has been reported to selectively innervate the ipsilateral gland (49). Since transection of the vagosympathetic trunk disrupts vagal afferents and efferents in addition to the sympathetic relays of interest, the daytime-restricted feeding experiment was repeated with a group of rats (n = 5) in which the left sympathetic chain was carefully dissected from adjacent nerves before transection. Left and right submaxillary gland explants from these animals peaked at an average of ZT 10.93 ± 0.68, which was not significantly different from day-fed animals that had undergone transection of the whole vagosympathetic trunk (P = 0.38, by t-test; data are combined as “Denervated” for Fig. 3). [There was no significant difference between the left and right submaxillary glands explanted from this group (P = 0.85, by t-test).] Livers from these animals also exhibited the anticipated phase shift, with an average peak phase at ZT 8.55 ± 0.65 (Fig. 2).
Sham-Sympathectomized Rats' Submaxillary Glands Show Entrainment Patterns Similar to Those of Intact Rats
To rule out the possibility that nonspecific effects of the surgery were responsible for food entrainment in sympathectomized glands, sham surgeries were performed. After sham surgery, animals (n = 8) were placed on the 10-day daytime-restricted feeding regimen ending in explantation and culture of the liver and left and right submaxillary gland. All liver explants from this group phase shifted to peak at an average of ZT 8.64 ± 0.38 (Fig. 2), indicating that food entrainment did take place. As with intact rats that received no surgery, 50% (4 of 8) of the rats showed no effect on submaxillary gland Per1 expression, peaking at (average) ZT 19.93 ± 0.05 (Fig. 3). One of the remaining rats showed complete arrhythmicity in explants of its left and right submaxillary gland, and two more had asymmetric results between the left submaxillary gland, which was arrhythmic, and the right, which peaked at ZT 21.02 ± 0.36. The eighth animal's gland exhibited a phase shift, peaking at ZT 8.92. This one outlier indicates that sham surgery can affect the results. However, it does not create the conditions that allowed all submaxillary glands of sympathetically denervated animals to entrain to the food schedule.
DISCUSSION
The submaxillary glands are unique among investigated peripheral oscillators in their failure to entrain to daytime-restricted feeding. In other previously studied peripheral tissues, rhythms of gene expression exhibited entrainment to time of feeding. These tissues include the liver (46), kidney, heart, and pancreas (10, 31) and esophagus, antral stomach, body of the stomach, and colon (12). Among central structures, only the SCN and the ventromedial nucleus of the hypothalamus are reported to have shown no change in phase of gene expression after daytime-restricted feeding (2). However, in certain mouse strains, even gene expression in the SCN responds to a feeding schedule in constant-lighting conditions (1, 8).
The results of the present study suggest newfound complexity in the entrainment of peripheral oscillators. A given tissue may entrain consistently to light or to food timing information, but it may also, as is the case with submaxillary glands, entrain inconsistently. Per1 expression in submaxillary gland explants from half the animals on a daytime-feeding regimen entrained to light, whereas rhythms in explants from the other half were arrhythmic. This result suggests that some peripheral oscillators must integrate multiple entrainment cues. Under normal conditions, when rodents eat at night, in synchrony with their rest-activity cycle, food and light entrainment cues act in concert. In a disruptive regimen, such as daytime-restricted feeding, these entrainment signals may compete for control over the phase of gene expression in this peripheral oscillator.
If we interpret our results in the context of cues in different modalities competing for control over the phase of the oscillator, light “wins” and food “loses” in half of submaxillary glands in intact rats. In the other half of intact rats' submaxillary glands, there is a “draw” between light and food time cues; the glands do not entrain to either, but become arrhythmic, perhaps in response to signals of approximately equal strength in antiphase. In the liver, used here as a positive control, food wins and dominates the phase of Per1 expression under all circumstances. Such variety of peripheral oscillator responses to daytime feeding cannot be explained by the hypothesis that the SCN entrains the periphery via control of activity rhythms and, thus, feeding time and that those events downstream of feeding act as the only entrainment cues (41). Our results make it clear that light and food cues must be acting through distinct pathways and that at least some tissues are capable of integrating the different entrainment signals. Moreover, there does not appear to be a consistent hierarchy of time cues: whether light or food dominates the phase of a given tissue may vary from tissue to tissue (Fig. 4). In certain tissues (such as the liver), one entrainment signal is dominant; in other tissues, however, multiple signals appear to act in concert to set the phase of gene expression.
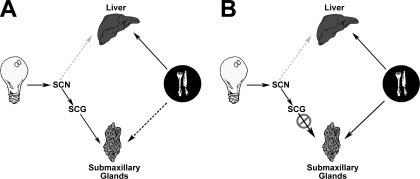
Fig. 4.
Pathways for light vs. food entrainment of peripheral oscillators. A: in intact animal, light information reaches the suprachiasmatic nucleus (SCN) via the retinohypothalamic tract. The SCN in turn modulates sympathetic outflow through a multisynaptic pathway. Sympathetic efferents then reach the submaxillary gland via the superior cervical ganglion (SCG). Information carried by this series of neural connections (solid black arrows) is strong enough to control the phase of submaxillary gland Per1 expression. When faced with competing food entrainment cues, 50% of submaxillary glands remain entrained to the light cycle and the other 50% lose rhythmicity. This disruption in half of the salivary glands may be due to signals from the competing food entrainment pathway (dotted black arrow). Per1 expression in the liver robustly entrains to feeding schedule (solid black arrow). Hepatic sympathetic innervation, possibly carrying light entrainment information from the SCN, does not offer any competition to food entrainment pathways (dotted gray arrow). B: after disruption of the sympathetic chain carrying light entrainment information to the submaxillary glands, their Per1 expression entrains robustly to the feeding schedule, very similar to the liver (solid black arrow).
Adrenergic signals from the sympathetic nervous system have been shown to underlie certain physiological responses to light, including phase shifting of important rhythms. The most thoroughly studied of these are rhythms of pineal melatonin synthesis (35) and adrenal corticosteroid release (20). Sympathetic efferent fibers responsible for these rhythms receive input from SCN neurons, which are entrained to the ambient light cycle via the retinohypothalamic tract (15). Moreover, there are many examples of light stimuli acutely affecting sympathetically controlled or regulated functions, presumably via sympathetic nerve activity [e.g., ambulatory heart rate (39, 40), adrenal corticosterone secretion (5), pineal melatonin release (21–23), and salivary secretion (4)].
If sympathetic projections from the SCN do specifically relay light information to peripheral oscillators, it follows that a disruption of neural projections from the SCN to submaxillary glands may diminish the extent to which light cues can control their phase. This is precisely what we observe in animals whose sympathetic chain was transected: light is no longer an effective cue and Per1 rhythms in 100% of the denervated submaxillary glands entrain to time of feeding (i.e., food always wins). Our data indicate strongly that sympathetic efferents contribute to light entrainment of clock gene expression in peripheral oscillators. When pathways through which light information may reach these tissues are severed and the (unknown) pathways through which information about feeding schedules is transferred remain intact, food entrainment can dominate or at least more strongly affect phase.
The sympathetic outflow circuit sends information from the SCN to the submaxillary glands via neurons in the PVN of the hypothalamus, sympathetic preganglionic neurons in the intermediolateral column of the spinal cord, and postganglionic neurons in the SCG (49). This ganglion gives rise to the sympathetic chain neurons that innervate the salivary glands. However, one anatomic consideration complicates the interpretation of our findings: these sympathetic relays are thought to innervate submaxillary glands ipsilaterally, and in our experiments only the left submaxillary glands were denervated. Unexpectedly, we observed the same results in left and right submaxillary glands. The mechanism underlying this bilateral phase shift remains to be elucidated. It is possible that chemical pathways couple the phase of gene expression in the left and right submaxillary glands, which lie directly adjacent to each other in the neck. In that case, a phase shift on one side could chemically precipitate the same shift on the other. It is also possible that further investigation of submaxillary gland innervation is warranted, inasmuch as some neurons may fail to take up viral tracers, or conservative injection of tracer may miss subpopulations of neurons (7).
Perspectives and Significance
Although our results demonstrate that sympathetic neural efferents to the salivary glands are important for their entrainment to light cycles, they also make clear that phase control of peripheral oscillators can be complex. Instead of one absolute “master” clock in the SCN driving “slave” oscillators everywhere else, peripheral oscillators may act more like integrators of multiple different time cues. The manner in which peripheral tissues respond to our disruptive feeding regimen may provide some insight into what happens in human feeding rhythm disorders. Such disturbances in the normal relationship between light cycle and food intake, whether related to shift work, night eating syndrome, or insomnia, have been linked to digestive and cardiovascular problems and increased risk for obesity and cancer. Understanding the effects of uncoupling light and food entrainment cues on rhythms in individual tissues may help elucidate the mechanism by which feeding rhythm disorders can predispose us to disease.
GRANTS
This research was supported by National Space Biomedical Research Institute Grant NCC9-58-167 and National Institute of Mental Health Grant RO1 MH-56647 (to M. Menaker), National Institute on Aging Grant F32 AG-22741-01 (to A. J. Davidson), and the David A. Harrison III Undergraduate Research Award (to N. Vujović).
Acknowledgments
We thank Drs. Patrice Guyenet and Ruth Stornetta for invaluable assistance in development of the surgical methodology and for many helpful ideas and suggestions, Dr. Arthur Loewy for sharing expert insight into the neuroanatomy underlying sympathetic outflow from the SCN, and Dr. Michael Sellix for artistic execution of Fig. 4. We acknowledge Tomoko Yoshikawa and Eva Papadimas for technical assistance, as well as Jeffery Hager, Dr. Jeffery Wimsatt, Amy O'Coin, Matthew Baer, Ozgur Tataroglu, and Denise Holmes.
Present addresses: N. Vujović, Department of Neurobiology, Harvard Medical School, 220 Longwood Ave., Boston, MA 02115; A. J. Davidson, Neuroscience Institute, Morehouse School of Medicine, 720 Westview Dr. SW, Atlanta, GA 30310.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe H, Honma S, Honma KI. Daily restricted feeding resets the circadian clock in the suprachiasmatic nucleus of CS mice. Am J Physiol Regul Integr Comp Physiol 292: R607–R615, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Angeles-Castellanos M, Aguilar-Roblero R, Escobar C. c-Fos expression in hypothalamic nuclei of food-entrained rats. Am J Physiol Regul Integr Comp Physiol 286: R158–R165, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythms 16: 196–204, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bellavia S, Gallara R. Effect of photic stimuli on rat salivary glands. Role of sympathetic nervous system. Acta Odontol Latinoam 13: 3–19, 2000. [PubMed] [Google Scholar]
- 5.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 11: 1535–1544, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, Feenstra M, Pevet P, Buijs RM. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur J Neurosci 22: 2531–2540, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Card JP Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci Biobehav Rev 22: 685–694, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Castillo MR, Hochstetler KJ, Tavernier RJ Jr, Greene DM, Bult-Ito A. Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol 287: R551–R555, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Challet E, Caldelas I, Graff C, Pevet P. Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol Chem 384: 711–719, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson AJ, Castanon-Cervantes O, Stephan FK. Daily oscillations in liver function: diurnal vs. circadian rhythmicity. Liver Int 24: 179–186, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav 2: 32–39, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Davidson AJ, Stokkan KA, Yamazaki S, Menaker M. Food-anticipatory activity and liver per1-luc activity in diabetic transgenic rats. Physiol Behav 76: 21–26, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Engeland WC, Arnhold MM. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine 28: 325–332, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284: 502–504, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA 102: 3111–3116, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J Neurosci 26: 6406–6412, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 4: 649–661, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Inouye SI Restricted daily feeding does not entrain circadian rhythms of the suprachiasmatic nucleus in the rat. Brain Res 232: 194–199, 1982. [DOI] [PubMed] [Google Scholar]
- 20.Jasper MS, Engeland WC. Splanchnic neural activity modulates ultradian and circadian rhythms in adrenocortical secretion in awake rats. Neuroendocrinology 59: 97–109, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Kalsbeek A, Cutrera RA, Van Heerikhuize JJ, Van Der Vliet J, Buijs RM. GABA release from suprachiasmatic nucleus terminals is necessary for the light-induced inhibition of nocturnal melatonin release in the rat. Neuroscience 91: 453–461, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Kalsbeek A, Drijfhout WJ, Westerink BH, van Heerikhuize JJ, van der Woude TP, van der Vliet J, Buijs RM. GABA receptors in the region of the dorsomedial hypothalamus of rats are implicated in the control of melatonin and corticosterone release. Neuroendocrinology 63: 69–78, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Kalsbeek A, Garidou ML, Palm IF, Van Der Vliet J, Simonneaux V, Pevet P, Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci 12: 3146–3154, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 24: 7604–7613, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalsbeek A, Perreau-Lenz S, Buijs RM. A network of (autonomic) clock outputs. Chronobiol Int 23: 521–535, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Krieger DT, Hauser H. Comparison of synchronization of circadian corticosteroid rhythms by photoperiod and food. Proc Natl Acad Sci USA 75: 1577–1581, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci 7: 1626–1638, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140: 207–218, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Mistlberger RE Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 18: 171–195, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42: 201–206, 1972. [DOI] [PubMed] [Google Scholar]
- 31.Oishi K, Miyazaki K, Ishida N. Functional CLOCK is not involved in the entrainment of peripheral clocks to the restricted feeding: entrainable expression of mPer2 and BMAL1 mRNAs in the heart of Clock mutant mice on Jcl:ICR background. Biochem Biophys Res Commun 298: 198–202, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Okamura H Clock genes in cell clocks: roles, actions, and mysteries. J Biol Rhythms 19: 388–399, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, van der Vliet J, van Heijningen C, Simonneaux V, Pevet P, Buijs RM. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur J Neurosci 17: 221–228, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Perreau-Lenz S, Kalsbeek A, Pevet P, Buijs RM. Glutamatergic clock output stimulates melatonin synthesis at night. Eur J Neurosci 19: 318–324, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Perreau-Lenz S, Kalsbeek A, Van Der Vliet J, Pevet P, Buijs RM. In vivo evidence for a controlled offset of melatonin synthesis at dawn by the suprachiasmatic nucleus in the rat. Neuroscience 130: 797–803, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Perreau-Lenz S, Pevet P, Buijs RM, Kalsbeek A. The biological clock: the bodyguard of temporal homeostasis. Chronobiol Int 21: 1–25, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Reilly DF, Curtis AM, Cheng Y, Westgate EJ, Rudic RD, Paschos G, Morris J, Ouyang M, Thomas SA, FitzGerald GA. Peripheral circadian clock rhythmicity is retained in the absence of adrenergic signaling. Arterioscler Thromb Vasc Biol 28: 121–126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63: 647–676, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Scheer FA, Ter Horst GJ, van Der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol 280: H1391–H1399, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Scheer FA, van Doornen LJ, Buijs RM. Light and diurnal cycle affect human heart rate: possible role for the circadian pacemaker. J Biol Rhythms 14: 202–212, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms 18: 250–260, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell 111: 919–922, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382: 810–813, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Stephan FK The “other” circadian system: food as a zeitgeber. J Biol Rhythms 17: 284–292, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol 25: 545–554, 1979. [DOI] [PubMed] [Google Scholar]
- 46.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Teclemariam-Mesbah R, Kalsbeek A, Pevet P, Buijs RM. Direct vasoactive intestinal polypeptide-containing projection from the suprachiasmatic nucleus to spinal projecting hypothalamic paraventricular neurons. Brain Res 748: 71–76, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci USA 100: 6795–6800, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, Mettenleiter TC, Loewy AD. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci 2: 1051–1053, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]