Abstract
Although many feeding protocols induce obesity, few use multiple foods to analyze diet selection within a single group of animals. To this end, we describe a protocol using time-limited access to a dessert that induces hyperphagia and body weight gain while allowing simple analysis of diet selection. Female retired breeder Sprague-Dawley rats were provided with ad libitum access to standard moist chow (1.67 kcal/g) and daily 8-h nocturnal access to either a sugar gel (SG; 0.31 kcal/g) or sugar fat whip (SFW; 7.35 kcal/g) for 15 days, and food intake and body weight were measured daily. Rats given SFW reduced moist chow intake but not enough to compensate for the large amount of calories consumed from SFW, and thus gained weight. We use this SFW overconsumption protocol to investigate the hypothesis that cannabinoid (CB)1 receptor antagonists reduce caloric intake by selectively decreasing consumption of palatable foods. In two experiments, female retired breeder Sprague-Dawley rats were injected with either Rimonabant (1 mg/kg ip) or vehicle (equal parts polyethylene glycol and saline, 1 ml/kg ip) for 7 days, or one of three doses of AM251 (0.3, 1.0, or 3.0 mg/kg ip), or vehicle for 15 days; food intake and body weight were measured daily. Both Rimonabant and AM251 decreased 24-h caloric intake, but the reduction was specific to a decrease in SFW consumption. This supports the hypothesis that these CB1 receptor antagonists impact feeding by modulating the perception of palatability.
Keywords: diet-induced obesity, overconsumption, Rimonabant, AM251, body weight
incidence of human obesity and the associated metabolic syndrome is increasing at an alarming rate. The single factor that produces obesity is the overconsumption of calories in relation to expenditure. While decreased physical activity plays some role, there is substantial evidence that easier access to high-calorie foods correlates with the surge in obesity (12, 13). In this opportunistic food environment, the cycles of food craving due to dietary restriction followed by overconsumption have behavioral qualities similar to addiction (8, 9).
Overconsumption can be modeled in rodents that are provided chronically with the sole option of a palatable high-calorie diet, and this is referred to as diet-induced obesity (27). In contrast, humans normally have diverse food choices, and we believe that an element of choice in diet selection is necessary to explore the limits of caloric compensation in animal models. “Cafeteria style” diets (39) presented ad libitum to rodents promote overconsumption, but there are substantial individual differences in dietary preferences that often complicate the analysis of these results. A dessert protocol can be used to avoid these complications while still allowing a choice, as well as promoting overconsumption. In dessert protocols, rodents are given time-restricted access to a preferred, often nutritionally incomplete optional calorie source (the dessert) in conjunction with ad libitum access to a bland but nutritionally complete standard diet (7, 11). In a dessert protocol, caloric compensation refers to a decrease in standard diet consumption that is comparable to the caloric intake from dessert so that daily total caloric intake remains statistically unchanged. Overconsumption is the lack of caloric compensation that occurs when caloric intake of the dessert is not accompanied by an equivalent decrease in standard diet intake, with the result that the total intake is increased. In our first experiment, we sought to establish a model of overconsumption using a dessert protocol in which rats were given daily time-limited access to a dessert containing sugar and fat and with a high caloric density. We hypothesized that presenting rats with this dessert would lead to a high intake of this commodity and a failure to reduce intake of a standard diet by an equivalent amount; this incomplete caloric compensation would result in increases in body weight. We compared this to daily time-limited presentation of a dessert that also contained some sugar but no fat and so was not as calorically dense. We hypothesized that presentation of this dessert would lead to compensatory reductions in standard diet intake and no weight gain.
The stimulatory effect of cannabinoid (CB) receptor agonists, principally Δ9-tetrahydrocannabinol, on appetite has been extensively documented (25, 49). The characterization of an endogenous CB system in the brain working via the CB1 receptor (CB1R) (20) has led to studies to determine the feasibility of CB1R antagonists in weight loss treatments (3, 24). Agents that decrease CB1R signaling, such as Rimonabant (formerly SR141716A) and AM251, acutely decrease food consumption and behaviors associated with feeding in many rodent models (41), including moderately obese Lewis rats prefed with Ensure (4); obese and lean Zucker rats fed laboratory chow ad libitum (47); and mice on standard diet-induced obesity protocols (36). Most of these studies used acute administration and examined the effect for less than a week; few long-term studies of the effect of CB1R antagonists in animals have been reported. Also, one study (18) reported the lack of a hypophagic effect of CB1R antagonists and attributed it to the within-subjects nature of the experiment, suggesting that the effect of CB1R antagonists may be sensitive to the experimental design.
Similar to opioids and benzodiazepines (6), activity at CB1R may affect feeding by modulating the hedonic evaluation of foods, especially sweet solutions. The literature is inconsistent; some studies show a CB1R agonist-dependent increase and antagonist-dependent decrease in appetitive behavior via taste reactivity and brief-access tests (19, 22), and others report behavior akin to effect on satiation or motivation (21, 45). This inconsistency is mirrored regarding the effect of CB1R agonists and antagonists on preference for more complete food types in rodents; some studies report a selective decrease in the intake of palatable substances (1, 15, 26, 33, 44, 48), and other studies report equal suppression between diets of varying palatability (14, 17, 31, 46; but see 32). Most of these studies examined consumption of diets between groups. The dessert model described in experiment 1 allows exploration of selection between a complete diet and a palatable diet within a group. In experiments 2 and 3, we used our dessert model of overconsumption to examine the effect of long-term CB1R antagonist administration on diet selection and the development and maintenance of caloric imbalance. We hypothesized that administration of a CB1R antagonist will decrease food intake by selectively reducing consumption of a palatable dessert.
METHODS
Animals and housing.
All experiments were conducted using female retired breeder Sprague-Dawley rats (Harlan, Indianapolis, IN) ∼9 mo of age and weighing 300–350 g. Adult female rats were used because their body weights under conventional baseline conditions are stable, and so changes are readily detected. Data were taken without regard to the stage of the estrous cycle; visual inspection of the data revealed no systematic differences in group results as a result of such cyclicity. All rats were housed individually in conventional polycarbonate tubs (∼ 48 × 27 × 20 cm) containing 2- to 3-cm SaniChips (Teklad, Madison, WI) bedding. They were given ad libitum access to tap water and a complete maintenance diet as described below. The vivarium was temperature and humidity controlled (23 ± 2°C, 45–55%) with a reverse light cycle (lights off from 0930–2130 h). Procedures were performed just prior to the dark cycle, when rats are most active and consume the majority of their daily food. Each animal was used in only one experiment, and the experiments were performed serially. All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
Diets.
Moist chow was used as the ad libitum maintenance diet throughout the study to minimize spillage and thus provide an accurate measure of food consumption. Spillage of moist chow, estimated by visual inspection, was almost always minimal (<1 g). Moist chow is also more similar in texture to the desserts used (see below) than pelleted and powdered chow, and was used to reduce this difference among the diets. Moist chow (1.67 kcal/g) was made daily by combining one part powdered standard chow (Purina 5001) with one part tap water. The moist chow was brought to room temperature and spooned into 50-ml glass jars. The jars were attached to a metal stirrup and suspended in a corner of the home cage. Food consumption was measured daily for a 4- to 7-day period prior to the experimental phases of each experiment to insure stability of intake and to provide a baseline for assignment into groups such that big and small eaters were equally represented in each group. Evaporation of water from the moist chow was measured prior to the experiment and was found to be negligible (<0.5 g in 24 h) so was not considered in the analysis. Fresh jars of chow were provided daily at the beginning of the experimental session.
Some rats were also presented with optional palatable food sources during portions of the experiments. Some rats were given a sugar gel (SG; 0.31 kcal/g) dessert made by dissolving 60 g of commercial white sugar and 10 g gelatin in 1,000 ml of warm tap water. This solution was poured into 50-ml glass jars, covered, and allowed to solidify in a refrigerator overnight. The jar of SG was brought to room temperature, attached to a metal stirrup, and suspended in a corner of the home cage adjacent to chow. The effect of evaporation of water from the SG was measured prior to the experiment and was found to be negligible (<0.1 g in 8 h) so was not considered in the analysis. Other rats were given a sugar fat whip (SFW; 7.35 kcal/g) dessert made daily by combining two parts softened vegetable shortening with one part white sugar, both standard brands purchased from a local supermarket. The SFW mixture was brought to room temperature and spooned into 50-ml glass jars. The jars were attached to a metal stirrup and suspended in a corner of the home cage adjacent to the moist chow. Spillage of either of the desserts was never seen in our experiments.
Experimental design.
Experiment 1 sought to establish a model of overconsumption by comparing the effect of the two different desserts on caloric compensation. Two groups of eight rats were given access to one of the two desserts (SG or SFW) during an 8-h nocturnal session (0930–1730 h). Another group of eight rats was given no dessert and used as a control for subsequent comparison. Dessert and chow intakes were measured by subtracting the remaining weight of the diet from that originally presented. Total caloric intakes, as well as individual caloric intakes from moist chow and SG or SFW, were calculated daily for 15 days. Body weights were measured every 2–3 days and were analyzed as changes since the start of access to the dessert regimen.
Experiment 2 used the SFW overconsumption protocol detailed in experiment 1 to determine in rats the effect of repeated administration of Rimonabant (SR141716A), a CB1R antagonist, on diet selection and caloric compensation. Two groups of 12 rats were given daily 8-h nocturnal SFW and received daily injections of either Rimonabant (1 mg/kg ip) or the vehicle (1 ml/kg ip) 30 min prior to SFW access. Total caloric intakes, as well as individual caloric intakes from moist chow and SFW, and body weight changes from baseline were assessed daily for 7 days, which was the period in experiment 1 during which an increase in caloric intake was most evident.
Experiment 3 used the SFW overconsumption protocol detailed in experiment 1 to determine the effect of another CB1R antagonist, AM251, on the same variables as in experiment 2, but over a longer time course and with a range of doses. Four groups of six rats received daily 8-h nocturnal SFW access and received daily injections of one of three doses of AM251 (0.3, 1.0, or 3.0 mg/kg ip) or the vehicle (1 ml/kg ip) 30 min prior to SFW access. Total caloric intakes, as well as individual caloric intakes from moist chow and SFW, and body weight changes from baseline were assessed daily for 15 days.
Drugs.
Rimonabant was obtained through the National Institute on Drug Abuse experimental drug distribution program. The dose of Rimonabant (1 mg/kg) was chosen because it was not expected to eliminate feeding completely and would also be unlikely to produce cumulative effects with chronic dosing. Rimonabant was dissolved in warm polyethylene glycol solution (mol wt = 400; Sigma, St. Louis, MO), and an equal volume of saline was slowly added, creating a stable suspension in which no precipitate was visible; this was made the day prior to dosing and kept at room temperature until injection. The vehicle was chosen on the basis of preliminary studies showing this had little or no effect on food intake. AM251 was purchased from Tocris (Ellisville, MO). The doses chosen (0.3, 1.0, and 3.0 mg/kg) encompassed and expanded the doses of Rimonabant used in the previous study. AM251 was also dissolved in warm polyethylene glycol solution; however, the suspension precipitated when saline was added. The drug and vehicle mixture was sonicated immediately prior to injection; this provided a suitable suspension in which no precipitate was visible at the time of injection. Both Rimonabant and AM251 may have inverse agonist properties, but for simplicity they will be considered antagonists in this manuscript (29, 35).
Statistical analysis.
The daily individual total caloric intakes, as well as the component intakes from moist chow and dessert were analyzed via two-way ANOVA with groups and days as main factors. When the analysis revealed a significant (P ≤ 0.05) effect of days and/or a significant group × day interaction, the data were analyzed further with one-way ANOVA followed by Tukey post hoc comparisons to examine daily differences between groups and within-group differences across days. For brevity, only those post hoc analyses that were significant will be reported. The cumulative body weight changes between the first and last day of each experiment were analyzed with two-way ANOVA, and significant differences between or within groups were further analyzed with one-way ANOVA followed by Tukey post hoc comparisons of each day's average cumulative body weight change.
RESULTS
Experiment 1: model of overconsumption.
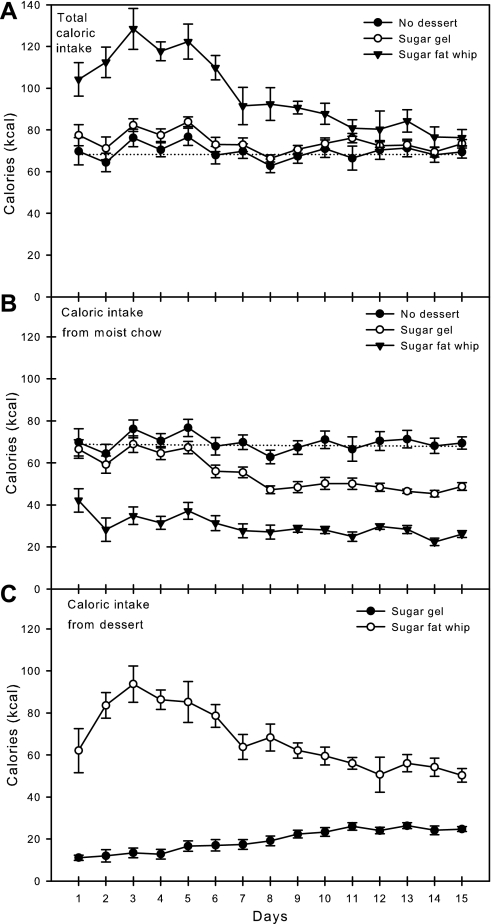
The average daily total caloric intakes across the days of the experiment are presented in Fig. 1A. Two-way ANOVA revealed a significant effect of group (F[2,21] = 137.7, P < 0.001), days (F[14,21] = 7.2, P < 0.001), and a group × day interaction (F[28,294] = 3.4, P < 0.001); the overall averages are presented in Table 1. Post hoc analysis revealed that rats given 8-h access to SFW consumed significantly more calories than rats given either no dessert or rats given 8-h access to SG. Daily one-way ANOVA revealed that rats given SFW consumed significantly more calories than rats given SG or rats given no dessert during days 1–10 of the experiment. The total intakes of rats given SFW showed significant differences across days, with intakes decreasing by almost 50% (from 128 to 67 kcal/day) between days 3 and 15.
Fig. 1.
Means ± SE daily caloric intake of rats given ad libitum access to moist chow and 8 h/day access to either sugar gel (SG; n = 8), sugar fat whip (SFW; n = 8), or no additional diet (n = 8). A: total caloric intake. B: caloric intake from moist chow. C: caloric intake from dessert. The horizontal dotted line is the mean baseline consumption of moist chow only of all the rats before experimental manipulations and is shown for comparison. Rats given SFW consumed significantly (P < 0.05) more total calories than the other groups on days 1–10 and significantly fewer calories from chow than the other groups on all days. Rats given SG consumed fewer calories from moist chow than rats given no dessert on days 7–15. Rats given SFW consumed significantly more calories from SFW than rats given SG consumed from SG on all days.
Table 1.
Average daily total intakes, intakes from moist chow and dessert, and cumulative body weight changes from experiment 1
| No Dessert | Sugar Gel | Sugar Fat Whip | |
|---|---|---|---|
| Total intake, kcal; g | 69.5±1.3*; 41.6±1.1 | 74.2±1.3*; 95.2±1.1 | 97.2±1.3†; 27.1±1.1 |
| Moist chow intake, kcal; g | 69.5±0.9*; 41.6±0.5 | 54.9±0.9†; 32.8±0.5 | 29.9±0.9‡; 17.9±0.5 |
| Dessert intake, kcal; g | NA | 19.3±1.2*; 62.4±1.0 | 67.3±1.2†; 9.2±1.0 |
| ΔBody weight, g | −2.3±1.0* | −3.0±1.0* | 29.7±1.0† |
Intake values are expressed as means ± SE; n = 8 rats/group. Changes in body weight values are expressed as cumulative means ± SE. NA, not applicable.
*,†,‡Significant differences (P < 0.05) in caloric intakes and body weight changes between groups as revealed by two-way ANOVA and Tukey post hoc tests.
The average daily caloric intakes from moist chow across days are presented in Fig. 1B. Two-way ANOVA revealed a significant effect of group (F[2,21] = 488.3, P < 0.001) and days (F[14,21] = 6.3, P < 0.001), and no group × day interaction (F[28,294] = 1.3, P = 0.144); the overall averages are presented in Table 1. Post hoc analysis revealed that rats given SFW consumed significantly fewer calories from chow than rats given SG. Daily one-way ANOVA revealed that rats given SFW consumed significantly fewer calories from chow than rats given SG or rats that received no dessert during all 15 days of the experiment. Rats given SG consumed as many calories from chow as rats given no dessert during days 1–6, but they consumed significantly less than rats given no dessert during days 7–15.
The average daily caloric intakes from dessert across days are presented in Fig. 1C. Two-way ANOVA revealed a significant effect of group (F[2,21] = 1221.3, P < 0.001), days (F[14,21] = 2.6, P = 0.002), and a group × day interaction (F[28,294] = 6.7, P < 0.001); the overall averages are presented in Table 1. Post hoc analysis revealed that rats given SFW consumed significantly more calories from SFW than rats given SG consumed from SG (P < 0.001). Daily one-way ANOVA revealed that rats given SFW consumed more calories from SFW than rats given SG consumed from SG on all 15 days. Rats given SFW ate increasing amounts of SFW for the first 3 days, and then slowly decreased SFW consumption until stabilizing consumption at day 8. Rats given SG consumed modestly increasing amounts of SG across the entire 15-day experiment.
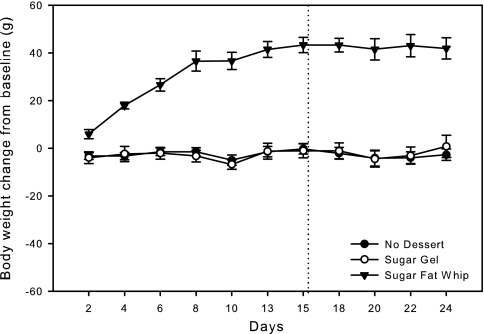
The average body weight changes from baseline across days are presented in Fig. 2. Two-way ANOVA revealed a significant effect of group (F[2,21] = 368.9, P < 0.001), days (F[14,21] = 11.5, P < 0.001), and a group × day interaction (F[28,294] = 8.9, P < 0.001); the cumulative averages are presented in Table 1. Post hoc analysis revealed that rats given SFW gained significantly more weight than rats given SG or rats that received no dessert. Daily one-way ANOVA revealed that rats given SFW weighed more than rats in the other groups on every day of the experiment. The body weight of rats given SFW increased rapidly between days 1–10 and then seemed to plateau.
Fig. 2.
Means ± SE daily change in body weight from baseline in rats fed the desserts described in Fig. 1. The vertical dotted line represents when the rats were taken off study and returned to access to moist chow only. Rats given SFW gained significantly more weight than rats given no dessert or rats given SG. Rats given SFW gained weight every day and weighed more than rats given no dessert or given SG on all days. Rats given no dessert and rats given SG did not significantly change in body weight from baseline.
Experiment 2: Rimonabant and overconsumption.
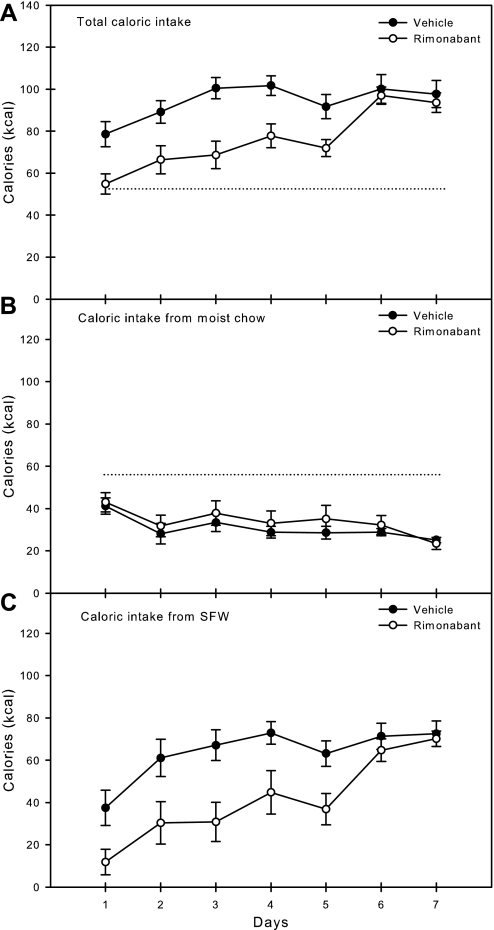
The average daily total intakes across the 7 days of the experiment are presented in Fig. 3A. Two-way ANOVA revealed a significant effect of group (F[1,22] = 40.5, P < 0.001) and days (F[6,22] = 7.9, P < 0.001), and no group × day interaction (F[6,132] = 2.0, P = 0.72); the overall averages are presented in Table 2. Rats injected daily with Rimonabant prior to SFW access consumed significantly fewer total calories than rats injected with vehicle prior to SFW access. Daily one-way ANOVA revealed that rats injected with Rimonabant consumed fewer total calories than rats injected with vehicle on days 1–5.
Fig. 3.
Means ± SE daily caloric intake of rats injected with either vehicle (n = 12) or cannabinoid (CB) receptor (CB1R) antagonist Rimonabant (n = 12) while given ad libitum access to moist chow and 8 h access to SFW. A: total caloric intake. B: caloric intake from moist chow. C: caloric intake from SFW. The horizontal dotted line represents the mean baseline consumption of moist chow only of all the rats prior to experimental manipulations and is shown for comparison. Rats injected with Rimonabant consumed significantly fewer total calories than rats injected with vehicle on days 1–5. Rats injected with Rimonabant consumed an equivalent number of calories from moist chow as rats injected with vehicle on all days, and both groups consumed less chow than during baseline. Rats injected with Rimonabant consumed significantly fewer calories from SFW than rats injected with vehicle on days 1–5.
Table 2.
Average daily total intakes, intakes from moist chow and sugar fat whip, and cumulative body weight changes from experiment 2
| Vehicle | Rimonabant | |
|---|---|---|
| Total intake, kcal; g | 94.2±2.2*; 25.5±0.7 | 75.1±2.2†; 25.3±0.7 |
| Moist chow intake, kcal; g | 30.6±1.7*; 17.4±0.9 | 33.8±1.6*; 18.8±0.9 |
| Dessert intake, kcal; g | 63.6±2.9*; 8.1±0.3 | 41.4±2.7†; 6.4±0.3 |
| ΔBody weight, g | 6.9±0.8* | −2.6±0.8† |
Intake values are expressed as means ± SE; n = 12 rats/group. Changes in body weight values are expressed as cumulative means ± SE.
*,†Significant differences (P < 0.05) in caloric intakes and body weight changes between groups as revealed by two-way ANOVA.
The average daily intakes from moist chow across days are presented in Fig. 3B. Two-way ANOVA revealed no significant effect of group (F[1,22] = 1.822, P = 0.179), a significant effect of days (F[6,22] = 3.1, P = 0.007), and no group × day interaction (F[6,132] = 1.7, P = 0.984); the overall averages are presented in Table 2. Daily one-way ANOVA revealed that although the chow intakes of rats injected with Rimonabant did not differ from that of rats injected with vehicle, both groups decreased chow consumption across all days and consumed significantly less chow than during baseline.
The average daily caloric intakes from SFW across days are presented in Fig. 3C. Two-way ANOVA revealed a significant effect of group (F[1,22] = 31.3, P < 0.001) and days (F[6,22] = 8.9, P < 0.001) and no group × day interaction (F[6,132] = 1.5, P = 0.198); the overall averages are presented in Table 2. Rats injected with Rimonabant consumed fewer calories from SFW than rats injected with vehicle. Daily one-way ANOVA revealed that rats injected with Rimonabant consumed significantly fewer calories from SFW than rats injected with vehicle during days 1–5.
The average daily body weight changes across days are presented in Fig. 4. Two-way ANOVA revealed a significant effect of group (F[1,22]=66.1, P < 0.001), days (F[6,22] = 13.9, P < 0.001), and a group × day interaction (F[6,132] = 2.2, P = 0.045); the cumulative averages are presented in Table 2. Rats injected with Rimonabant gained significantly less weight than rats injected with vehicle. Daily one-way ANOVA revealed that rats injected with Rimonabant weighed less than rats injected with vehicle during days 4–7, reaching a positive weight gain only on day 7. Rats injected with vehicle gained weight on all days but day 2.
Fig. 4.
Means ± SE daily change in body weight from baseline in rats on the drug regimen and dessert protocol described in Fig. 3. Rats injected with Rimonabant while given SFW gained significantly less weight than rats injected with vehicle while given SFW. Rats injected with Rimonabant while given SFW weighed less than those injected with vehicle while given SFW on every day except day 2, and maintained a negative body weight change until day 5.
Experiment 3: AM251 and overconsumption.
The average daily total intakes of the groups across days are presented in Fig. 5A. Two-way ANOVA revealed a significant effect of group (F[3,20] = 27.3, P < 0.001) and days (F[14,20] = 2.5, P = 0.002), and no group × day interaction (F[42,280] = 0.9, P = 0.59). The overall averages are presented in Table 3. Post hoc analysis revealed that rats injected daily with the 3.0 mg/kg AM251 consumed significantly fewer total calories than rats injected with vehicle across all days. Rats injected with 3.0 mg/kg AM251 consumed significantly fewer total calories than rats injected with 0.3 mg/kg AM251. Daily one-way ANOVA revealed that a dose of 3.0 mg/kg AM251 reduced total intakes on days 1–8.
Fig. 5.
Means ± SE daily caloric intake of rats injected with either vehicle (n = 6) or 1 of 3 doses of CB1 receptor antagonist AM251 (each, n = 6) while on the dessert protocol described in Fig. 3. A: total caloric intake. B: caloric intake from moist chow. C: caloric intake from dessert. The horizontal dotted line represents the mean baseline consumption of moist chow only of all the rats prior to experimental manipulations and is shown for comparison. Rats injected with 3.0 mg/kg AM251 consumed significantly fewer total calories than rats injected with vehicle on days 1–8. Rats injected with 3.0 mg/kg AM251 consumed significantly fewer total calories than rats injected with 0.3 mg/kg AM251 on days 4 and 5. Rats injected with 0.3 and 3.0 mg/kg AM251 consumed an equivalent number of calories from moist chow as rats injected with vehicle on all days. Rats injected with 1.0 mg/kg AM251 consumed significantly fewer calories from chow than rats in the other groups on days 7, 8, 10, and 12. Rats injected with 3.0 mg/kg AM251 consumed significantly fewer calories from SFW than rats injected with vehicle on days 1–3 and 5–8.
Table 3.
Average daily total intakes, intakes from moist chow and sugar fat whip dessert, and cumulative body weight changes from experiment 3
|
AM251 |
||||
|---|---|---|---|---|
| 0.0 mg/kg | 0.3 mg/kg | 1.0 mg/kg | 3.0 mg/kg | |
| Total intake, kcal; g | 97.4±2.0*; 30.1±0.5 | 80.9±2.0*,†; 27.9±0.5 | 78.1±2.0*,†,‡; 24.9±0.5 | 73.9±2.0‡; 26.5±0.5 |
| Moist chow intake, kcal; g | 20.3±0.6*; 12.1±0.4 | 19.9±0.6*; 11.9±0.4 | 15.2±0.6†; 9.1±0.4 | 20.1±0.6*; 12.0±0.4 |
| Dessert intake, kcal; g | 77.1±1.7*; 10.4±0.3 | 61.0±1.7*,†; 8.2±0.3 | 62.9±1.7*,†,†; 8.5±0.3 | 53.7±1.7†,‡; 7.4±0.3 |
| ΔBody weight, g | 30.9±1.4* | 16.9±1.4*,† | 14.1±1.4*,†,‡ | 6.4±1.4‡ |
Intake values are expressed as means ± SE; n = 6 rats/group. Changes in body weight values are expressed as cumulative means ± SE.
*,†,‡Significant differences (P < 0.05) in caloric intakes and body weight changes between groups as revealed by two-way ANOVA and Tukey post hoc tests.
The average daily intakes from moist chow only across days are presented in Fig. 5B. Two-way ANOVA revealed a significant effect of group (F[3,20] = 15.1, P < 0.001), no significant effect of days (F[14,20] = 1.1, P = 0.374), and no group × day interaction (F[42,280] = 1.2, P = 0.211); the overall averages are presented in Table 3. Post hoc analysis revealed that the moist chow intakes of rats injected with 1.0 mg/kg AM251 consumed significantly fewer calories from moist chow than rats injected with vehicle. Daily one-way ANOVA revealed that rats injected with 1.0 mg/kg AM251 consumed fewer calories from moist chow than rats injected with vehicle on days 7, 8, 10, and 12.
The average daily caloric intakes from SFW only across days are presented in Fig. 5C. Two-way ANOVA revealed a significant effect of group (F[3,20] = 31.7, P < 0.001) and days (F[14,20] = 2.6, P = 0.002), and no group × day interaction (F[42,280] = 0.8, P = 0.769); the overall averages are presented in Table 3. Post hoc analysis revealed that rats injected with 3.0 mg/kg AM251 consumed fewer calories from SFW than rats injected with vehicle. Daily one-way ANOVA revealed that rats injected with 3.0 mg/kg AM251 consumed significantly fewer calories from SFW than rats injected with vehicle during days 1–3 and 5–8.
The average daily body weight changes across days are presented in Fig. 6. Two-way ANOVA revealed a significant effect of group (F[3,20]=51.3, P < 0.001) and days (F[14,20]=11.7, P < 0.001), and no group × day interaction (F[42,280] = 0.5, P = 0.997); the cumulative averages are presented in Table 3. Post hoc analysis revealed that rats injected with 3.0 mg/kg AM251 gained less weight than rats injected with vehicle. Daily one-way ANOVA revealed that rats injected with any dose of AM251 gained less weight than rats injected with vehicle across all 15 days. Rats injected with 3.0 mg/kg AM251 maintained a negative change in body weight from baseline until day 8.
Fig. 6.
Means ± SE daily change in body weight from baseline in rats on the drug regimen and dessert protocol described in Fig. 5. Rats injected with any dose of AM251 gained less weight than rats injected with vehicle on days 6–15. Rats injected with 3.0 mg/kg AM251 gained less weight than rats injected with 0.3 or 1.0 mg/kg AM251 on days 1–8. Rats injected with 3.0 mg/kg while given SFW weighed less than those injected with vehicle while given SFW on every day and maintained a negative body weight change until day 8.
DISCUSSION
Access to foods that have a high caloric density and are also usually more palatable has been shown to increase total caloric intake in humans and animals (28). Our first experiment sought to develop in female rats a model of overconsumption, in which access to an optional calorie source (dessert) along with a basic diet (chow) would result in incomplete caloric compensation and a resultant increase in body weight. Many protocols influence the feeding and body weight gain of rats, but our dessert protocol allows efficient and simple exploration of the balance of caloric intake via selection from two different food sources. In this dessert protocol, caloric compensation was defined as a decrease in caloric intake from chow that matched the caloric intake from dessert such that caloric intake did not differ from no-dessert controls.
Our results depended on the type of dessert presented. Female rats given SG reduced chow consumption to compensate for calories consumed from SG; however, while large volumes of SG were consumed, its low caloric density yielded relatively few calories. Other protocols have found that access to carbohydrates can induce net hyperphagia and weight gain associated with consumption of a Polycose gel (43), and the differences from the present study may be due to a restricted rather than ad libitum access to the dessert, the use of SG rather than Polycose gel, or the use of female rats rather than males. We used female rats, which is an arguably more clinically relevant model since more women than men report and seek treatment for body weight disorders. We further compared intake of SG with that of a very sweet and fatty dessert (SFW), the type of food for which women often report craving and overconsumption (37). Rats given SFW reduced chow intake even more than the rats given SG, but this compensation was far less than the large caloric intake from SFW. This result is similar to the intermittent excessive eating that has been reported when female rats are given highly restrictive access to shortening (11). However, this latter binge eating protocol did not result in a net increase in calories consumed across days or weight gain. Thus, our SFW protocol of daily intermittent access may be more useful to study the etiology of overconsumption and weight gain, at least in females. Substantial weight gain from SFW was seen within days, making this dessert protocol suitable for screening potential treatments compared with other diet-induced obesity models. Although the effect of 8 h of uninterrupted access to SFW on total caloric intake lasted <2 wk, the substantial increase in body weight was observed for 9 days after access to dessert ceased (Fig. 2) and was noted by visual inspection to have persisted past the time that total intake had normalized. This protocol may be useful for examining the effects of diet selection on total intake and a means to evaluate the role of appetite-suppressant pharmaceuticals on these factors. In experiments 2 and 3, we examined this potential application using CB1R antagonists.
Although the acute appetite-reducing properties of CB1R antagonists have been studied extensively, few studies have examined repeated administration and the effect on long-term body weight regulation and within-group diet selection. When administered daily while rats were exposed to the SFW protocol developed in experiment 1, Rimonabant reduced total caloric intake for the first 5 days of experiment 2. This is similar to the hypophagic effect previously reported when rats were given only standard maintenance diet (5). However, rather than reducing caloric intake by further decreasing consumption of chow, Rimonabant administration selectively reduced intake of SFW. Since at the end of experiment 2 there was a suggestion of a decreasing effect of Rimonabant, we sought to extend our findings using several doses of a CB1R antagonist and a longer duration dosing regimen. We used commercially available AM251 rather than Rimonabant because we had exhausted our supply of Rimonabant. Both agents are structurally similar and have 100 times greater selectivity for CB1R over CB2R (16, 38). All doses of AM251 decreased total caloric intake throughout the study, and there was a trend for this to be dose dependent. There was little difference in chow intake between any of the groups. Rats injected with the middle dose of AM251 ate slightly, though significantly less. As with our results from experiment 2, the major difference in total caloric intake between groups in experiment 3 was due to differences in SFW intake.
The finding that CB1R antagonists Rimonabant and AM251 decrease palatable food consumption when presented with a choice between two food commodities is congruent with results from some feeding protocols in rodents and marmosets (26, 44), which suggests that CB1R activity plays a role in the motivation toward and hedonic evaluation of palatable foods, but not with some reports of equal suppression of all food types in rodents and baboons (46, 14). A difference between some of the former and latter results is the analysis of diet selection within a group. Although CB1R antagonists reduce intakes of all diets, when animals are presented with a choice between diets, differences among the diets may become clear. A more complete analysis of CB1R antagonist-mediated depression of appetite may require analysis of diet selection across multiple meals. Foltin and Haney (14) did provide a diet choice to their baboons, and suggest that the discrepancy in the effect of Rimonabant on diet selection may be due to the fact that animals consume much more of the palatable food choice than standard diet. The dessert we used in our study was much more calorically dense than in their study, and so, while rats consumed many calories from it, they consumed by weight a larger quantity of moist chow than SFW. In another study seeking to model night eating syndrome, we examined the effect of nocturnal vs. diurnal SFW presentation and found that rats decreased their consumption of chow slightly further when provided with longer SFW access. Female retired breeder SD rats were given ad libitum access to moist chow and 12-h access to SFW, either during the light phase or the dark phase of their daily cycle. Rats given SFW nocturnally consumed more SFW than rats given SFW diurnally, but there were no differences in total daily caloric intake or body weight change. Compared with rats in the present study that were given 8-h nocturnal access to SFW, rats given 12-h nocturnal access to SFW consumed statistically more SFW (85 kcal/day compared with 67 kcal/day) and less chow (15 kcal/day compared with 30 kcal/day) with no apparent deleterious effects stemming from a low protein-to-calorie ratio (Mathes CM and Rowland NE, unpublished observations). Thus, it is possible for rats to reduce their chow intake further, but they did not when injected with CB1R antagonists.
CB1R antagonist administration also reduced the body weight increase associated with access to SFW. Rats injected with Rimonabant in experiment 2 sustained a small weight loss for the first 5 days of the study, and, even on the last 2 days when their caloric intake was the same as controls, they did not show a net gain relative to these controls (Fig. 4). The decreased caloric intake in AM251-treated groups in experiment 3 resulted in a reduced or delayed body weight gain during SFW exposure. Unlike our results from experiment 2, none of the rats injected with any dose of AM251 lost weight, and their failure to gain as much weight as vehicle-treated controls seemed to match their decrease in caloric intake (Figs. 5 and 6). The decreases in body weight may not be the result of changes in feeding alone, but study of the effect of cannabinoid antagonists on fat metabolism, gastric emptying, nausea, and other variables (see Ref. 42 for review) was beyond the scope of this experiment. Minor differences in our results from Rimonabant and AM251 may be due to different bioavailabilities of the CB1R antagonists and/or individual variability in consumption between the batches of rats, but despite these possibilities, our findings with both drugs support our hypothesis that CB1R antagonists decrease caloric intake by reducing selection and consumption of palatable foods.
Perspectives and Significance
These data add to the debate concerning the mechanisms by which CB1R antagonists reduce caloric intake and whether this reduction is dependent on the palatability of the commodity. We explored this question using a novel dessert protocol that provides the important element of analyzing within-group diet selection. Our results support the suggestion that areas in the brain associated with reward (i.e., nucleus accumbens, ventral tegmental area) interact with areas associated with homeostatic drives (i.e., hypothalamic and brain stem nuclei), and that in some environmental situations, hedonism may prevail over regulation (40). CB1R are found in areas of the brain associated with both of these functions, and a recent demonstration suggests that CB1R in the nucleus accumbens may play a key role in “liking” responses to sweet tastes (30). The role of CB1R in reward mirrors the rich literature on opioid and benzodiazepine modulation of reward (2, 34), and structural, and functional similarities and synergisms seen between the opioid, GABA, and cannabinoid systems suggest that these systems interact and impinge on dopamine circuits to define the perception of rewarding stimuli and maintain behavior associated with them (10, 23). Further studies using clinically relevant methods, such as dessert protocols, are necessary to define the physiological and behavioral effects of CB1R antagonists and their potential interactions with other systems that affect food intake and reward. This knowledge may lead to the development of pharmacological treatment of obesity that is better suited to human physiological reaction to today's food environment.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1RO1-DK-064712 (to Neil Rowland).
Acknowledgments
The authors thank Kim Robertson for technical support and Deepak Suresh for assistance in statistical analysis for experiment 1.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arnone M, Maruani J, Chaperon F, Thiebot M, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology 132: 104–106, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC, Pecina S. Benzodiazepines, appetite, and taste palatability. Neurosci Biobehav Rev 19: 121–131, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Carai MA, Colombo G, Maccioni P, Gessa GL. Efficacy of rimonabant and other cannabinoid CB1 receptor antagonists in reducing food intake and body weight: preclinical and clinical data. CNS Drug Rev 12: 91–99, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers AP, Sharkey KA, Koopmans HS. Cannabinoid (CB)1 receptor antagonist, AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav 82: 863–869, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci 63: PL113–PL117, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Cooper SJ Endocannabinoids and food consumption: comparisons with benzodiazepine and opioid palatability-dependent appetite. Eur J Pharmacol 500: 37–49, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav 82: 123–130, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Corwin RL, Hajnal A. Too much of a good thing: neurobiology of non-homeostatic eating and drug abuse. Physiol Behav 86: 5–8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corwin RL Bingeing rats: a model of intermittent excessive behavior? Appetite 46: 11–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cota D, Tschop MH, Horvath TL, Levine AS. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev 51: 85–107, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord 28: 436–445, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Drewnowski A The real contribution of added fats and sugars to obesity. Epidemiol Rev 29: 160–171, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Ello-Martin JA, Ledikwe JH, Rolls BJ. The influence of food portion size and energy density on energy intake: implications for weight management. Am J Clin Nutri 82, Suppl 1: 236S–241S, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Foltin RW, Haney M. Effects of the cannabinoid antagonist SR141716 (Rimonabant) and d-amphetamine on palatable food and food pellet intake in non-human primates. Pharmacol Biochem Behav 86: 766–773, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav 67: 265–270, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis.A. 123I-Labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol 307: 331–338, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Gessa CL, Orru A, Lai P, Maccioni P, Lecca R, Lobina C, Carai MA, Colombo G. Lack of tolerance to the suppressing effect of rimonabant on chocolate intake in rats. Psychopharmacology (Berl) 185: 248–254, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Ginsburg BC, Lamb RJ. Cannabiniod effects on behaviors maintained by ethanol or food: a within-subjects comparison. Behav Pharmacol 17: 249–257, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Higgs S, Williams CM, Kirkham TC. Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after Δ9-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology (Berl) 165: 370–377, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hapson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 47: 345–358, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Jarrett MM, Limebeer CL, Parker LA. Effect of Δ9-tetrahydrocannabinol on sucrose palatability as measured by the taste reactivity test. Physiol Behav 86: 475–479, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Jarrett MM, Scantlebury J, Parker LA. Effect of Δ9-tetrahydrocannabinol on quinine palatability and AM251 on sucrose and quinine palatability using the taste reactivity test. Physiol Behav 90: 425–430, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86: 773–795, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kirkham TC, Williams CM. Endocannabinoid receptor antagonists: potential for obesity treatment. Treat Endocrinol 3: 345–360, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Kirkham TC Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol 16: 297–313, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Koch JE δ9-THC stimulates food intake in Lewis rats: effects on chow, high-fat and sweet high-fat diets. Pharmacol Biochem Behav 68: 539–543, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 282: R46–R54, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE Factors promoting and ameliorating the development of obesity. Physiol Behav 86: 633–639, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Maclennan SJ, Reynen PH, Kwan J, Bonhaus DW. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br J Pharmacol 124: 619–622, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances “liking” of a sweet reward. Neuropsychopharmacology 32: 2267–2278, 2007. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol 14: 583–588, 2003. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, Ishiwari K, Betz AJ, Pandarinathan L, Xu W, Makriyannis A, Salamone JD. Suppression of food intake and food-reinforced behavior produced by the novel CB1 antagonist/inverse agonist AM 1387. Pharmacol Biochem Behav 83: 396–402, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav 80: 611–616, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav 91: 506–512, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Pertwee RG Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76: 1307–1324, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284: R345–R353, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Raymond NC, Neumeyer B, Warren CS, Lee SS, Peterson CB. Energy intake patterns in obese women with binge eating disorder. Obes Res 11: 869–879, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Rinaldi-Carmona M, Barth F, Heaulme M, Shire B, Calandra B, Congy C, Martinez S, Maruani J, Nelliat G, Caput D, Ferrara B, Soubrie P, Breliere JC, Lefur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350: 240–244, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Rothwell NJ, Stock MJ. The cafeteria diet as a tool for studies of thermogenesis. J Nutr 118: 925–928, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Rowland NE, Vaughan CH, Mathes CM, Mitra A. Feeding behavior, obesity, and neuroeconomics. Physiol Behav 93: 97–109, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav 91: 383–388, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanger GJ Endocannabinoids and the gastrointestinal tract: what are the key questions? Br J Pharmacol 152: 663–670, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sclafani A Carbohydrate-induced hyperphagia and obesity in the rat: effects of saccharide type, form, and taste. Neurosci Biobehav Rev 11: 155–162, 1987. [DOI] [PubMed] [Google Scholar]
- 44.Simiand J, Keane M, Keane PE, Soubrie P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol 9: 179–181, 1998. [PubMed] [Google Scholar]
- 45.Thornton-Jones ZD, Kennett GA, Vickers SP, Clifton PG. A comparison of the effects of the CB1 receptor antagonist SR141716A, pre-feeding and changed palatability on the microstructure of ingestive behaviour. Psychopharmacology (Berl) 193: 1–9, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Verty AN, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett 354 217–220, 2004. [DOI] [PubMed]
- 47.Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 167: 103–111, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940). Behav Pharmacol 16: 381–388, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Williams CM, Kirkham TC. Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol Behav 76: 241–150, 2002. [DOI] [PubMed] [Google Scholar]