Abstract
The effect of ovalbumin (Ova) sensitization on pulmonary C-fiber sensitivity was investigated. Brown-Norway rats were sensitized by intraperitoneal injection of Ova followed by aerosolized Ova three times per week for 3 wk. Control rats received the vehicle. At the end of the third week, single-unit fiber activities (FA) of pulmonary C fibers were recorded in anesthetized, artificially ventilated rats. Our results showed the following: 1) Ova sensitization induced airway inflammation (infiltration of eosinophils and neutrophils) and airway hyperresponsiveness in rats; 2) baseline FA in sensitized rats was significantly higher than that in control ones; 3) similarly, the pulmonary C-fiber response to right atrial injection of capsaicin was markedly higher in sensitized rats, which were significantly amplified after the acute Ova inhalation challenge; and 4) similar patterns, but smaller magnitudes of the differences in C-fiber responses to adenosine and lung inflation, were also found between sensitized and control rats. In conclusion, Ova sensitization elevated the baseline FA and excitability of pulmonary C fibers, and the hypersensitivity was further potentiated after the acute Ova inhalation challenge in sensitized rats. Chronic allergic inflammatory reactions in the airway probably contributed to the sensitizing effect on these lung afferents.
Keywords: airway hyperresponsiveness, inflammatory mediators, allergen, vagus, asthma
asthma is a disease characterized by episodic and reversible airway obstruction and inflammation, accompanied by airway hyperresponsiveness (AHR) (42). AHR is defined as exaggerated bronchomotor responses to various nonallergic and nonspecific stimuli, and its pathogenic mechanism is not fully understood, but is known to involve an interaction among multiple types of cells in the airways during allergic inflammatory reaction.
Nonmyelinated (C fiber) vagal afferents represent a major type of sensory nerves innervating the airways and lung. Activation of these afferents by inhaled irritants or by endogenous mediators is known to induce pronounced cardiorespiratory reflex responses, via both centrally mediated reflex pathways and local axon-reflex mechanism (9, 38). These responses include, but are not limited to, bronchoconstriction, airway hypersecretion, cough, tachypnea, etc. Increasing evidence has suggested that hypersensitivity of these afferents plays an important role in the manifestation of various symptoms associated with airway inflammatory diseases (9, 38). However, the changes in bronchopulmonary C-fiber sensitivity during allergic airway inflammatory reaction have not been fully characterized.
Several animal models of asthma have been developed and reported in the literature for studying the pathogenic mechanisms of AHR. One of the extensively used models is Brown-Norway (BN) rats actively sensitized with ovalbumin (Ova); it has been demonstrated that chronic allergic inflammation develops in the airways of sensitized rats, and many of their pathophysiological features closely resemble those observed in patients suffering from allergic asthma (13, 14). For example, in sensitized animals, acute inhalation challenge with aerosol Ova triggers early- and late-phase airway responses in a pattern similar to those found in asthmatic patients after acute exposure to allergens (11, 12, 14). Therefore, the present study was carried out in this animal model of allergen-induced asthma to investigate the following: 1) whether the excitability of pulmonary C fibers in the responses to mechanical and chemical stimulants was altered in the rats that were sensitized with Ova; and 2) if so, whether the responses were altered by acute Ova inhalation challenge in sensitized rats.
METHODS
The experimental procedures were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health, and were also approved by the University of Kentucky Institutional Animal Care and Use Committee.
Animal sensitization and allergen exposure.
Pathogen-free male BN rats weighing 250–350 g were divided into two groups (control and sensitized groups). Sensitized rats were actively sensitized by an initial intraperitoneal injection of a suspension containing 2 mg Ova in 1 ml Imject Alum as adjuvant. Three days after the initial intraperitoneal injection, sensitized rats were exposed to Ova aerosol for 15 min each time, three times per week for 3 wk, following the protocol established by previous investigators (14). During exposure, the unanesthetized rat was placed in a Plexiglas restrainer (University of Kentucky, Center for Manufacturing), and it breathed spontaneously and continuously through a nose cone connected to a free stream of air-aerosol mixture under a negative-pressure exhaust hood. Ova solution (wt/vol concentration: 1.25% in saline) was nebulized and delivered by an ultrasonic nebulizer (model 099HD, Devilbiss, Somerset, PA) at a droplet size ranging from 0.5 to 5 μm. Control rats received the intraperitoneal injection and aerosol inhalation of the vehicle (isotonic saline) following the identical procedures.
Animal preparation.
After 3 wk of either Ova or saline exposure, rats were initially anesthetized with an intraperitoneal injection of α-chloralose (100 mg/kg) and urethane (500 mg/kg) dissolved in a 2% borax solution; whenever necessary, supplemental doses (one-tenth of the initial dose) of the same anesthetics were injected intravenously to maintain abolition of pain reflexes elicited by paw pinch. The right femoral artery and the left jugular vein were cannulated for recording arterial blood pressure (ABP) and administration of pharmacological agents, respectively; the latter was advanced until its tip was positioned just above the right atrium. A short tracheal cannula was inserted just below the larynx via a tracheotomy. Tracheal pressure (Ptr) was measured (MP45-28; Validyne, Northridge, CA) via a side port of the tracheal cannula. After a midline thoracotomy was performed, the lung was artificially ventilated with a respirator (model 7025, UGO Basile, Comerio-Varese, Italy), and the expiratory outlet of the respirator was placed under 3 cmH2O pressure to maintain a near-normal functional residual capacity. Tidal volume (Vt) and respiratory frequency were set at ∼8 ml/kg and 48–50 breaths/min, respectively, to mimic those of unilaterally vagotomized rats. Body temperature was maintained at ∼36°C by means of a heating pad placed under the animal lying in a supine position.
Electrophysiological recording of pulmonary C-fiber activity.
Single-unit pulmonary C-fiber activity was recorded as previously described (25). Briefly, the right cervical vagus nerve was separated from right carotid artery. The caudal end of the cut right vagus nerve was placed on a small dissecting platform and immersed in a pool of mineral oil. A thin filament was teased away from the desheathed nerve trunk and placed on a platinum-iridium hook electrode. Action potentials were amplified (model P511K, Grass Instruments, Quincy, MA), monitored by an audio monitor (model AM8RS, Grass Instruments), and displayed on an oscilloscope (model 2211, Tektronix, Wilsonville, OR). The thin filament was further split until the afferent activity arising from a single unit was electrically isolated. The nerve trunk was ligated just above the diaphragm to eliminate afferent signals arising from lower visceral organs. The afferent activity of a single unit was first searched by hyperinflation (3–4 × Vt) and then identified by the immediate (delay <1 s) response to a bolus injection of capsaicin (1.0 μg/kg) into the right atrium. Finally, the general locations of pulmonary C fibers were identified by their responses to the gentle pressing of the lungs with a blunt-ended glass rod. The signals of the afferent activity, Ptr and ABP were recorded on a thermal writer (model TW11, Gould Instrument Systems, Valley View, OH) and on a videocassette recorder format data recorder (model SLV-N900, Sony Electronics, Park Ridge, NJ). Fiber activities (FA) in impulses per second (imp/s) were analyzed by an online data acquisition system (model TS-100, Biocybernetics, Taipei, Taiwan) in 0.5-s intervals.
Inflammatory cell analysis in bronchoalveolar lavage fluid.
Bronchoalveolar lavage fluid (BALF) was collected from another two groups (control and Ova sensitized) of rats after 3 wk of vehicle and Ova exposures, respectively. BALF was obtained by injecting 10 ml of sterile saline (5 ml, twice) into the lungs of the anesthetized rat via a tracheal cannula. The collected BALF (∼7 ml) was centrifuged at 1,500 rpm for 10 min, and the pelleted cells were treated with Tris-buffered ammonium chloride solution (pH 7.2) to lyse red blood cells. The remaining cells were washed once with phosphate-buffered saline, supplemented with 1% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin (GIBCO, Grand Island, NY). Total cell counts were determined by using a hemocytometer. Differential leukocyte counts were then performed on cytospin slides after Wright-Giemsa staining. A minimum of 500 leukocytes were counted by using standard morphological criteria and determined by two different individuals. The data were then averaged.
Measurements of lung mechanics.
Control and sensitized rats were anesthetized and artificially ventilated in the same manner as that described earlier day after the last Ova exposure or vehicle. A catheter for measuring intrapleural pressure (Ppl) was inserted into the intrapleural cavity via a surgical incision between the fifth and sixth ribs; this incision was subsequently sutured and further sealed airtight with silicone jelly. The pneumothorax was then corrected by briefly opening the intrapleural catheter to ambient air during a held hyperinflation (3 × Vt). During the experiment, the animals were paralyzed with pancuronium bromide (50 μg/kg intravenously). Transpulmonary pressure was measured as the difference between Ptr and Ppl with a differential pressure transducer (model MP 45-28, Validyne, Northridge, CA). Respiratory flow was measured with a heated pneumotachograph and a differential pressure transducer (model MP 45-14, Validyne). All signals were recorded on a chart recorder (model 7, Grass, Quincy, MA); total pulmonary resistance (Rl) and dynamic lung compliance (Cdyn) were analyzed continuously by an on-line computer on a breath-by-breath basis (TS-100 series, Biocybernetics, Taipei, Taiwan).
Acute Ova inhalation challenge.
Ova aerosol (3%) was delivered directly into the lungs for 5 min; aerosol was generated by a ultrasonic nebulizer (Lumiscope 6610, Lumiscope, East Brunswick, NJ) that was connected between the outlet of the respirator and the tracheal cannula to prevent contamination of the breathing circuit by the residual Ova. The mass mean diameter of aerosol under our experimental setting was estimated to be 3–4 μm. To shorten the protocol to maintain the signal stability of single-fiber recording, acute inhalation Ova challenge was administrated for a shorter duration (5 min) at a higher concentration of Ova solution (3%).
Methacholine dose-response curve.
To verify airway hyperresponsiveness, responses of Rl and Cdyn to inhalation challenge with increasing concentrations of methacholine (MCh) aerosol (0.1, 0.3, 1, 3 mg/ml solutions) were determined in each animal. MCh aerosol was generated and delivered in the same manner as described above for 1 min. There was a 15-min interval between two consecutive MCh aerosol challenges. The lungs were hyperinflated (3 × Vt) at 2 min before each MCh aerosol challenge.
Experimental protocol.
Three series of experiments were carried out. Study series 1 was carried out to determine the presence and severity of airway inflammation by differential leukocyte counts of the BALF on day 21 at 4–6 h after the last inhalation exposure to Ova aerosol. Study series 2 was designed to investigate whether the bronchomotor responses to inhaled MCh aerosol and acute Ova inhalation challenge were altered by Ova sensitization in anesthetized rats. On day 24, the dose-response curve to MCh aerosol was obtained in each of the animals. Subsequently, responses of Rl and Cdyn were measured continuously in each animal before and for 30 min immediately after acute Ova inhalation challenge. Study series 3 was carried out to determine whether the excitability of pulmonary C fibers was altered by chronic Ova sensitization and to determine whether the changes were further augmented by acute Ova inhalation challenge. The baseline activities and the responses to lung inflation and chemical stimulation were determined in anesthetized, open-chest, and artificially ventilated rats before and within 45 after acute Ova challenge in both control and sensitized rats on day 24. The baseline FA was recorded first, followed by the response to lung inflation, and 5 min later the response to chemical stimulation. There was always a 10-min interval between two chemical stimulation trials. Baseline FA was averaged over 60 s in each fiber before and 5–15 min after acute Ova challenge (3%, 5 min). Constant-pressure lung inflation was applied by inflating the lung with a constant airflow (∼12 ml/s) until Ptr reached 15 or 30 cmH2O, and it was maintained at that pressure for 10 s after the respirator was turned off. Two chemical agents (capsaicin 0.25 and 0.5 μg/kg; adenosine 75 and 150 μg/kg) were selected as stimulants of pulmonary C fibers for the following reasons: capsaicin is a potent stimulant of pulmonary C fibers and a selective agonist of the transient receptor potential vanilloid type 1 receptor (TRPV1) (6); adenosine is a metabolite of ATP and another pulmonary C-fiber stimulant, but its action is mediated by activating adenosine A1 receptors (24). The volume of each bolus injection was 0.15 ml, which was first slowly injected into the catheter (dead space ∼0.2 ml) and then flushed into the right atrium by an injection of 0.4 ml saline before and within 45 min after the acute Ova challenge (3%, 5 min). Mean ABP (MABP) and heart rate (HR) were measured as 30-s averages before and after acute Ova challenge in both control and sensitized rats.
Materials.
Solution of Ova was prepared daily at the concentration described earlier. Imject Alum as adjuvant was purchased from Pierce Biotechnology (Rockford, IL). Stock solution of capsaicin (250 μg/ml) was prepared in 1% Tween 80, 1% ethanol, and 98% saline and stored at −20°C. Stock solutions of adenosine (10 mg/ml) and MCh (250 mg/ml) were prepared in saline and stored at −20°C. Solutions of these chemical agents at desired concentrations for injection or aerosolization were then prepared daily by dilution with isotonic saline based on the animal's body weight. All chemical agents were purchased from Sigma Chemical (St. Louis, MO).
Statistical analysis.
Data were analyzed with a two-way repeated-measures ANOVA, unless mentioned otherwise. One factor was the treatment effect of Ova, and the other factor was the effect of lung inflation or chemical agents. When the ANOVA showed a significant interaction, pairwise comparisons were made with a post hoc analysis (Fisher's least significant difference). A P value <0.05 was considered significant. Data are reported as means ± SE.
RESULTS
A total of 52 rats (control, n = 26; sensitized, n = 26) were used in this study. The average body weight of control rats (300.8 ± 3.7 g) was significantly higher than sensitized rats (279.6 ± 4.4 g).
Study series 1: effect of chronic Ova sensitization on airway inflammatory cells infiltration.
The total number of leukocytes in the BALF collected from sensitized rats (n = 7) exceeded that of control ones (n = 7) by 73%. In particular, the percentage of eosinophils [change (Δ) = 1,633%; P < 0.01; paired t-test], neutrophils (Δ = 1,159%; P < 0.01), and lymphocytes (Δ = 183%; P < 0.05) in the BALF of sensitized rats was distinctly higher than that in control rats (Table 1).
Table 1.
Effect of Ova sensitization on leukocyte counts in BALF collected at 4–6 h after the acute Ova inhalation challenge
| Group | Total Cells, ×105 | Basophils, % | Eosinophils, % | Lymphocytes, % | Monocytes, % | Neutrophils, % |
|---|---|---|---|---|---|---|
| Control (n = 7) | 12.32±2.89 | 0.00±0.00 | 0.26±0.07 | 4.63±1.03 | 92.97±1.06 | 2.20±0.71 |
| Sensitized (n = 7) | 21.46±3.95 | 0.00±0.00 | 4.46±0.41† | 13.14±2.54* | 54.71±8.13* | 27.71±8.68† |
Values are means ± SE; n, no. of animals. Ova, ovalbumin; BALF, bronchoalveolar lavage fluid.
P < 0.05 and
P < 0.01, significant difference between control and sensitized rats (paired t-test).
Study series 2: effect of chronic Ova sensitization on bronchial reactivity.
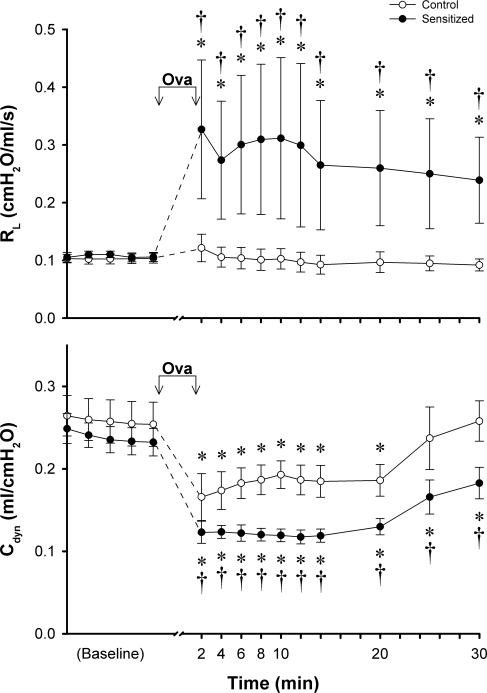
There was no significant difference in the average baseline Rl or Cdyn between control and sensitized rats, but the bronchomotor response to acute Ova aerosol challenge was significantly higher in sensitized rats (Fig. 1). The same Ova inhalation challenge evoked an intense increase of the Rl, which sustained and remained above the baseline for 30 min after challenge in sensitized rats; in contrast, no detectable change in Rl was found in control rats. The decrease in Cdyn after the Ova inhalation challenge was also significantly greater in sensitized rats (Fig. 1). Even in control rats, Cdyn decrease significantly below the baseline after acute Ova challenge, which was presumably caused by the aerosol (liquid) deposition in the lung because the decrease in Cdyn could be readily reversed by hyperinflation of the lung (data not shown).
Fig. 1.
Responses of total pulmonary resistance (Rl) and dynamic lung compliance (Cdyn) to acute inhalation of ovalbumin (Ova) aerosol (3%, 5 min). ○, Responses in control animals; •, responses in sensitized animals. Ova aerosol (3%; 5 min) was delivered into the lung by respirator between the 2 arrows. The peak responses were averaged over 20 consecutive breaths at every 2 min after acute inhalation of Ova in each animal. Values are means ± SE of 5 rats in each group. *P < 0.05, significant difference comparing between the baseline and the response to Ova at different time points in either control or sensitized group. †P < 0.05, significant difference comparing corresponding data between control and sensitized groups.
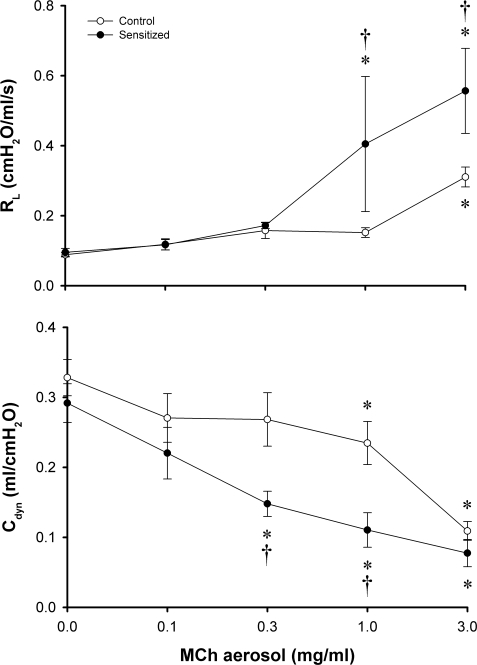
Inhalation of MCh aerosol induced an increase in Rl and a decrease in Cdyn in a dose-dependent manner in both control and sensitized rats (Fig. 2). The bronchomotor responses to MCh aerosol were significantly higher in sensitized rats at the higher concentrations of MCh (1.0 and 3.0 mg/ml; Fig. 2), verifying the presence of nonspecific AHR in the sensitized group.
Fig. 2.
Dose responses of Rl and Cdyn to aerosolized methacholine (MCh) in control and sensitized rats. MCh aerosol was delivered by respirator for 1 min. Peak response to each dose of MCh aerosol in each animal was averaged over 10 consecutive breaths that occurred within 30 s after the MCh aerosol exposure (1 min). Baseline data (zero concentration of MCh) represent responses to vehicle (isotonic saline). ○, Responses in control animals; •, responses in sensitized animals. Values are means ± SE of 5 rats in each group. *P < 0.05, significant difference comparing the baseline and the response to MCh in either control or sensitized group. †P < 0.05, significant difference comparing corresponding data between control and sensitized groups.
Study series 3: effect of Ova sensitization on the excitability of pulmonary C fibers.
A total of 35 pulmonary C fibers were studied in 28 rats (control rats: 311.9 ± 5.7 g, n = 14; sensitized rats: 294.4 ± 4.7 g, n = 14). The distribution of these receptors was as follows: 2 in upper lobe; 7 in middle lobe; 12 in lower lobe, and 6 in accessory lobe; all were located in the right lung. The locations of remaining eight pulmonary C fibers were not identified, but all of them were activated by lung inflation and a bolus injection of capsaicin with a latency of <1 s; these receptors were therefore considered as pulmonary C fibers (23).
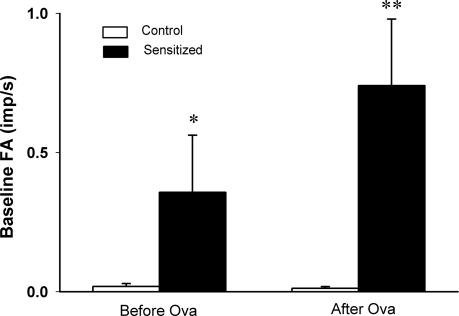
Pulmonary C fibers showed either no or very low and irregular baseline FA in both control and sensitized rats before acute Ova challenge. However, baseline FA in sensitized rats (0.36 ± 0.21 imp/s; n = 11) was significantly higher than that in control rats (0.02 ± 0.01 imp/s; n = 12; P < 0.05; Fig. 3). After the acute Ova challenge, FA in sensitized rats was further elevated (0.74 ± 0.24 imp/s) and significantly higher than that in control rats (0.01 ± 0.01 imp/s; P < 0.01; Fig. 3). There was no significant difference in FA, compared between before and after acute Ova challenge in either control or sensitized rats, despite that the average baseline FA in sensitized rats was almost doubled after acute Ova challenge (Fig. 3). There was no significant difference in either baseline MABP or HR between control and sensitized rats (Table 2).
Fig. 3.
Average baseline fiber activities (FA) before and after acute Ova challenge in anesthetized, open-chest, and artificially ventilated rats. Baseline FA was averaged over 60 s in each fiber before and 5–15 min after acute Ova inhalation challenge (3%, 5 min). Values are means ± SE. Open bars, control rats (n = 12); filled bars, sensitized rats (n = 11). imp/s, Impulses per second. *P < 0.05 and **P < 0.01, significantly different between corresponding data in control and sensitized rats.
Table 2.
Effects of acute Ova inhalation challenge on average baseline MABP and HR in both control and sensitized rats
| MABP, mmHg |
HR, beats/min | |||
|---|---|---|---|---|
| Before Ova | After Ova | Before Ova | After Ova | |
| Control (n = 14) | 86.1±3.3 | 96.9±3.4 | 390.3±5.5 | 386.9±6.0 |
| Sensitized (n = 14) | 91.3±3.1 | 102.5±4.5 | 407.1±6.4 | 401.3±8.0 |
Values are means ± SE; n, no. of animals. Mean arterial blood pressure (MABP) and heart rate (HR) were measured before and within 45 min after acute Ova challenge (3%, 5 min).
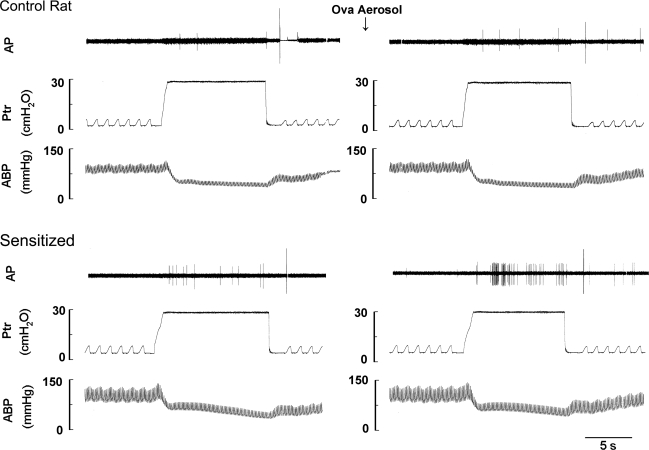
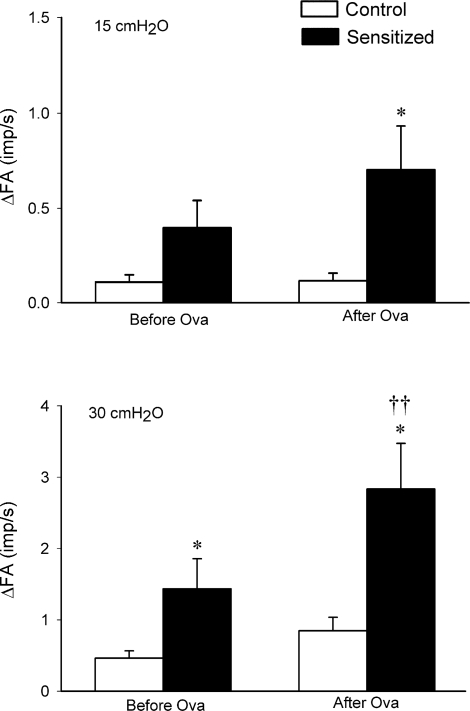
Pulmonary C-fiber response to lung inflation was also markedly greater in sensitized rats. The change in FA (ΔFA) in response to lung inflation was calculated as the difference between the average FA over the 10-s interval during lung inflation and the baseline FA (60-s average) in each fiber. Before acute Ova challenge, the response to lung inflation (Ptr = 30 cmH2O) in sensitized rats (ΔFA = 1.43 ± 0.42 imp/s) was significantly higher than that in control rats (ΔFA = 0.46 ± 0.1 imp/s; P < 0.05); after acute Ova challenge, the same lung inflation evoked a greater response in both groups, but the response (ΔFA) was greater in sensitized rats (2.83 ± 0.64 imp/s) than in control rats (0.85 ± 0.19 imp/s; P < 0.05). The FA response to lung inflation was markedly augmented by acute Ova challenge in sensitized rats (P < 0.01) but not in control rats (Figs. 4 and 5). The response to lower intensity of lung inflation (Ptr = 15 cmH2O) in sensitized rats was significantly higher than that in control rats after acute Ova challenge but not before the Ova challenge (Fig. 5).
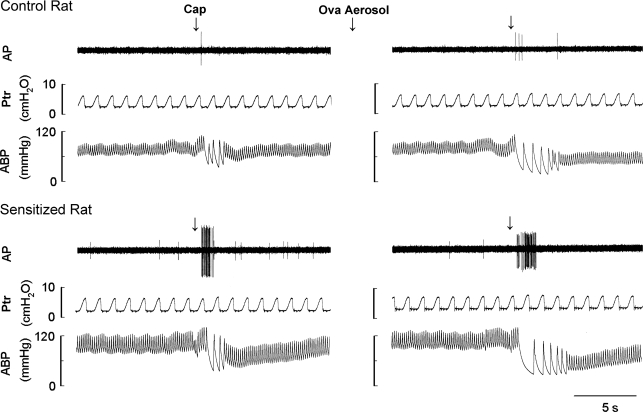
Fig. 4.
Experimental records illustrating the effects of acute Ova challenge on the pulmonary C-fiber responses to lung inflation in anesthetized, open-chest, and artificially ventilated rats. Left and right: responses to lung inflation [tracheal pressure (Ptr)= 30 cmH2O for 10 s] before and at ∼15 min after acute Ova challenge (3%, 5 min), respectively. AP, action potential; ABP, arterial blood pressure. Receptor location in the control rat (297 g): right lower lobe. Receptor location in the sensitized rat (298 g): right accessory lobe. The large spike at the end of lung inflation in each panel was electrical noise generated by turning on the respirator.
Fig. 5.
Effect of Ova sensitization on the average responses of pulmonary C fibers to lung inflation in anesthetized, open-chest, and artificially ventilated rats. Top and bottom: average responses to lung inflation at Ptr of 15 and 30 cmH2O, respectively. Open bars, control rats (n = 13); filled bars, sensitized rats (n = 16 in left, and n = 15 in right). Responses were tested both before and 5–25 min after acute Ova challenge (3%, 5 min) in both control and sensitized rats. Values are means ± SE. ΔFA, difference between the average FA over the 10-s interval during lung inflation and the average baseline FA (over 60 s) in each fiber. *P < 0.05, significant difference in responses between control and sensitized rats. †P < 0.05 and ††P < 0.01, significant difference in responses between before and after acute Ova challenge in either control or sensitized rats.
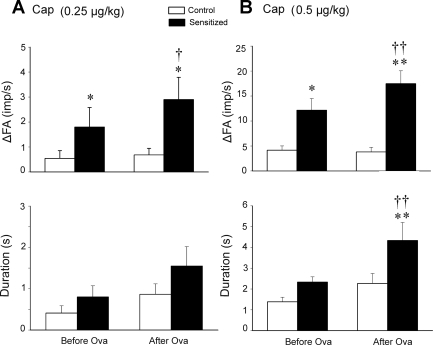
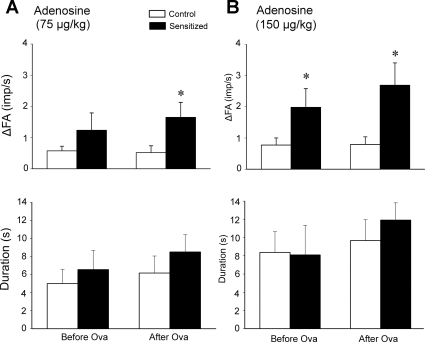
Responses of pulmonary C fibers to chemical stimulants (capsaicin and adenosine) were also clearly elevated in sensitized rats. The ΔFA in response to a stimulus was calculated as the difference between the peak FA and the baseline FA (60-s average) in each fiber. The peak response of FA was averaged over 2- and 10-s intervals after injections of capsaicin and adenosine, respectively, because the fiber discharges evoked by adenosine lasted substantially longer. Right atrial injection of capsaicin (0.5 μg/kg) consistently evoked an abrupt burst of C-fiber activity in both control and sensitized rats, accompanied by reflex bradycardia and hypotension, presumably mediated through the C-fiber afferents conducted in the left vagus nerve (e.g., Fig. 6). The responses of pulmonary C fibers to the high dose (0.5 μg/kg) of capsaicin (ΔFA = 12.2 ± 2.3 and 17.5 ± 2.6 imp/s, before and after acute Ova challenge, respectively) in sensitized rats were distinctly higher than those in control rats (ΔFA = 4.15 ± 0.86 imp/s and 3.81 ± 0.93 imp/s, before and after acute Ova challenge, respectively; P < 0.05 and P < 0.01) (Figs. 6, 7, and 8). In sensitized rats, the duration of discharge in response to capsaicin (0.5 μg/kg) was also significantly increased after acute Ova challenge (P < 0.01; Fig. 8B). Interestingly, the decrease in HR and MABP induced by capsaicin appeared to last a longer duration after acute Ova challenge in both control and sensitized rats (e.g., Figs. 6 and 7). The pulmonary C-fiber response to the lower dose of capsaicin (0.25 μg/kg) was also distinctly different between control and sensitized rats, both before and after acute Ova challenge. These results further showed that the FA responses to capsaicin were significantly augmented by acute Ova challenge in sensitized rats (P < 0.05; Fig. 8) but not in control rats. The differences in response to right atrial injection of adenosine between control and sensitized rats were similar, but less pronounced, compared with the difference in the response to capsaicin between the two groups. The response to the high dose of adenosine (150 μg/kg) in sensitized rats was higher than that in control rats both before and after acute Ova challenge, whereas the response to the low dose (75 μg/kg) was significantly higher in the sensitized rats only after acute Ova inhalation challenge (P < 0.05; Fig. 9). There was no significant difference in the duration of fiber discharge after the adenosine injection either between control and sensitized rats, or before and after acute challenge (Fig. 9).
Fig. 6.
Experimental records illustrating the effects of acute Ova inhalation challenge on the pulmonary C-fiber responses to capsaicin (Cap) in anesthetized, open-chest, and artificially ventilated rats. Left: responses to capsaicin (0.5 μg/kg in 0.15-ml volume) that was slowly injected into the catheter and then flushed (at arrow) into the right atrium as a bolus with saline (0.4 ml) before acute Ova challenge (3%, 5 min). Right: responses to the same dose of capsaicin at ∼30 min after acute Ova inhalation challenge. Receptor location in control rat (300 g): right lower lobe. Receptor location in sensitized rat (285 g): right lower lobe. For detailed explanations, see legend of Fig. 4.
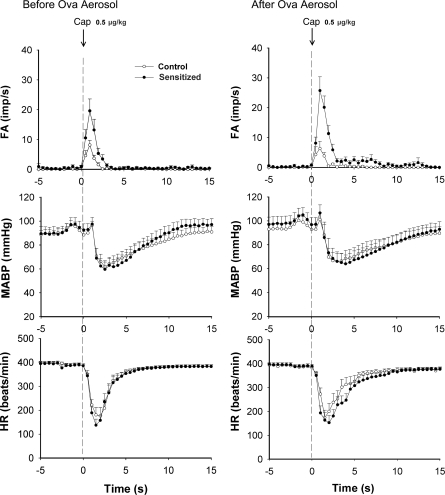
Fig. 7.
Effects of acute Ova inhalation challenge on pulmonary C-fiber activity and cardiovascular responses to capsaicin in anesthetized and open-chest rats. Left and right: FA and cardiovascular responses to right atrial injection of capsaicin (0.5 μg/kg) in both control (○, n = 13) and sensitized (•, n = 15) rats before and 15–30 min after acute Ova challenge (3%, 5 min), respectively. HR, heart rate; MABP, mean arterial blood pressure. Dashed vertical line, time of capsaicin injection.
Fig. 8.
Effect of Ova sensitization on the average peak response and the duration of discharge of pulmonary C fibers in response to right atrial bolus injection of capsaicin in anesthetized, open-chest, and artificially ventilated rats. A and B: average peak responses (top) and durations of discharge (bottom) of pulmonary C fibers in response to right atrial injections of capsaicin at 0.25 and 0.5 μg/kg, respectively. Open bars, control rats (n = 11 in A, n = 13 in B); filled bars, sensitized rats (n = 10 in A, n = 15 in B). Values are means ± SE. ΔFA, difference between the peak FA (average over 2-s interval) within 5 s after the injection and the baseline FA (average over 60-s interval) in each fiber. Duration of discharge was calculated as the time when FA exceeded the average baseline FA by 1 imp/s. Responses were tested before and 15–30 min after the acute Ova challenge (3%, 5 min) in both control and sensitized rats. *P < 0.05 and **P < 0.01, significant difference in the response or the duration of discharge between control and sensitized rats. †P < 0.05 and ††P < 0.01, significant difference in the response or the duration of the discharge between before and after acute Ova challenge.
Fig. 9.
Effect of Ova sensitization on the average peak response and the duration of discharge of pulmonary C fibers in response to right atrial bolus injection of adenosine in anesthetized, open-chest, and artificially ventilated rats. A and B: average peak responses (top) and durations of discharge (bottom) of pulmonary C fibers in response to right atrial injections of adenosine at 75 and 150 μg/kg, respectively. Values are means ± SE. Open bars, control rats (n = 12); filled bars, sensitized rats, n = 12. ΔFA, difference between the peak FA (average over 10-s interval) and the baseline FA (average over 60-s interval) in each fiber. Duration of discharge was calculated as the time when FA exceeded the average baseline FA by 0.5 imp/s. Responses were tested before and 25–45 min after acute Ova challenge (3%, 5 min) in both control and sensitized rats. *P < 0.05, significant difference in the response between control and sensitized rats.
DISCUSSION
Results of the present study showed that baseline activity of pulmonary C fibers was significantly elevated in the rats sensitized by chronic inhalation of aerosolized Ova, compared with that in the control rats. Chronic Ova exposure also produced a distinctly higher sensitivity of these afferents to chemical stimulants and lung inflation. The increase in fiber sensitivity to capsaicin was demonstrated by pronounced increases in both the peak activity and discharge duration in response to the same chemical challenge (Figs. 6–8). In addition, after an acute Ova inhalation challenge, the increase in baseline activity and the excitability were further augmented in sensitized rats. In sharp contrast, no significant change in the fiber activity or sensitivity was detected in control rats following the acute Ova inhalation challenge.
BN rats have been established as an animal model of allergic asthma by previous investigators (13, 21, 52). Many features induced by Ova sensitization are very similar to those observed in human allergic asthma, including production of an antigen-specific immunoglobulin (Ig) E antibody; upregulation of T helper 2 cytokines such as interleukin (IL)-4 and IL- 5; early- and late-phase airway reaction to the inhaled antigen; inflammatory cell (e.g., eosinophils, neutrophils) infiltration; and AHR to nonspecific bronchoactive challenge. Our study showed a significant and sustained increase of airway resistance to acute Ova challenge in sensitized rats, which demonstrated the AHR to the antigen challenge (Fig. 1). At the same time, differential cell counts of the BALF revealed inflammatory cell (eosinophils, neutrophils) infiltration in the airways of sensitized animals (Table 1). These results are therefore in agreement with those reported by previous investigators in the BALF of sensitized rats (13) and in the sputum of asthmatic patients during asthmatic attack (49). Furthermore, elevated bronchomotor response and sensitivity to MCh challenge indicated AHR in sensitized rats (Fig. 2), which are consistent with those reported by previous investigators using the same animal model (13, 15).
In Ova-sensitized animals, acute inhalation challenge with aerosolized Ova triggers early- and late-phase airway responses in the pattern and time course similar to those found in asthmatic patients after acute exposure to allergens (11, 12, 14). The early-phase response, which usually subsides within an hour, is exemplified by severe bronchoconstriction, mediated primarily by mast cell-derived mediators, including histamine and leukotrienes (LTs). Four to six hour later, a more sustained late-phase reaction, characterized by airway obstruction and inflammatory reaction, develops as a result of the action of various endogenous inflammatory mediators, cytokines and chemokines generated by the resident inflammatory cells (e.g., mast cells, macrophages, and epithelial cells) as well as the recruited inflammatory cells (lymphocytes, neutrophils, and eosinophils) (5).
Extensive evidence suggests a close link of the inflammatory mediators released from eosinophils and neutrophils to the airway hypersensitivity developed during allergic airway inflammatory reaction. The eosinophil is one of the primary effector cells in airway inflammation, and it produces a wide range of inflammatory mediators, including granule-derived cationic proteins such as eosinophil major basic protein (MBP), lipid mediators such as prostaglandins and LTC4, and cytokines (32). Eosinophils may also localize to airway nerves by recognizing neuronal adhesion molecules (34), which allows the mediators released from esoinophils to act directly on the airway nerves. Previous studies demonstrated that activated eosinophils acted directly on dorsal root ganglion neurons to stimulate their neuropeptides release and implicated MBP as a key mediator of this direct effect (17). Recent studies in our laboratory showed that intratracheal instillation of cationic proteins consistently induced intense stimulation and sensitization of pulmonary C fibers, and the cationic charge carried by these proteins is primarily responsible for generating the stimulatory and sensitizing effects on pulmonary C-fiber afferents (18, 20, 36).
Activated eosinophils can also synthesize and secret newly formed lipid mediators, and some of these mediators are known to exert potent sensitizing effects on pulmonary C fibers. For example, prostaglandin (PG) E2 has been reported to enhance the cough sensitivity to capsaicin in healthy human volunteers (7). Indeed, it has been clearly demonstrated that PGE2 can sensitize pulmonary C fibers in anesthetized rats (22), as well as isolated vagal pulmonary sensory neurons (35). Besides prostaglandins, LTC4, a potent bronchoconstrictor, can also stimulate the release of tachykinins from airway afferent nerve fibers in isolated trachea/bronchus of guinea pigs (39).
The neutrophil is one of the first inflammatory cells to be recruited into the airway after allergen exposure (48, 50). The neutrophil contains an array of products, including lipids, cytokines, proteases, microbicidal products, reactive oxygen species (ROS), and nitric oxide (47). Many of these endogenous substances are possible contributors to the AHR. For example, it has been reported that airway challenge of aerosolized H2O2, one of ROS known to be released in the airways from eosinophils, neutrophils, and epithelial cells (27), stimulated capsaicin-sensitive vagal pulmonary afferents in a dose-dependent manner, and that its sensory transduction was mediated through both TRPV1 and P2X purinergic receptors (45). In addition, several cytokines have also been suggested to be involved in the sensitization of nociceptors (46). For example, IL-1β, IL-6, and tumor necrosis factor-α can be produced by many cell types, including mast cells, eosinophils, neutrophils, and macrophages in the lung and airways. More recently, Yu et al. (53) demonstrated that IL-1β stimulated airway nociceptors in anesthetized rabbits.
After acute Ova inhalation challenge, the enhanced sensitivity of pulmonary C fibers was further augmented in sensitized rats. The increased sensitivity occurred coinciding with the period of the early-phase response reported previously in sensitized rats (11, 41, 54), and mast cell degranulation presumably played a major part of the reaction; on exposure to antigen, IgE binds to the high-affinity IgE receptor FcɛRI expressed on the mast cells, and triggers the release of mediators such as histamine, PGs LTs, and cytokines (4). Histamine, as one of primary autacoids secreted from mast cells, is known to have profound effects on a number of cells in the airways, including sensory nerves (4). A previous study in our laboratory has shown that inhalation of a low dose of aerosolized histamine augmented the afferent responses of vagal pulmonary C fibers to both lung inflation and right-atrial injection of capsaicin in anesthetized, open-chest dogs (37).
Tryptase is frequently considered as a marker of mast cell degranulation (43). Increased concentration of this protease has been found in BALF of asthmatic patients (1). A recent study by Gu and Lee (19) showed that intratracheal instillation of trypsin significantly amplified the capsaicin-induced pulmonary chemoreflex responses mediated by pulmonary C-fiber afferents. In isolated vagal pulmonary sensory neurons, pretreatment with trypsin potentiated the capsaicin-induced whole cell inward current (19), and this effect by trypsin is probably mediated though an activation of protease-activated receptor-2 and the protein kinase C-dependent transduction pathway. Bradykinin, a nonapeptide released from mast cells and found in the BALF of asthmatic patients (2), has been shown to stimulate bronchial C-fiber afferents in anesthetized dogs (33). Similarly, in an isolated preparation of guinea pig trachea and bronchus, bradykinin activated the C-fiber sensory terminals innervating these airways, particularly those originating from the jugular ganglia (31). Bradykinin is also known to increase the sensitivity of C fibers in isolated guinea pig airways and in Ova-sensitized guinea pigs (3, 16). In addition, 5-hydroxytryptamine, another autacoid derived from mast cells, stimulated vagal bronchopulmonary C fibers in guinea pig lungs (8). Together, these studies have provided strong evidence suggesting that these mediators derived from mast cell degranulation are probably responsible for the stimulation and sensitization of pulmonary C fibers after acute inhalation challenge with Ova in sensitized rats.
Our results illustrating the Ova-induced increase in excitability of these C-fiber endings to lung inflation have important physiological implications because these endings are relatively insensitive to changes in lung volume under normal conditions (23). Furthermore, expansion of the lung is a natural stimulus that occurs commonly in physiological conditions; for example, an increase in Vt can occur on taking a deep inspiration (sigh) at rest or during hyperventilation in heavy exercise. Activation of bronchopulmonary C fibers is known to play a part in evoking the sensation of airway irritation, cough, and reflex bronchoconstriction (9, 38). Therefore, the hypersensitivity of pulmonary C fibers to lung inflation may be involved, at least in part, in generating these symptoms and AHR found in patients with allergic airway inflammation.
Capsaicin is a potent stimulant of the pulmonary C fibers, and a selective activator of the TRPV1 receptor; the sensitivity to capsaicin is a characteristic feature of pulmonary C-fiber afferents in various animal species (9, 23, 38). Previous investigators have reported increases in the levels of airway acidity, airway temperature, and lipooxygenase metabolites of arachidonic acid in asthmatic patients (26, 44, 51), all of which are known to activate the TRPV1 (29). Other inflammatory mediators released in asthmatic airways (e.g., PGs, bradykinin, etc.) do not activate the TRPV1 directly, but they can lower its activation threshold (18, 28). It has been reported that patients with asthma are more sensitive to the tussive effect of TRPV1 agonists (10). In addition, selective TRPV1 antagonists can inhibit cough elicited by Ova inhalation challenge in sensitized guinea pigs (40). The present study further demonstrated a distinctly elevated sensitivity of pulmonary C fibers to capsaicin in Ova-sensitized rats (Figs. 7 and 8). Because capsaicin is a selective activator of TRPV1, these results seem to suggest a possible involvement of an increase in the response of TRPV1 channels, although direct evidence has not been established in this study. We speculate that the enhanced response may have resulted from an increase in the expression or excitability of TRPV1, or both, in the sensitized rats; the precise mechanisms were not determined in the present study. Furthermore, our results suggested that other ion channels (e.g., adenosine A1 receptors) were also involved in the Ova-induced sensitizing effect on pulmonary C fibers because there were significant increases in the C-fiber responses to adenosine and lung inflation in sensitized rats (24), and stimulation of C fibers by neither of these stimuli is known to involve TRPV1 activation.
It is well documented that stimulation of pulmonary C fibers elicits a number of reflex responses mediated through the cholinergic pathway, such as bronchoconstriction, airway hypersecretion, and bronchial vasodilation. In addition, certain neuropeptides, such as tachykinins and calcitonin gene-related peptide, released locally from these nerve endings can produce airway smooth muscle contraction, protein extravasation, and chemotactic effects on inflammatory cells (e.g., mast cells, leukocytes, etc) (30); sustained stimulation of these afferents may, therefore, lead to the development of neurogenic inflammation (30). Based on the results obtained in this study, it seems reasonable to suggest that an increase in the sensitivity of pulmonary C-fiber endings may play an important role in the development and manifestation of AHR induced by allergen sensitization.
In conclusion, the present study demonstrated that Ova sensitization induces a pronounced sensitizing effect on vagal pulmonary C-fiber afferents. Potent effects of certain chemical mediators released from inflammatory cells (eosinophils, neutrophils, and mast cells) are possibly involved in enhancing the sensitivity of pulmonary C fibers in sensitized rats. Further studies will be required to evaluate the relative contributions of specific types of inflammatory cells and endogenous mediators in the development of pulmonary C-fiber hypersensitivity, and to identify the signaling pathways possibly involved in the interaction between inflammatory cells and pulmonary C-fiber endings in allergen-sensitized airways.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant HL-67379.
Acknowledgments
The authors thank Robert F. Morton and Michael Dodd for their technical assistance in this study, and Dr. Qihai Gu for critical reading and comments on the manuscript.
Results of this study have been presented in an abstract in the Experimental Biology meeting at Washington, DC in April 2007.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barnes PJ The role of inflammation and anti-inflammatory medication in asthma. Respir Med 96, Suppl A: S9–S15, 2002. [PubMed] [Google Scholar]
- 2.Baumgarten CR, Lehmkuhl B, Henning R, Brunnee T, Dorow P, Schilling W, Kunkel G. Bradykinin and other inflammatory mediators in BAL-fluid from patients with active pulmonary inflammation. Agents Actions 38: 475–481, 1992. [PubMed] [Google Scholar]
- 3.Bergren DR Enhanced lung C-fiber responsiveness in sensitized adult guinea pigs exposed to chronic tobacco smoke. J Appl Physiol 91: 1645–1654, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bloemen K, Verstraelen S, Van Den Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunol Lett 113: 6–18, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 344: 350–362, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Choudry NB, Fuller RW, Pride NB. Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis 140: 137–141, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Chuaychoo B, Lee MG, Kollarik M, Undem BJ. Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm Pharmacol Ther 18: 269–276, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 55: 643–649, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du T, Xu LJ, Lei M, Wang NS, Eidelman DH, Ghezzo H, Martin JG. Morphometric changes during the early airway response to allergen challenge in the rat. Am Rev Respir Dis 146: 1037–1041, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Eidelman DH, Bellofiore S, Martin JG. Late airway responses to antigen challenge in sensitized inbred rats. Am Rev Respir Dis 137: 1033–1037, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Elwood W, Barnes PJ, Chung KF. Airway hyperresponsiveness is associated with inflammatory cell infiltration in allergic brown-Norway rats. Int Arch Allergy Immunol 99: 91–97, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Elwood W, Lotvall JO, Barnes PJ, Chung KF. Characterization of allergen-induced bronchial hyperresponsiveness and airway inflammation in actively sensitized brown-Norway rats. J Allergy Clin Immunol 88: 951–960, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Elwood W, Sakamoto T, Barnes PJ, Chung KF. Allergen-induced airway hyperresponsiveness in Brown-Norway rat: role of parasympathetic mechanisms. J Appl Physiol 75: 279–284, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nature Med 2: 814–817, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Garland A, Necheles J, White SR, Neeley SP, Leff AR, Carson SS, Alger LE, McAllister K, Solway J. Activated eosinophils elicit substance P release from cultured dorsal root ganglion neurons. Am J Physiol Lung Cell Mol Physiol 273: L1096–L1102, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 533: 207–214, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Gu Q, Lee LY. Hypersensitivity of pulmonary chemosensitive neurons induced by activation of protease-activated receptor-2 in rats. J Physiol 574: 867–876, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu QWM, Gleich GJ, Lee LY. Sensitization of isolated rat vagal pulmonary sensory neurons by eosinophil-derived cationic proteins. Am J Physiol Lung Cell Mol Physiol 294: L544–L552, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Haczku A, Macary P, Haddad EB, Huang TJ, Kemeny DM, Moqbel R, Chung KF. Expression of Th-2 cytokines interleukin-4 and -5 and of Th-1 cytokine interferon-gamma in ovalbumin-exposed sensitized Brown-Norway rats. Immunology 88: 247–251, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho CY, Gu Q, Hong JL, Lee LY. Prostaglandin E2 enhances chemical and mechanical sensitivities of pulmonary C fibers in the rat. Am J Respir Crit Care Med 162: 528–533, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Hong JL, Ho CY, Kwong K, Lee LY. Activation of pulmonary C fibres by adenosine in anaesthetized rats: role of adenosine A1 receptors. J Physiol 508: 109–118, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong JL, Kwong K, Lee LY. Stimulation of pulmonary C fibres by lactic acid in rats: contributions of H+ and lactate ions. J Physiol 500: 319–329, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 161: 694–699, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Jackson RM Pulmonary oxygen toxicity. Chest 88: 900–905, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta 1772: 915–927, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Jia Y, McLeod RL, Hey JA. TRPV1 receptor: a target for the treatment of pain, cough, airway disease and urinary incontinence. Drug News Perspect 18: 165–171, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Joos GF, Germonpre PR, Pauwels RA. Role of tachykinins in asthma. Allergy 55: 321–337, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Kajekar R, Proud D, Myers AC, Meeker SN, Undem BJ. Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. J Pharmacol Exp Ther 289: 682–687, 1999. [PubMed] [Google Scholar]
- 32.Kariyawasam HH, Robinson DS. The eosinophil: the cell and its weapons, the cytokines, its locations. Semin Respir Crit Care Med 27: 117–127, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman MP, Coleridge HM, Coleridge JC, Baker DG. Bradykinin stimulates afferent vagal C-fibers in intrapulmonary airways of dogs. J Appl Physiol 48: 511–517, 1980. [DOI] [PubMed] [Google Scholar]
- 34.Kingham PJ, Costello RW, McLean WG. Eosinophil and airway nerve interactions. Pulm Pharmacol Ther 16: 9–13, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Kwong K, Lee LY. PGE2 sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol 93: 1419–1428, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Lee LY, Gu Q. Mechanisms of bronchopulmonary C-fiber hypersensitivity induced by cationic proteins. Pulm Pharmacol Ther 16: 15–22, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Lee LY, Morton RF. Histamine enhances vagal pulmonary C-fiber responses to capsaicin and lung inflation. Respir Physiol 93: 83–96, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001. [DOI] [PubMed] [Google Scholar]
- 39.McAlexander MA, Myers AC, Undem BJ. Inhibition of 5-lipoxygenase diminishes neurally evoked tachykinergic contraction of guinea pig isolated airway. J Pharmacol Exp Ther 285: 602–607, 1998. [PubMed] [Google Scholar]
- 40.McLeod RL, Fernandez X, Correll CC, Phelps TP, Jia Y, Wang X, Hey JA. TRPV1 antagonists attenuate antigen-provoked cough in ovalbumin sensitized guinea pigs. Cough (London, England) 2: 10, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagase T, Moretto A, Dallaire MJ, Eidelman DH, Martin JG, Ludwig MS. Airway and tissue responses to antigen challenge in sensitized brown Norway rats. Am J Respir Crit Care Med 150: 218–226, 1994. [DOI] [PubMed] [Google Scholar]
- 42.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol 116: 477–486; quiz 487, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Payne V, Kam PC. Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia 59: 695–703, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Piacentini GL, Peroni D, Crestani E, Zardini F, Bodini A, Costella S, Boner AL. Exhaled air temperature in asthma: methods and relationship with markers of disease. Clin Exp Allergy 37: 415–419, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Ruan T, Lin YS, Lin KS, Kou YR. Sensory transduction of pulmonary reactive oxygen species by capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol 565: 563–578, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sachs D, Cunha FQ, Poole S, Ferreira SH. Tumour necrosis factor-alpha, interleukin-1beta and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain 96: 89–97, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Sampson AP The role of eosinophils and neutrophils in inflammation. Clin Exp Allergy 30, Suppl 1: 22–27, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Smith HR, Larsen GL, Cherniack RM, Wenzel SE, Voelkel NF, Westcott JY, Bethel RA. Inflammatory cells and eicosanoid mediators in subjects with late asthmatic responses and increases in airway responsiveness. J Allergy Clin Immunol 89: 1076–1084, 1992. [DOI] [PubMed] [Google Scholar]
- 49.Spahn JD Asthma biomarkers in sputum. Immunol Allergy Clin North Am 27: 607–622, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Teran LM, Carroll M, Frew AJ, Montefort S, Lau LC, Davies DE, Lindley I, Howarth PH, Church MK, Holgate ST. Neutrophil influx and interleukin-8 release after segmental allergen or saline challenge in asthmatics. Int Arch Allergy Immunol 107: 374–375, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol 25: 40–46, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Waserman S, Olivenstein R, Renzi P, Xu LJ, Martin JG. The relationship between late asthmatic responses and antigen-specific immunoglobulin. J Allergy Clin Immunol 90: 661–669, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol 156: 116–119, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Zhang XD, Fedan JS, Lewis DM, Siegel PD. Asthmalike biphasic airway responses in Brown Norway rats sensitized by dermal exposure to dry trimellitic anhydride powder. J Allergy Clin immunol 113: 320–326, 2004. [DOI] [PubMed] [Google Scholar]