Abstract
Presynaptic blockade of cutaneous vasoconstrictor nerves (VCN) abolishes the axon reflex (AR) during slow local heating (SLH) and reduces the vasodilator response. In a two-part study, forearm sites were instrumented with microdialysis fibers, local heaters, and laser-Doppler flow probes. Sites were locally heated from 33 to 40°C over 70 min. In part 1, we tested whether this effect of VCN acted via nitric oxide synthase (NOS). In five subjects, treatments were as follows: 1) untreated; 2) bretylium, preventing neurotransmitter release; 3) NG-nitro-l-arginine methyl ester (l-NAME) to inhibit NOS; and 4) combined bretylium + l-NAME. At treated sites, the AR was absent, and there was an attenuation of the ultimate vasodilation (P < 0.05), which was not different among those sites (P > 0.05). In part 2, we tested whether norepinephrine and/or neuropeptide Y is involved in the cutaneous vasodilator response to SLH. In seven subjects, treatments were as follows: 1) untreated; 2) propranolol and yohimbine to antagonize α- and β-receptors; 3) BIBP-3226 to antagonize Y1 receptors; and 4) combined propranolol + yohimbine + BIBP-3226. Treatment with propranolol + yohimbine or BIBP-3226 significantly increased the temperature at which AR occurred (n = 4) or abolished it (n = 3). The combination treatment consistently eliminated it. Importantly, ultimate vasodilation with SLH at the treated sites was significantly (P < 0.05) less than at the control. These data suggest that norepinephrine and neuropeptide Y are important in the initiation of the AR and for achieving a complete vasodilator response. Since VCN and NOS blockade in combination do not have an inhibition greater than either alone, these data suggest that VCN promote heat-induced vasodilation via a nitric oxide-dependent mechanism.
Keywords: bretylium, local control of blood flow, BIBP-3226, yohimbine, axon reflex
direct local heating or cooling of the skin can maximally vasodilate or maximally vasoconstrict the skin (17, 27). Barcroft and Edholm (3) were the first to examine thoroughly the effects of local heating on skin blood flow. Since then, there has been a significant number of factors implicated in the vasodilator response of the cutaneous circulation to direct local heating of the skin. Current thinking is that the initial rise in skin blood flow during local heating is elicited by an axon reflex, with a secondary rise and plateau heavily dependent on nitric oxide (NO) (20, 23). Houghton et al. (15) noted that NO synthase (NOS) inhibition eliminated the axon reflex response to slow local skin heating (+0.1°C/min from 33 to 40°C). They also made the provocative finding that presynaptic blockade of transmitter release from adrenergic vasoconstrictor nerves with bretylium tosylate (BT) also abolished the axon reflex and greatly attenuated the overall vasodilator response achieved with slow local heating. These findings imply that both NO and functional adrenergic vasoconstrictor nerves are required for a complete vasodilator response to slow local skin heating. The authors suggested that the neurotransmitter norepinephrine (NE) and possibly neuropeptide Y (NPY) were required for the activation of the axon reflex.
NPY is colocalized and released with NE from peripheral sympathetic nerves, including cutaneous vasoconstrictor nerves (33, 34). As such, it is plausible that NPY might be involved in modulating the vasodilator response to slow local heating, as BT blocks the release of NPY as well as NE (31). The possibility for a role for NPY is also raised by the inability of Houghton et al. (15) to restore the axon reflex vasodilation to slow local heating by infusing NE following presynaptic vasoconstrictor nerve blockade. Activation of endothelial α2- and Y1 receptors by NE and NPY, respectively, stimulates the release of NO (2, 4, 24, 36). This arrangement might explain the somewhat counterintuitive finding that functional vasoconstrictor nerves are required for a full expression of the vasodilator response to slow local heating in human skin (15). Previous work by Kellogg et al. (20) and Minson et al. (23) showed that NOS inhibition led to a significant reduction in the magnitude of the vasodilator response to rapid local heating. Thus it might be that these two systems, sympathetic vasoconstrictor nerves and NO, are linked with respect to the support of the vasodilator response to local heating of the skin.
In this study, we tested whether any such attenuation of the vasodilator response we observed under conditions of the blockade of transmitter release by vasoconstrictor nerves was due to antagonism of the synthesis of NO. We did this by comparing the cutaneous vascular responses to slow local heating of skin with either presynaptic adrenergic vasoconstrictor nerve blockade or NOS inhibition to the response achieved with the combination blockade of both vasoconstrictor nerves and NOS. We hypothesized that the attenuation of the vasodilator response to the slow local heating would not differ among the treatments, which would be the case if these systems were working in series. We also sought to determine whether the initiation of the axon reflex and the secondary rise in skin blood flow in response to slow local heating involved NE, NPY, or a combination of the pair. To this end, we performed postsynaptic blockade of α- and β-adrenergic receptors with yohimbine (YOH) and propranolol (PRO) and Y1-receptor blockade with BIBP-3226 (18, 31–33), and combination treatment with YOH, PRO, and BIBP-3226. We hypothesized that antagonism of either NE or NPY receptors would raise the temperature threshold for the initiation of the axon reflex during slow local skin heating and would reduce the overall level of vasodilation achieved. Furthermore, we hypothesized that the combination of the three would eliminate the axon reflex and cause a further reduction of the ultimate vasodilator response to local skin heating.
METHODS
Subjects.
All studies were approved by the local Institutional Review Board and conformed to the Declaration of Helsinki. All subjects were fully informed of the methods and risks before written consent was obtained. A total of nine subjects participated (7 men and 2 women; age range 20–30 yr, part 1, 4 men and 1 woman; part 2, 6 men, 3 of whom participated in part 1, and 1 woman). All were healthy nonsmokers, not taking medications, and all refrained from alcoholic and caffeinated beverages for at least 12 h before the study. The menstrual status of the female participants was recorded. However, their responses did not differ perceptively from those of the men, and their results were combined.
Instrumentation.
Subjects had microdialysis probes placed intradermally on the ventral aspect of the forearm, as previously described (5, 20). These probes consisted of 1 cm of microdialysis tubing (regenerated cellulose, inner diameter 200 μm, 18-kDa nominal molecular mass cutoff) attached at each end to polyimide tubing. Before implantation, the area of skin was temporarily anesthetized by the application of an ice pack for 5 min. A 25-gauge needle was introduced aseptically for ∼2.5 cm into the skin before exiting. The microdialysis probe and the connecting tubing were introduced into the skin via the lumen of the needle, which was then removed, leaving the probe in place. All probes were placed in this manner. To allow for the effects of the insertion trauma to subside, we waited 1.5 h before any protocols began (1, 9). The different probes were placed 3–5 cm apart. The subject was supine during this procedure.
Measurements.
All measurements were performed with the subjects resting in the supine posture. Skin blood flow was measured from the ventral aspect of the forearm by laser-Doppler flowmetry (MoorLAB, Moor Instruments, Axminster, UK), and expressed as laser-Doppler flow (LDF) (16, 25). LDF measures are exclusive to the skin and are not affected by underlying skeletal muscle blood flow (29). Local temperature control was achieved with custom-built Peltier cooling/heating metal probe holders (12, 13, 14, 18, 37). These controlled surface temperature over an area of 6.3 cm2, with the exception of a small aperture (0.28 cm2) in the center of the holder to enable measurement with the laser-Doppler probe. Laser-Doppler probes were placed precisely above the microdialysis membrane that had previously been inserted within the dermis. A thermocouple between the skin surface and the probe holder enabled local skin temperature assessment and feedback control. Local skin temperature can be precisely maintained within 0.05°C. Blood pressure was measured noninvasively and continuously by the Penaz method (26) from the middle finger of the experimental arm (Finapres, Ohmeda, Madison, WI). Mean arterial pressure was obtained from the electrical integration of the continuous blood pressure signal. Cutaneous vascular conductance (CVC), in arbitrary units, was calculated as the ratio of LDF to mean arterial pressure. Whole body skin temperature was recorded as the weighted mean from six thermocouples placed on the body surface and controlled by the use of a water-perfused suit (35). The suit covered the entire body surface, apart from the head, hands, feet, and the forearm used for the blood flow measurement. Whole body skin temperature was maintained at 34°C (thermoneutral). All variables were sampled by computer at 1-s intervals and stored as 20-s averages for offline analysis.
Blockade of transmitter release from adrenergic vasoconstrictor nerves was achieved by the iontophoresis of BT (Schweizerhall, South Plainfield, NJ) at a concentration of 10 mM at 250 μA for 10 min, to a 0.64-cm2 area of skin (19). Administration of BT causes a selective and localized blockade of transmitter release by the cutaneous adrenergic vasoconstrictor nerves lasting several hours (11, 19).
Agents delivered by microdialysis were filter sterilized with 0.2-μm micropore syringe filters (Acrodisc, Pall, Ann Arbor, MI). NOS activity was inhibited by the infusion of NG-nitro-l-arginine methyl ester (l-NAME) (Sigma Chemical, St Louis, MO) via microdialysis. A solution at a concentration of 20 mM of l-NAME in sterile saline was perfused at 4 μl/min. This level of l-NAME has been shown to produce a complete NOS inhibition after 50 min under resting conditions (14, 37).
Blockade of the α- and β-adrenergic receptors was achieved by the application of a combination of YOH and PRO to antagonize those receptors. The antagonists were applied continuously by microdialysis in a sterile saline solution of YOH (5 mM) and PRO (1 mM) at a rate of 4 μl/min. YOH is traditionally regarded as an α2-adrenergic antagonist; however, previously we found this combination and level of adrenergic antagonists to be effective in inhibiting the cutaneous vascular responses to exogenous NE (18, 31–33), suggesting that all α- and β-adrenergic receptors are blocked.
A 10 μM solution of the NPY Y1 antagonist N2-(diphenylacetyl)-N-([4-hydroxyphenyl]methyl)-d-arginine amide (BIBP-3226) (Sigma Chemical), in sterile saline was perfused at 4 μl/min (6, 10, 18, 28, 33). This concentration and administration have proven effective in revealing an NPY involvement in cutaneous vasoconstrictor responses (33).
To attain maximal skin blood flow levels, local skin temperature was increased to 42°C, and sodium nitroprusside (SNP; Sigma Chemical), dissolved in sterile saline at a concentration of 28 mM, was administered via microdialysis at a rate of 4 μl/min, which has previously been shown to elicit maximal skin blood flow (20).
Protocols.
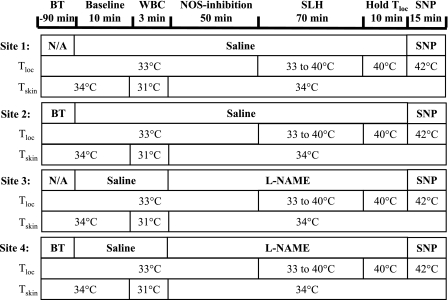
Part 1 was performed to test for an involvement of NOS in any role of vasoconstrictor nerves in the responses to slow local heating. Four sites on the ventral aspect of the forearm, complete with microdialysis fibers, LDF probes, and holders, as described above, were selected and treated as follows: site 1, untreated control; site 2, iontophoretically treated with BT; site 3, treated with l-NAME; and site 4, treated with both BT and l-NAME (Fig. 1). The protocol began with all sites being perfused with saline for 10 min (baseline measures). A 3-min phase of aggressive whole body cooling (∼3°C decrease in mean whole body skin temperature) was performed to confirm adequate cutaneous vasoconstrictor nerve blockade with BT (19). Following this, l-NAME was infused at sites 3 and 4 for 50 min to inhibit NO production by NOS (14, 37). The local skin temperature at all sites was then raised from 33 to 40°C over a 70-min period at a rate of 0.1°C/min and maintained at the final temperature for 10 min (see Fig. 1). Local heating was performed in this manner to match the protocol used by Houghton et al. (15). l-NAME continued to be infused during this time. All sites were then heated further to 42°C, and 28 mM of SNP were infused through the microdialysis fibers to all sites to obtain maximal CVC values.
Fig. 1.
Protocol for part 1. Four forearm skin sites were prepared with microdialysis fibers local cooling/heating probe holders and laser-Doppler probes. Sites 2 and 4 were previously treated with bretylium tosylate (BT). All sites were perfused with saline for 10 min for baseline measurements. At 10 min, a 3-min bout of whole body cooling (WBC) was performed to test the adequacy of the BT blockade. Sites 3 and 4 changed to perfusion with NG-nitro-l-arginine methyl ester (l-NAME). This combination yielded an untreated control (site 1), a site with vasoconstrictor nerve block (site 2), a site with nitric oxide (NO) synthase (NOS) inhibition (site 3), and a site with a combination of vasoconstrictor nerve blockade and NOS inhibition (site 4). l-NAME was infused for 50 min, and then local skin temperature (Tloc) at all four sites was raised from 33 to 40°C at 0.1°C/min and held at 40°C for 10 min. The perfusate was then changed to sodium nitroprusside (SNP), and Tloc was increased to 42°C to attain maximal vascular conductance levels. SLH, slow local heating; Tskin, skin temperature; N/A, not applicable.
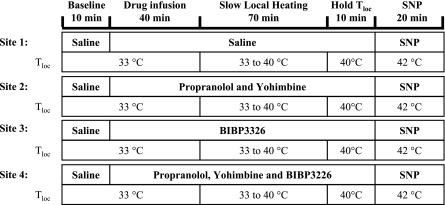
Part 2 was designed to test whether the axon reflex timing or the ultimate vasodilator response to slow local skin heating depended on NE and/or NPY. To this end, four sites were instrumented as in part 1 and treated as follows: site 1, untreated control (saline only); site 2, treated with PRO and YOH to antagonize α- and β-receptors; site 3, treated with BIBP-3226 to antagonize Y1 receptors; and site 4, treated with a combination of BIBP-3226, PRO, and YOH (Fig. 2). The protocol began with perfusion of all four microdialysis probes with sterile saline solution for 10 min at 4 μl/min (baseline measures). The perfusates for three probes were then changed: site 2 PRO and YOH, site 3 BIBP-3226, and site 4 PRO, YOH, and BIBP-3226, with sterile saline solution continuing at the control site. As indicated in Fig. 2, this arrangement provided one site with all receptors functional, a second site with α- and β-adrenergic receptor antagonism, a third site with Y1-receptor antagonism, and fourth site with α-, β-, and Y1 receptors all antagonized. After 40 min, slow local heating (+0.1°C/min) commenced at all sites. The temperature at all sites was raised from 33 to 40°C over a 70-min period and maintained at the final temperature (40°C) for 10 min (see Fig. 2). All sites were then heated further to 42°C, and 28 mM of SNP were infused to all sites to obtain maximal CVC values.
Fig. 2.
Protocol for part 2. Four forearm skin sites were prepared with microdialysis fibers, local cooling/heating probe holders, and laser-Doppler probes. All probes were perfused with saline for 10 min, and then sites 2, 3, and 4 were changed from saline to propranolol (PRO) and yohimbine (YOH) (site 2), BIBP-3226 (site 3), and PRO, YOH, and BIBP-3226 (site 4), respectively. After 40 min, Tloc at all four sites was raised from 33 to 40°C at 0.1°C/min. The temperature at all four sites was maintained at 40°C for 10 min. The perfusate was then changed to SNP, and Tloc was increased to 42°C to attain maximal vascular conductance levels.
Statistical analysis.
Data are presented as means ± SE and analyzed with statistical software (SPSS version 15 for Windows). We evaluated the response to slow local heating at the four sites for both the axon reflex and the ultimate vasodilation achieved at the end of the slow local heating protocol (10-min stable skin temperature of 40°C). The axon reflex response was characterized as a pronounced transient increase in red blood cell flux of at least 10–15% of maximal CVC (15) and thus easily distinguishable from movement or other artifacts. The temperature threshold for the axon reflex was defined as the local skin temperature at which the onset of the axon reflex occurred. The data for the net vasodilator response were expressed as a percentage of the maximal CVC achieved at that site, as produced from the infusion of 28 mM of SNP, combined with a local skin temperature of 42°C. Analysis was by paired statistics or, when appropriate, repeated-measures ANOVA. Statistical significance was assumed when P < 0.05.
RESULTS
Part 1.
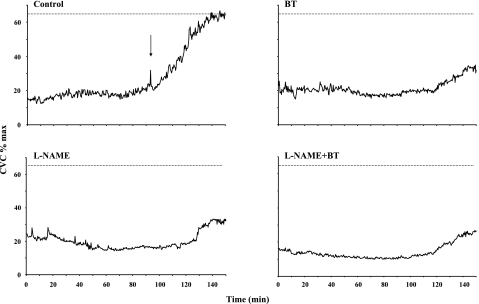
In part 1, we tested the effects of NOS blockade, vasoconstrictor nerve blockade, and their combination on the cutaneous vascular response to slow local heating. Figure 3 shows results for part 1 from a representative subject. No axon reflexes were observed in response to the slow local heating protocol at sites treated with BT, l-NAME, or BT + l-NAME, and the ultimate vasodilator response was reduced to the same extent at all treated sites.
Fig. 3.
Results for part 1 from a representative subject. Note that no axon reflexes were observed in response to the SLH protocol at sites treated with BT, l-NAME, or BT + l-NAME. Importantly, the ultimate vasodilator response was reduced to the same extent at all treated sites. Arrow indicates the presence of an axon reflex. CVC, cutaneous vascular conductance.
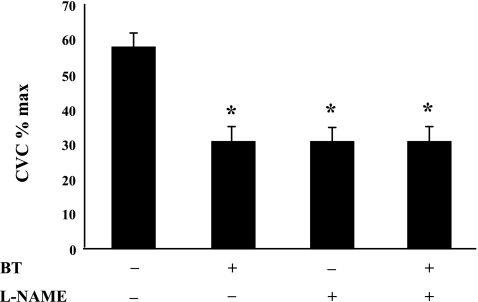
Figure 4 shows the average data for the final CVC as a percentage of maximal CVC reached during the slow local heating protocol in part 1. Each of the three treatments significantly reduced the level of vasodilation achieved, compared with that at the untreated control (P < 0.05). Importantly, the levels reached among the three treated sites did not differ statistically (P > 0.05).
Fig. 4.
The overall vasodilation in CVC in response to the SLH in part 1. Blockade of transmitter release from vasoconstrictor nerves with BT, inhibition of NOS with l-NAME, or a combination of the pair significantly decreases the vasodilator response to the SLH. The decreases in the response among the treated sites are not significantly different. The + and − indicate sites were or were not treated with antagonists, respectively. Values are means ± SE; n = 5 subjects. *P < 0.05 vs. untreated site.
Part 2.
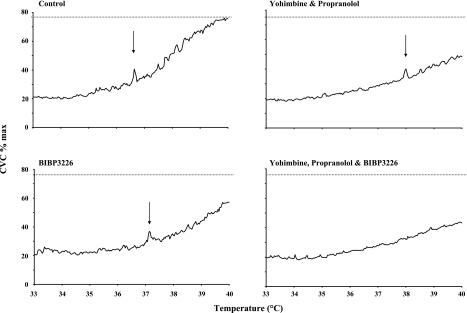
In part 2, we tested for roles for NE and NPY in the vasodilator response to slow local skin heating. Figure 5 shows data from part 2 for a representative subject. Axon reflexes were either offset to a higher local temperature, as in this case, or were abolished at the YOH/PRO and BIBP-3226 sites. They were uniformly abolished at sites treated with YOH, PRO, and BIBP-3226. Importantly, note the reduced responses in CVC at all treated sites.
Fig. 5.
Data from part 2 for a representative subject. Axon reflexes were either offset to a higher local temperature, as in this case, or were abolished at the YOH/PRO and BIBP-3226 sites. They were uniformly abolished at sites treated with the combination of YOH, PRO, and BIBP-3226. Importantly, note the reduced responses in CVC at all treated sites. Arrows indicate the presence of an axon reflex.
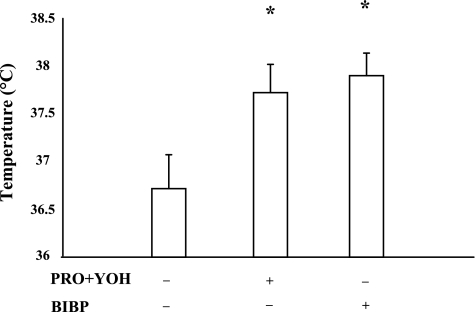
Figure 6 shows a summary of the temperature thresholds at which the axon reflex occurred. It is important to note that these data are from only four subjects, as the remaining three (of the total seven) did not display an axon reflex at either the PRO + YOH or BIBP-3226-treated sites; however, axon reflexes were present at the untreated control sites of all seven subjects. The untreated control site had the lowest threshold for the onset of the axon reflex, with the PRO + YOH and BIBP-3226-treated sites both being offset to elevated temperatures (P < 0.05 relative to the control site). In no subject did the site with a combined treatment of PRO, YOH, and BIBP-3226 exhibit an axon reflex in response to slow local heating. Hence, adrenergic and/or NPY receptor antagonism delayed or abolished this axon reflex response to local heating.
Fig. 6.
Temperature threshold for the initiation of the axon reflex. The α- and β-receptor or Y1-receptor antagonism with PRO and YOH or BIBP-3226, respectively, increases the temperature threshold of the axon reflex compared with the untreated control. These data are only from 4 of the 7 subjects in part 2, as the remaining 3 did not display an axon reflex at either the PRO and YOH or BIBP-3226-treated sites. No sites exhibited an axon reflex in the presence of combined adrenergic and neuropeptide Y (NPY) receptor blockade. The + and − indicate sites were or were not treated with antagonists, respectively. Values are means ± SE; n = 4 subjects. *P < 0.05 vs. untreated site.
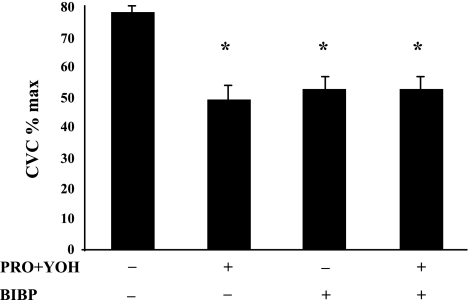
Figure 7 shows the final level of CVC as a percentage of maximal CVC for all four sites, averaged across all seven subjects, reached at the end of the slow local heating protocol in part 2. Final CVC levels from those sites with axon reflexes vs. those without were not different; therefore, the data were pooled. All treated sites achieved a significantly (P < 0.05) lower level of CVC than did the untreated control site. The final CVC values achieved among the treated sites were statistically not different (P > 0.05).
Fig. 7.
Overall vasodilation in response to the SLH in part 2. Antagonism of α- and β-receptors, Y1 receptors, or α-, β-, and Y1 receptors significantly decreases the overall level of vasodilation (expressed as CVC %max) achieved in response to SLH to 40°C in part 2. The decreases in the response among the treated sites were not significantly different. The + and − indicate sites were or were not treated with antagonists, respectively. Values are means ± SE; n = 7 subjects. *P < 0.05 vs. untreated control.
DISCUSSION
The major findings from this study are as follows. 1) presynaptic vasoconstrictor nerve blockade and/or NOS inhibition consistently eliminated the axon reflex and significantly reduced the overall vasodilator response to slow local heating and, when performed in combination, did not have an effect greater than that with either antagonist alone. This latter observation is consistent with the proposal that the mechanisms affecting vasodilation during local heating via vasoconstrictor nerves and by NOS have a series arrangement. 2) Combined antagonism of α- and β-adrenergic receptors or of NPY Y1 receptors significantly reduced the ultimate cutaneous vasodilation produced in response to slow local skin heating, suggesting that NE and NPY are both required for the full vasodilator response. 3) The combined antagonism of α-, β-, and Y1 receptors did not further reduce the response in CVC to slow local heating, suggesting that both systems need to be functional for a complete vasodilator response. 4) Combined antagonism of α- and β-adrenergic receptors or of Y1 receptors significantly increased the temperature threshold for the axon reflex (or abolished it), suggesting that NE and NPY both participate in the initiation of the axon reflex with slow local heating.
Houghton et al. (15) examined the contribution of sympathetic vasoconstrictor nerves and NO to the temperature threshold for the axon reflex vasodilation during slow local heating in human skin. The authors found that BT (presynaptic vasoconstrictor nerve blockade) or l-NAME (NOS inhibition) administration removed the axon reflex, implying that NOS is required and, somewhat counterintuitively, that functional adrenergic nerves are also necessary for the axon reflex vasodilator response to slow local heating. NE and NPY are both released from vasoconstrictor nerve terminals (33, 34). Houghton et al. (15) failed to restore the axon reflex and the ultimate vasodilator response to slow local heating with NE infusion under conditions of presynaptic sympathetic vasoconstrictor nerve blockade. Therefore, we sought to determine whether both transmitters were important in initiating the axon reflex and the subsequent vasodilation, which is what we found. Postsynaptic blockade of α- and β-adrenergic receptors and/or Y1 receptors either raised the temperature threshold for the initiation of the axon reflex, or abolished it. The overall level of vasodilation achieved was also reduced under these conditions, irrespective of the occurrence of an axon reflex.
Although it is somewhat counterintuitive that adrenergic nerve transmitters (NE and NPY are traditionally thought of as vasoconstrictors) should promote the vasodilator response to slow local heating, there are data that show NE and NPY bind to α2- and Y1 receptors on endothelial cells and stimulate endothelial NOS (eNOS), leading to the production of NO (2, 4, 36). In keeping with this possibility, we tested whether the effects of NE- and NPY-induced vasodilation were dependent on NOS function. We postulated that, if the adrenergic and the NOS systems were working in a series fashion, the increases in CVC in response to slow local heating among sites treated for presynaptic adrenergic blockade with BT, treated with NOS inhibition via l-NAME treatment, or treated with combined BT + l-NAME treatment would not be different. This is, indeed, what we found, suggesting that these systems work in a series fashion. If they were not working in series, it would be expected that NOS blockade, in combination with presynaptic vasoconstrictor nerve blockade, would cause a greater inhibition of heat-induced vasodilation than that from either antagonist alone. Additionally, as noted here and previously by Houghton et al. (15), inhibition of adrenergic transmitter release and/or NOS function abolished the axon reflex and significantly reduced the overall vasodilation. It should be noted that part 1 of this investigation is similar to the study performed by Houghton et al., with the exception of the combination of the BT + l-NAME treatment in the present study, and for the current investigation this was the key condition. As the results with this combination of treatments do not differ from those of the separate treatments, a series linkage is suggested as hypothesized.
A link between the delayed onset of the axon reflex and the attenuation of vasodilation was not fully seen in this investigation. For the seven subjects who participated in part 2, axon reflexes were observed in all cases at the untreated control site. However, only four displayed axon reflexes in response to the slow local skin heating protocol at the PRO + YOH (α- and β-receptor antagonism) and BIBP-3226 (Y1-receptor antagonism) treated sites; these were the same four subjects. The axon reflexes for those four subjects were offset to higher temperatures relative to control. Yet the remaining three subjects without observable axon reflexes had similar ultimate vasodilator responses compared with the four subjects with axon reflexes. Furthermore, those with the combination of antagonists (PRO + YOH + BIBP-3226), who, in all cases, did not have any axon reflexes, had similar levels of vasodilation as the sites with separate antagonism. We take this as good evidence that the axon reflex and the ultimate vasodilator response to slow local skin heating are not directly related, i.e., the absence or delay of the axon reflex does not cause a decrease in the overall vasodilator response to local heating. This contention is reinforced by the work of Houghton et al. (15) and Minson et al. (23). Houghton et al. (15) showed a greatly attenuated CVC response to slow local heating with NOS inhibition, regardless of the presence or absence of the axon reflex. Minson et al. (23) showed that treatment with local anesthesia reduced the magnitude of the axon reflex, but did not affect the ultimate vasodilator response to local skin warming.
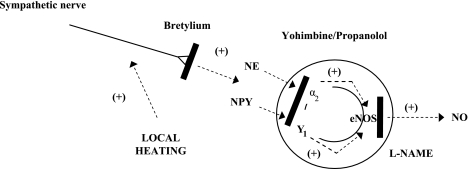
We observed that, in response to slow local skin heating, the effects of postsynaptic adrenergic receptor blockade and Y1-receptor blockade are indistinguishable from each other and from that of combination blockade; we find this outcome perplexing. One idea we have considered, however, is that, if NE and NPY are acting via endothelial α2- and Y1 receptors to stimulate eNOS, then perhaps there is a threshold of stimulation required to increase eNOS activity to produce NO, as outlined in Fig. 8. Thus blocking either adrenergic receptors or Y1 receptors removes a sufficient degree of stimulation of eNOS during slow local heating so that there are no increases in NO production. This could explain why combined blockade of adrenergic and Y1 receptors does not cause a further decrease in the level of vasodilation achieved in response to the slow local heating. Findings by Houghton et al. (15) support this speculation. Moreover, recent work by Kellogg et al. (21) shows that the cutaneous vascular responses to local heating is dependent only on eNOS, and that there is little, if any, involvement of neuronal NOS. Under conditions of presynaptic adrenergic blockade, infusion of exogenous NE did not restore the vasodilator response to local heating (15). This could be because NE alone does not provide a sufficient stimulus to increase eNOS activity during slow local heating. However, addition of NE did sensitize adrenergically intact skin to local warming (15). Exogenous NE, when delivered by iontophoresis, is known to cause an axon reflex cutaneous vasodilation in immediately adjacent skin (7). It has been suggested that NE has this vasodilator effect by sensitizing heat-sensitive nociceptors (7). Indeed, local anesthesia blocks axon reflex-mediated vasodilation from the iontophoretic application of NE. If this applies to local warming, as well, it would suggest the activity of NE is via its action on afferents, causing the release of calcitonin gene-related peptide and/or substance P, which then act to increase NOS activity (8). Moreover, it is also possible that the roles for NE and NPY in the cutaneous vascular responses to local heating are in a series arrangement. This possibility arises from the observation that one action of NPY is to enhance NE release (22, 30). In this scenario, either BIBP-3226 or YOH/PRO would reduce the contribution of NE to the response to local heating.
Fig. 8.
Proposed model of the factors contributing to the vasoconstrictor nerve involvement in the cutaneous vascular responses to SLH. From the data in this investigation, we suggest that local heating stimulates sympathetic vasoconstrictor nerves, which causes an increase in the release of the transmitters norepinephrine (NE) and NPY. These bind to endothelial α2- and Y1 receptors, respectively, that stimulate endothelial NOS (eNOS) and generate NO. This model provides one possible explanation as to how vasoconstrictor nerves are involved in the cutaneous vasodilation associated with SLH in human skin. Not shown are factors contributing to the response to local heating that are not associated with vasoconstrictor nerve activity. +, Excitatory; solid bars, sites and methods of blockade used in this study.
Experimental considerations.
The perplexing findings regarding the similar degree of involvement of NPY and NE in part 2 might be due to insufficient blockade of Y1 receptors. The effectiveness of the blockade at this concentration of BIBP-3226 has never been directly tested due to the difficulties of infusing NPY into human skin via microdialysis. Stephens et al. (33), however, showed that, with reflex whole body cooling, this concentration of BIBP-3226 delivered at this rate of infusion via microdialysis (i.e., exactly the same protocol) was, in conjunction with adrenergic blockade with PRO and YOH, effective in abolishing the reflex cutaneous vascular response to whole body cooling. Although this is not direct evidence for a complete blockade, we take it as strong evidence of a highly effective blockade of the Y1 receptors.
In summary, we found that the combined antagonism of α- and β-adrenergic receptors or of Y1 receptors either raises the temperature threshold for the initiation of the axon reflex, or removes it entirely, and, in either case, there is a reduced overall vasodilator response to slow local skin heating. These data suggest that NE and NPY are both important in the initiation of the cutaneous axon reflex and for achieving a complete vasodilator response. Furthermore, presynaptic blockade of adrenergic vasoconstrictor nerves or NOS inhibition abolished the axon reflex and greatly reduces the vasodilator response to slow local skin heating. Importantly, when performing vasoconstrictor nerve blockade and NOS blockade in combination, they do not have an effect greater than with either antagonist alone, suggesting that NE and NPY are working via NO-dependent mechanisms to elicit both the axon reflex and their contribution to the subsequent vasodilator response to slow local skin heating.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant RO1 HL-059166.
Acknowledgments
The authors thank the volunteers for their participation.
Present address of G. J. Hodges: The University of Western Ontario, London, Ontario, Canada N6A 5B8.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol 102: 807–811, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Angus JA, Cocks TM, Satoh K. The alpha adrenoceptors on endothelial cells. Fed Proc 45: 2355–2359, 1986. [PubMed] [Google Scholar]
- 3.Barcroft H, Edholm OG. The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol 102: 5–20, 1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocks TM, Angus JA. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature 305: 627–630, 1983. [DOI] [PubMed] [Google Scholar]
- 5.Crandall CG, Etzel RA, Johnson JM. Evidence of functional beta-adrenoceptors in the cutaneous vasculature. Am J Physiol Heart Circ Physiol 273: H1038–H1043, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Doods HN, Wieland HA, Engel W, Eberlein W, Willim KD, Entzeroth M, Wienen W, Rudolf K. BIBP 3226, the first selective neuropeptide Y1 receptor antagonist: a review of its pharmacological properties. Regul Pept 65: 71–77, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Drummond PD, Lipnicki DM. Noradrenaline provokes axon reflex hyperaemia in the skin of the human forearm. J Auton Nerv Syst 77: 39–44, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Drummond PD Repeated cycles of electrical stimulation decrease vasoconstriction and axon-reflex vasodilation to noradrenaline in the human forearm. Br J Clin Pharmacol 64: 421–427, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groth L, Serup J. Cutaneous microdialysis in man: effects of needle insertion trauma and anaesthesia on skin perfusion, erythema and skin thickness. Acta Derm Venereol 78: 5–9, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Grundemar L, Ekelund M. Effects of the neuropeptide Y (NPY)-receptor antagonist BIBP3226 on vascular NPY-receptors with different ligand requirements. Pharmacol Toxicol 79: 266–269, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Haeusler G, Haefely W, Huerlimann A. On the mechanism of the adrenergic nerve blocking action of bretylium. Naunyn Schmiedebergs Arch Pharmacol 265: 260–277, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Hodges GJ, Traeger JA 3rd, Tang T, Kosiba WA, Zhao K, Johnson JM. Role of sensory nerves in the cutaneous vasoconstrictor response to local cooling in humans. Am J Physiol Heart Circ Physiol 293: H784–H789, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Hodges GJ, Kosiba WA, Zhao K, Alvarez GE, Johnson JM. The role of baseline in the cutaneous vasoconstrictor responses during combined local and whole body cooling in humans. Am J Physiol Heart Circ Physiol 293: H3187–H3192, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol 574: 849–857, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JM The cutaneous circulation. In: Laser-Doppler Blood Flowmetry, edited by Shepherd AP and Öberg PA. New York: Springer, 1990, p. 121–139.
- 17.Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect 4, vol. I, chapt. 11, p. 215–243. [Google Scholar]
- 18.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol 288: H1573–H1579, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg DL, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol 257: H1599–H1606, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Kellogg DL, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg DL, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg JM Pharmacology of cotransmission in the autonomic nervous system: integrative aspects of amines, neuropeptides, adenosine triphosphate, amino acids, and nitric oxide. Pharmacol Rev 48: 113–178, 1996. [PubMed] [Google Scholar]
- 23.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson T, Lind H, Brunkvall J, Edvinsson L. Vasodilation in human subcutaneous arteries induced by neuropeptide Y is mediated by neuropeptide Y Y1 receptors and is nitric oxide dependent. Can J Physiol Pharmacol 78: 251–255, 2000. [PubMed] [Google Scholar]
- 25.Öberg PA Laser-Doppler flowmetry. Crit Rev Biomed Eng 18: 125–163, 1990. [PubMed] [Google Scholar]
- 26.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Rowell LB Cardiovascular aspects of human thermoregulation. Circ Res 52: 367–379, 1983. [DOI] [PubMed] [Google Scholar]
- 28.Rudolf K, Eberlein W, Engel W, Wieland HA, Willim KD, Entzeroth M, Wienen W, Beck-Sickinger AG, Doods HN. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur J Pharmacol 271: R11–R13, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Saumet JL, Kellogg DL Jr, Taylor WF, Johnson JM. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol 65: 478–481, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Songping H, Yang C, Chen X, Naes L, Cox BF, Westfall T. Direct evidence for the role of neuropeptide Y in sympathetic nerve stimulation-induced vasoconstriction. Am J Physiol Heart Circ Physiol 274: H290–H294, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol 280: H1496–H1504, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Stephens DP, Bennett LA, Aoki K, Kosiba WA, Charkoudian N, Johnson JM. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am J Physiol Heart Circ Physiol 282: H264–H272, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol 287: H1404–H1409, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Sundler F, Böttcher G, Ekblad E, Håkanson R. PP, PYY, and NPY: occurrence and distribution in the periphery. In: The Biology of Neuropeptide Y and Related Peptides, edited by Colmers WF and Wahlestedt C. Totowa, NJ: Humana, 199, p. 157–196.
- 35.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Vanhoutte PM, Miller VM. Alpha 2-adrenoceptors and endothelium-derived relaxing factor. Am J Med 87: 1S–5S, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki F, Sone R, Zhao K, Alvarez GE, Kosiba WA, Johnson JM. Rate dependency and role of nitric oxide in the vascular response to direct cooling in human skin. J Appl Physiol 100: 42–50, 2006. [DOI] [PubMed] [Google Scholar]