Abstract
A decrease in maximal exercise heart rate (HRmax) is a key contributor to reductions in aerobic exercise capacity with aging. However, the mechanisms involved are incompletely understood. We sought to gain insight into the respective roles of intrinsic heart rate (HRint) and chronotropic β-adrenergic responsiveness in the reductions in HRmax with aging in healthy adults. HRmax (Balke treadmill protocol to exhaustion), HRint (HR during acute ganglionic blockade with intravenous trimethaphan), and chronotropic β-adrenergic responsiveness (increase in HR with incremental intravenous infusion of isoproterenol during ganglionic blockade) were determined in 15 older (65 ± 5 yr) and 15 young (25 ± 4 yr) healthy men. In the older men, HRmax was lower (162 ± 9 vs. 191 ± 11 beats/min, P < 0.0001) and was associated with a lower HRint (58 ± 7 vs. 83 ± 9 beats/min, P < 0.0001) and chronotropic β-adrenergic responsiveness (0.094 ± 0.036 vs. 0.154 ± 0.045 ΔHR/[isoproterenol]: P < 0.0001). Both HRint (r = 0.87, P < 0.0001) and chronotropic β-adrenergic responsiveness (r = 0.61, P < 0.0001) were positively related to HRmax. Accounting for the effects of HRint and chronotropic β-adrenergic responsiveness reduced the age-related difference in HRmax by 83%, rendering it statistically nonsignificant (P = 0.2). Maximal oxygen consumption was lower in the older men (34.9 ± 8.1 vs. 48.6 ± 6.7 ml·kg−1·min−1, P < 0.0001) and was positively related to HRmax (r = 0.62, P < 0.0001), HRint (r = 0.51, P = 0.002), and chronotropic β-adrenergic responsiveness (r = 0.47, P = 0.005). Our findings indicate that, together, reductions in HRint and chronotropic responsiveness to β-adrenergic stimulation largely explain decreases in HRmax with aging, with the reduction in HRint playing by far the greatest role.
Keywords: exercise, tachycardia, ganglionic blockade
aerobic exercise capacity decreases progressively with age and is associated with reductions in physical functional capacity, increases in disability, and decreases in independence and quality of life (18, 30, 36a, 37). Thus the mechanisms underlying the decline in maximal exercise capacity with aging have important physiological implications for middle-aged and older adults.
The most consistently reported mechanism contributing to the decrease in aerobic exercise capacity with aging is a reduction in maximal heart rate (HRmax) (17, 26, 30). This progressive decline in HRmax with age is independent of sex, habitual physical activity, and other factors (26, 30). Thus it appears to be a nonmodifiable, inevitable consequence of aging. The decrease in HRmax with aging contributes to a reduction in maximal cardiac output (17, 26), the key determinant of aerobic exercise capacity (32). However, despite its importance, the mechanisms responsible for the decrease in HRmax with aging remain incompletely understood.
Jose and Collison (22) noted in 1970 that the rate of decline in HRmax with age appears to be similar to that reported for intrinsic heart rate (HRint), i.e., the HR observed in the absence of autonomic influences. On the basis of data obtained on small groups (n = 6) of young (23 yr) and middle-aged (45–55 yr) men who performed strenuous submaximal cycle ergometry under control conditions and autonomic (vagal and β-adrenergic) blockade, in a separate report, Jose and colleagues (23) also found that most of the age group difference in exercise heart rate could be explained by a lower intrinsic heart rate.
Differences in vagal (parasympathetic) activity do not appear to contribute to reductions in HRmax with aging. Vagal activity does not influence HRmax under normoxic conditions (31) because cardiac vagal tone is progressively decreased during incremental exercise, with little or none existing at maximal workloads (31, 33). Moreover, aging is associated with reductions in cardiac vagal tone at rest and during exercise in both men and women (8, 13), which, if anything, would act to reduce parasympathetic inhibition of pacemaker activity and increase exercise HRmax in older adults. That inhibition of vagal activity does not influence the HRmax evoked by pharmacological stimulation in young and older beagles is consistent with these observations (38).
It has been suggested that a reduction in β-adrenergic responsiveness to sympathoadrenal stimulation also may contribute to or even explain the decrease in HRmax with aging (12, 34). However, β-adrenergic responsiveness and HRmax have not been determined in the same groups of young and older subjects, and the results of previous studies aimed at determining the contribution of the β-adrenergic system are inconsistent (6, 12).
Accordingly, the purpose of the present study was to gain further insight into the roles of reductions in HRint and chronotropic β-adrenergic responsiveness in the age-associated reductions in HRmax in healthy adults. We hypothesized that both mechanisms would contribute and, together, would largely explain the reductions in HRmax with age. To test this hypothesis, we measured HRmax during true maximal treadmill exercise (performed to exhaustion) and HRint and chronotropic responsiveness to β-adrenergic stimulation at rest during complete ganglionic blockade in properly sized groups (n = 15) of healthy, unmedicated men differing widely in age (mean 25 vs. 65 yr). Finally, we measured maximal oxygen consumption so that we could determine the relation between this measure of maximal aerobic exercise capacity and maximal heart rate, HRint, and chronotropic responsiveness to β-adrenergic stimulation.
METHODS
Ethical Approval
The study conformed to the standards set by the Declaration of Helsinki. Procedures were approved by the Colorado Multiple Institutional Review Board and the University of Colorado at Boulder Human Research Committee and were in accordance with their respective regulations. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Subjects
A total of 30 men were studied: 15 older (65 ± 5 yr, range 57–76 yr) and 15 young (25 ± 4 yr, range 18–30 yr). Subjects were normotensive (blood pressure <140/90 mmHg) nonsmokers who were not taking any medications. All subjects were free of clinical disease as assessed by medical history, physical examination, resting ECG, urinalysis, blood chemistries, and hematological evaluation. Men older than 40 yr of age demonstrated normal ECG (i.e., no ST segment depression indicative of ischemia) and blood pressure responses to incremental treadmill exercise based on previously described guidelines (14). Subjects ranged from sedentary to regularly physically active, with the young and older subjects having a similar range of activity behavior.
Study Procedures
Weight, height, and body mass index.
Body weight was measured to the nearest 0.1 kg with a physician's balance scale (Detecto, Webb City, MO). Subjects were weighed barefoot wearing light clothing. Height was measured to the nearest millimeter using a stadiometer. Body mass index was determined as weight divided by height squared (kg/m2).
Maximal exercise responses.
Maximal oxygen consumption was used as a measure of aerobic exercise capacity and was determined using on-line computer-assisted open-circuit spirometry during incremental treadmill exercise as previously described (10). Briefly, after a 6- to 10-min warm-up period, each subject ran or walked at a comfortable speed that corresponded to 70–80% of age-predicted HRmax. The treadmill grade was increased 2.5% every 2 min until volitional exhaustion. To ensure that each subject attained maximal oxygen consumption, at least three of the following four criteria were met: 1) plateau in oxygen consumption with increasing exercise intensity; 2) a maximal respiratory exchange ratio (measure of hyperventilation and, therefore, volitional effort during maximal exercise) of at least 1.15; 3) achievement of age-predicted HRmax; and 4) a rating of perceived exertion of at least 18 on the Borg scale. Maximal exercise HR was determined as the peak HR attained during the exercise.
Autonomic-cardiovascular function.
All measurements were performed after subjects had abstained from caffeine and after a 12-h overnight fast. Subjects were studied during supine rest according to procedures previously described (19). HR was measured beat to beat via electrocardiogram, and the average HR over a 5-min period was used in the analyses. Arterial blood pressure was continuously monitored by a pressure transducer (radial or brachial artery catheter). Intrinsic HR was determined during ganglionic blockade achieved by blockade of NN-cholinergic receptors via continuous intravenous infusion of trimethaphan (on average 6–7 mg/min). Complete cardiac autonomic blockade was documented by an absence of change in HR (<5 beats/min) in response to the acute increase in blood pressure (≥15 mmHg) produced by bolus administration of phenylephrine (25, 50, and/or 100 μg). Chronotropic β-adrenergic responsiveness was determined during ganglionic blockade using incremental intravenous infusions of isoproterenol in 6-min steady-state doses (0.0025, 0.005, 0.01, 0.02 mg·kg−1·min−1) and was defined as the slope of the change in HR (beats/min) with increasing plasma concentration of isoproterenol (pg/ml). Plasma samples were collected during the final 15 s of each 6-min infusion dose and were analyzed for both isoproterenol and norepinephrine concentrations (27).
Data Analysis
Age-group comparisons were performed using t-tests for independent samples. To gain preliminary insight into the bivariate relations between HRmax, HRint, and chronotropic β-adrenergic responsiveness, Pearson product-moment correlation coefficients were examined. The contributions of HRint and chronotropic β-adrenergic responsiveness to the age-related reductions in HRmax were determined using analysis of covariance (ANCOVA). In this univariate general linear model, HRmax was entered as the dependent variable, whereas the categorical variable coding for age was entered as a fixed factor and HRint and β-adrenergic responsiveness as covariates. Analysis of the data using regression produced identical results to those reported here. Physical activity levels did not influence HRmax, HRint, or chronotropic β-adrenergic responsiveness. Statistical significance was set at P < 0.05. Statistical analyses were performed using SPSS (version 14.0). Data are presented as means ± SD unless otherwise specified.
RESULTS
Subject characteristics are presented in Table 1. There were no differences in body weight, body mass index, resting supine HR, or resting supine systolic or diastolic blood pressure between the groups (all P ≥ 0.14). Resting plasma norepinephrine was higher and maximal oxygen consumption was lower in the older men (P ≤ 0.001). The respiratory exchange ratio, an index of hyperventilation, was not different in the young and older men at the end of maximal exercise (1.18 ± 0.04 vs. 1.21 ± 0.05, respectively, P = 0.13), nor were there group differences in the peak ratings of perceived exertion (19.4 ± 0.9 vs. 18.9 ± 1.1 units, P = 0.14). These data indicate that there were no differences in voluntary effort during maximal exercise in the young and older men.
Table 1.
Subject characteristics
| Young | Older | P | |
|---|---|---|---|
| Age, yr | 25±4 | 65±5 | <0.0001 |
| Body weight, kg | 79.2±8.2 | 80.4±10.9 | 0.74 |
| Body mass index, kg/m2 | 25.1±2.8 | 25.8±3.0 | 0.55 |
| Resting supine HR, beats/min | 58±9 | 54±7 | 0.19 |
| Resting supine SBP, mmHg | 126±11 | 124±13 | 0.71 |
| Resting supine DBP, mmHg | 66±6 | 62±8 | 0.14 |
| V̇o2max, ml·kg−1·min−1 | 48.6±6.7 | 34.9±8.1 | <0.0001 |
| Plasma norepinephrine, pg/ml | 238.2±65.3 | 392.1±142.4 | 0.001 |
Data are means ± SD. HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; V̇o2max, maximal oxygen consumption.
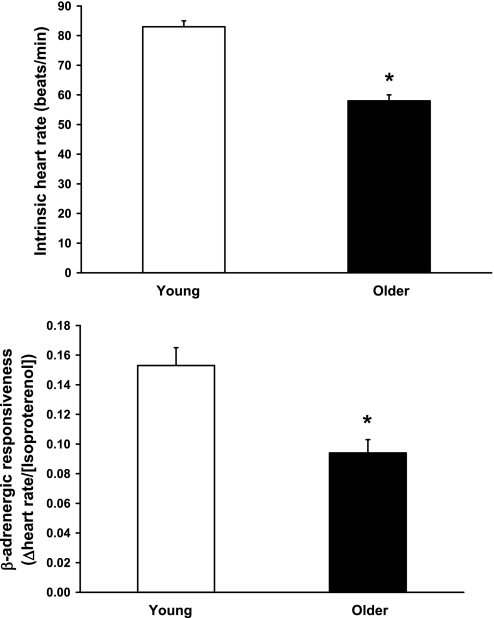
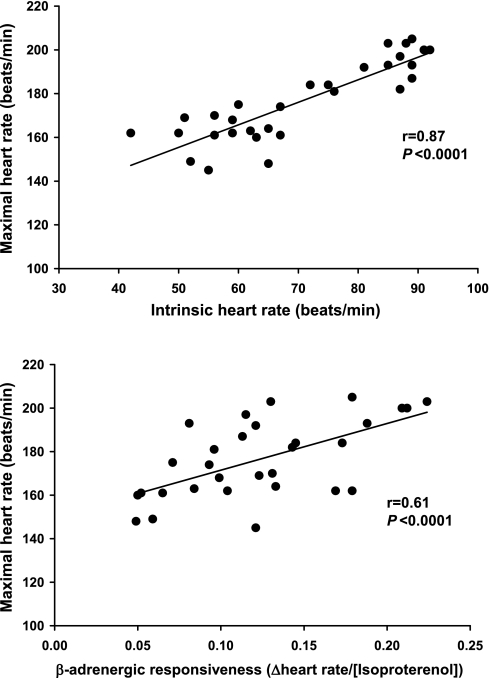
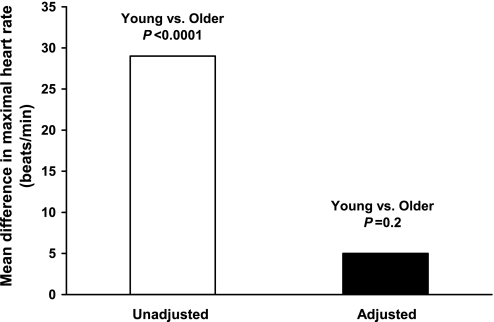
HRmax was 29 beats/min lower in the older men (162 ± 9 vs. 191 ± 11 beats/min, P < 0.0001). This was associated with a 25-beats/min lower HRint (58 ± 7 vs. 83 ± 9 beats/min, P < 0.0001) and a 39% lower chronotropic β-adrenergic responsiveness (0.094 ± 0.036 vs. 0.154 ± 0.045 ΔHR/[isoproterenol], P < 0.0001) (Fig. 1). In the entire group, HRmax was most strongly related to HRint (r = 0.87, P < 0.0001) but also was significantly related to chronotropic β-adrenergic responsiveness (r = 0.61, P < 0.0001) (Fig. 2). Group differences in HRmax remained significant (P = 0.04) after accounting for the effects of either factor alone. However, accounting for both HRint and chronotropic β-adrenergic responsiveness reduced the age-related difference in HRmax by 83%, which no longer was statistically significant (P = 0.2) (Fig. 3). In the overall group, age, HRint, and chronotropic responsiveness to β-adrenergic stimulation together accounted for ∼80% of the variance in HRmax.
Fig. 1.
Intrinsic heart rate (top) and chronotropic responsiveness to β-adrenergic stimulation (bottom) in young and older healthy men. Data are means ± SE. *P < 0.0001.
Fig. 2.
Relation of maximal exercise heart rate and intrinsic heart rate (top) and chronotropic responsiveness to β-adrenergic stimulation (bottom) in the overall group.
Fig. 3.
Mean difference in maximal exercise heart rate in the young and older men before and after adjusting for intrinsic heart rate and chronotropic responsiveness to β-adrenergic stimulation.
In the entire group, maximal oxygen consumption was related to HRmax (r = 0.62, P < 0.0001), HRint (r = 0.51, P = 0.002), and chronotropic β-adrenergic responsiveness (r = 0.47, P = 0.005).
DISCUSSION
The key findings of the present study are as follows. First, the lower mean HRmax in older compared with young healthy men is associated with lower mean levels of HRint and chronotropic responsiveness to β-adrenergic stimulation. Second, among individual subjects, HRint and chronotropic responsiveness to β-adrenergic stimulation are positively related to HRmax, with HRint showing a particularly strong correlation. Third, accounting for the combined, but not the individual, effects of HRint and chronotropic responsiveness to β-adrenergic stimulation abolishes the significant difference in HRmax between young and older healthy men. Fourth, maximal oxygen consumption was related to both HRint and chronotropic responsiveness to β-adrenergic stimulation.
Taken together, these findings support the hypothesis that reductions in HRint and chronotropic β-adrenergic responsiveness largely explain the reduction in HRmax with aging in healthy men, with reductions in HRint being the greatest contributor. Because the age-related decline in maximal attainable HR during exercise is the most consistent physiological determinant of the age-associated reduction in maximal oxygen consumption, our results provide evidence that decreases in HRint and chronotropic β-adrenergic responsiveness may be key mechanisms contributing to reductions in aerobic exercise capacity with aging.
Intrinsic HR
Jose and Collison (22) first reported that HRint decreases with age. They studied healthy men and women 16–70 yr of age using concurrent muscarinic and β-adrenergic receptor blockade with atropine and propranolol, respectively. More recently these findings were confirmed and extended to an older group (60–83 yr of age) of healthy men and women using the same pharmacological approach (7). The results of the present study obtained using complete inhibition of ganglionic transmission with trimethaphan are consistent with these earlier observations, as well as with previous findings using ganglionic blockade (35).
In their comments, Jose and Collison (22) noted that the rate of decline in HRint appeared to be similar to that for HRmax. In a separate paper, Jose and colleagues (23) extended this observation in two ways. First, they presented a figure that illustrated the slope of the age-related decline in HRint (taken from their observations) as a function of HRmax using previously published data in the literature. They also reported that the age-related differences in HRint during rest (15 beats/min) and during strenuous submaximal cycle ergometry exercise (13 beats/min) were similar in small groups of healthy young and middle-aged men.
The present findings are the first to our knowledge to measure both HRint and HRmax in groups of young and older subjects. Consistent with the findings of Jose and colleagues (23), we found that mean HRint was markedly lower in the older men, almost to the same degree as the decrease in HRmax. Moreover, there was a strong positive relation between intrinsic and maximal exercise HR, with the former accounting for ∼76% of the variance in the latter. Indeed, as observed previously (23), the difference between HRmax and HRint was similar in our two groups (older, 162 − 58 = 104 beats/min vs. young, 191 − 83 = 108 beats/min), suggesting that HR could increase to a similar extent above the HRint in response to maximal exercise. Thus, under conditions of maximal exercise in men separated in age by an average of 40 yr, our findings extend and confirm the observations of Jose et al. (23) demonstrating that the age-associated reduction in HRint is the primary mechanism mediating reductions in HRmax with aging in healthy adults.
Decreases in HRint with aging in healthy adults are thought to reflect the development of a broader subclinical sinoatrial node dysfunction (16, 24). The mechanisms mediating reduced HRint with aging are not fully understood. Increased collagen deposition (fibrosis) of the atrial tissue could contribute to reductions in HRint (16). However, sinoatrial node dysfunction can be observed in the absence of marked structural remodeling (2, 20, 24), implicating changes in the properties of pacemaker cells in the sinoatrial node. Recent experimental studies of the molecular changes involved have identified at least two mechanisms that could contribute to deceases in HRint with healthy aging: reductions in Cav1.2 channel protein, resulting in decreases in sinus node depolarization reserve with consequent suppression of action potential formation and propagation (20); and reductions in connexin43 protein, the primary protein in gap junctions, which would restrict the conduction of action potentials from the sinoatrial node to other regions of the heart (21).
We should point out that our study in men and that of White and Leenen (35) in men and women using ganglionic blockade with trimethaphan found lower absolute levels of HRint in both the young and older subjects compared with studies using coadministration of atropine and propranolol (22, 23). The reason for these differences is not clear. The completeness of the blockade in ganglionic transmission was established in both the present study and in White and Leenen (35), so residual vagal modulation of HR in those investigations is an unlikely explanation. In dogs, atropine can cause “excess tachycardia” beyond that resulting from the elimination of cardiovagal inhibition (4, 5, 28, 29). This nonsympathetic, vagally mediated effect may be the result of a positive chronotropic action on ionic pacemaker cells (4, 28, 29). Thus this potential positive chronotropic influence of atropine may lead to overestimation of HRint and contribute to, if not completely explain, the absolute differences reported in studies using the two methods. In any case, such differences do not alter the fundamental conclusions of this or the previous studies regarding aging and HRint.
Chronotropic Responsiveness to β-Adrenergic Stimulation
Infusion of vasoactive compounds that alter systemic arterial blood pressure results in reflex adjustments in cardiac autonomic activity that modulate HR. Therefore, to accurately assess cardiac β-adrenergic responsiveness in the absence of the counterregulatory baroreflex influences in humans, β-adrenergic receptor agonists must be administered either 1) during ganglionic blockade (19, 35), which interrupts autonomic nervous system activity; or 2) in the presence of muscarinic and β-adrenergic receptor antagonists, which block autonomic signaling at synapses in the heart (13). Reduced cardiac responsiveness to isoproterenol administered during ganglionic blockade has been reported previously in older compared with young healthy men and women (35). Moreover, the concentration of isoproterenol required to increase HR 25 beats/min was 29% higher in older than young healthy adults during combined autonomic blockade using atropine and clonidine (13), although the difference was not statistically significant, perhaps because the study was underpowered. Our findings support the earlier observations of White and Leenen (35) that chronotropic responsiveness to β-adrenergic stimulation is reduced in healthy older adults.
A novel finding of the present study was that chronotropic responsiveness to β-adrenergic stimulation is positively related to HRmax among healthy young and older men. These are the first data addressing this relation and suggest that a decrease in chronotropic β-adrenergic responsiveness is linked to age-associated reductions in HRmax in humans. We also found that maximal oxygen consumption is positively related to chronotropic β-adrenergic responsiveness. Overall, our results are consistent with the longstanding hypothesis that reduced cardiac responsiveness to β-adrenergic stimulation contributes to decreases in aerobic exercise capacity with aging (12, 34, 38). Part of the effect may be mediated by reduced β-adrenergic stimulation of left ventricular contractility (34).
Two previous investigations used systemic β-adrenergic blockade to gain insight into this issue, with mixed results. Conway and colleagues (6) found that the reductions in peak exercise HR in response to propranolol, a nonspecific β-adrenergic receptor antagonist, were not different in young compared with middle-aged/older men and women, suggesting a similar β-adrenergic influence on HRmax in these groups. In contrast, Fleg et al. (12) observed smaller reductions in peak exercise HR with advancing age in a group of healthy men treated with propranolol compared with the age-associated decreases observed in a separate group of untreated controls. The latter finding was interpreted as indicating that β-adrenergic stimulation plays a lesser role in determining HRmax in older compared with young men. The results of the present study may help explain the different conclusions of these two previous investigations and clarify the issue. Specifically, our findings suggest that reduced β-adrenergic responsiveness may contribute to age-associated decreases in HRmax, but the contribution is small relative to the effect of the reduction in HRint.
The mechanisms underlying age-associated reductions in cardiac responsiveness to β-adrenergic stimulation have been studied in cardiac tissue primarily from experimental animals and using lymphocytes from human subjects (1, 9, 11, 36). Reductions in post-receptor signaling have been reported consistently in older animals and cells, whereas observations concerning age-associated changes in β-adrenergic receptor density and/or agonist affinity have been more variable (1, 9, 11, 36). In a study of ventricular tissue from human hearts, White and colleagues (36) found several changes with age that could contribute to reduced chronotropic responsiveness, including reductions in β1-adrenergic receptor density, a decreased percentage of receptors in a high-affinity binding state, reduced isoproterenol-stimulated increases in adenylyl cyclase, increased uncoupling of β2-adrenergic receptors, and decreased G protein-mediated signal transduction.
Combined Effects of HRint and Chronotropic β-Adrenergic Responsiveness
We can only speculate as to how these mechanisms work in concert to reduce HRmax in older adults. During incremental exercise to exhaustion, cardiac vagal tone is progressively reduced, whereas cardiac sympathetic activity and circulating catecholamines are increased, collectively producing an exercise intensity-dependent tachycardia (33). Presumably these autonomic influences on the sinoatrial node have a finite ability to elevate HR during exercise above the HRint. If so, lowering the HRint would reduce the “baseline” on which these autonomically mediated increases are superimposed, thus reducing the maximal attainable HR during exercise. Reduced β-adrenergic responsiveness would further limit HRmax, presumably by restricting the augmentation of HR (i.e., above the HRint) that can be produced by activation of the sympathoadrenal system.
We wish to point out that in addition to reduced sinoatrial node responsiveness to sympathoadrenal stimulation, it is possible that less activation of the sympathetic nervous system and/or epinephrine release from the adrenal medulla could have contributed to the smaller augmentation of HR during maximal exercise in our older men. However, we have no plasma catecholamine data during maximal exercise and, thus, cannot provide insight into this possibility.
Limitations
The present study has several limitations. First, we associated two putative mechanisms with age group differences in HRmax instead of attempting to manipulate those mechanisms. However, we know of no method to experimentally alter HRint in humans. Cardiac chronotropic β-adrenergic responsiveness can be increased by short-term blockade of the sympathetic nervous system in young adults, but this intervention is not as effective in older adults (25). Second, we studied discrete groups of young and older adults but not a continuous adult age range. As such, the preliminary analysis based on bivariate correlations may contain inflated relations between the key variables of interest. However, these results are consistent with those derived from our ANCOVA. Third, we used a nonselective β-adrenergic agonist to assess β-adrenergic chronotropic responsiveness. As a result, we cannot determine if the reduction in β-adrenergic responsiveness with age was mediated by decreased β1- or β2-adrenoreceptor signaling (or both). Finally, we studied only healthy men. As such, our results cannot be generalized to patients with symptom-limited exercise capacity or to healthy women, although the same mechanisms likely are involved in reductions in HRmax with aging in women.
Physiological Significance
In 1938 Professor Sid Robinson (30) demonstrated that maximal oxygen consumption, the gold standard measure of aerobic exercise capacity, declined progressively with age and was associated with a parallel reduction in HRmax. Unlike the other determinants of maximal oxygen uptake, namely left ventricular stroke volume and systemic arteriovenous oxygen difference, the decline in HRmax with aging clearly is independent of factors such as sex and habitual exercise status, making it the most consistent contributor to reductions in aerobic exercise capacity with aging (17, 26, 30). Our results provide new insight into the mechanisms responsible for reductions in aerobic exercise capacity with aging. This has important physiological implications given the well-established associations between reduced aerobic exercise capacity and decreased physical functional capacity with adult aging (3, 36a).
In conclusion, the present findings provide novel experimental support for the concept that reductions in HRint and decreases in chronotropic responsiveness to β-adrenergic stimulation essentially account for the reductions in HRmax with aging in healthy men. Of these mechanisms, reductions in HRint, by far, play the greater role in mediating decreases in HRmax. We also show that reductions in HRint and decreases in chronotropic responsiveness to β-adrenergic stimulation are related to maximal oxygen consumption. Therefore, these two mechanisms may play an important role in the decline in maximal aerobic exercise capacity with aging.
GRANTS
This work was supported by National Institutes of Health Grants AG-06537, AG-13038, AG-22241, and RR-00051.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abrass IB, Scarpace PJ. Human lymphocyte beta-adrenergic receptors are unaltered with age. J Gerontol 36: 298–301, 1981. [DOI] [PubMed] [Google Scholar]
- 2.Alings AM, Abbas RF, Bouman LN. Age-related changes in structure and relative collagen content of the human and feline sinoatrial node. A comparative study (see Comment). Eur Heart J 16: 1655–1667, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality A prospective study of healthy men and women (see Comment). JAMA 262: 2395–2401, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Brunsting JR, Bennekers JH, Schuil HA, Zijlstra WG. Incomplete cardiac vagal blockade with atropine in the anesthetized dog. Pflügers Arch 381: 293–295, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Chassaing C, Godeneche D, Boucher M, Duchene-Marullaz P. A comparison of changes in atropine-induced tachycardia and atropine concentration in conscious dogs. Eur J Pharmacol 58: 433–441, 1979. [DOI] [PubMed] [Google Scholar]
- 6.Conway J, Wheeler R, Sannerstedt R. Sympathetic nervous activity during exercise in relation to age. Cardiovasc Res 5: 577–581, 1971. [DOI] [PubMed] [Google Scholar]
- 7.Craft N, Schwartz JB. Effects of age on intrinsic heart rate, heart rate variability, and AV conduction in healthy humans. Am J Physiol Heart Circ Physiol 268: H1441–H1452, 1995. [DOI] [PubMed] [Google Scholar]
- 8.de Marneffe M, Jacobs P, Haardt R, Englert M. Variations of normal sinus node function in relation to age: role of autonomic influence. Eur Heart J 7: 662–672, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Dillon N, Chung S, Kelly J, O'Malley K. Age and beta adrenoceptor-mediated function. Clin Pharmacol Ther 27: 769–772, 1980. [DOI] [PubMed] [Google Scholar]
- 10.Evans SL, Davy KP, Stevenson ET, Seals DR. Physiological determinants of 10-km performance in highly trained female runners of different ages. J Appl Physiol 78: 1931–1941, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Fan TH, Banerjee SP. Age-related reduction of beta-adrenoceptor sensitivity in rat heart occurs by multiple mechanisms. Gerontology 31: 373–380, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Fleg JL, Schulman S, O'Connor F, Becker LC, Gerstenblith G, Clulow JF, Renlund DG, Lakatta EG. Effects of acute beta-adrenergic receptor blockade on age-associated changes in cardiovascular performance during dynamic exercise. Circulation 90: 2333–2341, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Ford GA, James OF. Effect of “autonomic blockade” on cardiac beta-adrenergic chronotropic responsiveness in healthy young, healthy elderly and endurance-trained elderly subjects. Clin Sci 87: 297–302, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD, Winters WL Jr, Yanowitz FG, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Lewis RP, O'Rourke RA, Ryan TJ. ACC/AHA guidelines for exercise testing: executive summary A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation 96: 345–354, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Haqqani HM, Kalman JM. Aging and sinoatrial node dysfunction: musings on the not-so-funny side. Circulation 115: 1178–1179, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Higginbotham MB, Morris KG, Williams RS, Coleman RE, Cobb FR. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol 57: 1374–1379, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AS, Beard EF, Wier LT, Ross RM, Stuteville JE, Blair SN. Changes in aerobic power of men, ages 25–70 yr. Med Sci Sports Exerc 27: 113–120, 1995. [PubMed] [Google Scholar]
- 19.Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation 104: 2424–2429, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Jones SA, Boyett MR, Lancaster MK. Declining into failure: the age-dependent loss of the L-type calcium channel within the sinoatrial node (see Comment). Circulation 115: 1183–1190, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Jones SA, Lancaster MK, Boyett MR. Ageing-related changes of connexins and conduction within the sinoatrial node. J Physiol 560: 429–437, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res 4: 160–167, 1970. [DOI] [PubMed] [Google Scholar]
- 23.Jose AD, Stitt F, Collison D. The effects of exercise and changes in body temperature on the intrinsic heart rate in man. Am Heart J 79: 488–498, 1970. [DOI] [PubMed] [Google Scholar]
- 24.Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 44: 109–116, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Madden KM, Levy WC, Stratton JR. Normal aging impairs upregulation of the beta-adrenergic but not the alpha-adrenergic response: aging and adrenergic upregulation. J Cardiovasc Pharmacol 48: 153–159, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, Spina RJ, Martin WH, 3rd Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86: 494–503, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Peuler JD, Johnson GA. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci 21: 625–636, 1977. [DOI] [PubMed] [Google Scholar]
- 28.Rigel DF, Katona PG. Effects of antihistamines and local anesthetics on excess tachycardia in conscious dogs. J Pharmacol Exp Ther 238: 367–371, 1986. [PubMed] [Google Scholar]
- 29.Rigel DF, Lipson D, Katona PG. Excess tachycardia: heart rate after antimuscarinic agents in conscious dogs. Am J Physiol Heart Circ Physiol 246: H168–H173, 1984. [DOI] [PubMed] [Google Scholar]
- 30.Robinson S Experimental studies of physical fitness in relation to age. Arbeitsphysiologie 10: 251–323, 1938. [Google Scholar]
- 31.Robinson S, Pearcy M, Brueckman FR, Nicholas JR, Miller DI. Effects of atropine on heart rate and oxygen intake in working man. J Appl Physiol 5: 508–512, 1953. [DOI] [PubMed] [Google Scholar]
- 32.Saltin B, Calbet JA. Point: in health and in a normoxic environment, V̇o2max is limited primarily by cardiac output and locomotor muscle blood flow (see Comment). J Appl Physiol 100: 744–745, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Seals DR The autonomic nervous system. In: ACSM's Advanced Exercise Physiology, edited by Tipton C. Philadelphia, PA: Lippincott, Williams, and Wilkins, 2006, p. 197–245.
- 34.Stratton JR, Cerqueira MD, Schwartz RS, Levy WC, Veith RC, Kahn SE, Abrass IB. Differences in cardiovascular responses to isoproterenol in relation to age and exercise training in healthy men. Circulation 86: 504–512, 1992. [DOI] [PubMed] [Google Scholar]
- 35.White M, Leenen FH. Aging and cardiovascular responsiveness to beta-agonist in humans: role of changes in beta-receptor responses versus baroreflex activity. Clin Pharmacol Ther 56: 543–553, 1994. [DOI] [PubMed] [Google Scholar]
- 36.White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, Port JD, Anderson F, Campbell D, Feldman AM. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation 90: 1225–1238, 1994. [DOI] [PubMed] [Google Scholar]
- 36a.WHO Study Group. Aging and Working Capacity. Geneva, Switzerland: World Health Organization, 1993.
- 37.Wilson TM, Tanaka H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: relation to training status. Am J Physiol Heart Circ Physiol 278: H829–H834, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Yin FC, Spurgeon HA, Greene HL, Lakatta EG, Weisfeldt ML. Age-associated decrease in heart rate response to isoproterenol in dogs. Mech Ageing Dev 10: 17–25, 1979. [DOI] [PubMed] [Google Scholar]