Abstract
Leptin modulates energy metabolism and lung development. We hypothesize that the effects of leptin on postnatal lung development are volume dependent from 2 to 10 wk of age and are independent of hypometabolism associated with leptin deficiency. To test the hypotheses, effects of leptin deficiency on lung maturation were characterized in age groups of C57BL/6J mice with varying Lepob genotypes. Quasi-static pressure-volume curves and respiratory impedance measurements were performed to profile differences in respiratory system mechanics. Morphometric analysis was conducted to estimate alveolar size and number. Oxygen consumption was measured to assess metabolic rate. Lung volume at 40-cmH2O airway pressure (V40) increased with age in each genotypic group, and V40 was significantly (P < 0.05) lower in leptin-deficient (ob/ob) mice beginning at 2 wk. Differences were amplified through 7 wk of age relative to wild-type (+/+) mice. Morphometric analysis showed that alveolar surface area was lower in ob/ob compared with +/+ and heterozygote (ob/+) mice beginning at 2 wk. Unlike the other genotypic groups, alveolar size did not increase with age in ob/ob mice. In another experiment, ob/ob at 4 wk received leptin replacement (5 μg·g−1·day−1) for 8 days, and expression levels of the Col1a1, Col3a1, Col6a3, Mmp2, Tieg1, and Stat1 genes were significantly increased concomitantly with elevated V40. Leptin-induced increases in V40 corresponded with enlarged alveolar size and surface area. Gene expression suggested a remodeling event of lung parenchyma after exogenous leptin replacement. These data support the hypothesis that leptin is critical to postnatal lung remodeling, particularly related to increased V40 and enlarged alveolar surface area.
Keywords: leptin replacement, respiratory system mechanics, lung morphometry, metabolic rate, gene expression profiles
leptin, since its discovery in 1994 (56), has been considered an important peripheral signal that regulates satiety and energy metabolism (12, 27, 35). As an adipose-secreted protein, leptin has been shown to affect the function of other physiological systems, including respiratory system mechanics and breathing regulation (5, 46, 47). For example, lung volumes (Vl) in adult leptin-deficient (ob/ob) mice were significantly lower at incremental airway pressures (i.e., 0–30 cmH2O) compared with those in wild-type (+/+) mice (47). Furthermore, extended leptin replacement in the ob/ob mice led to a substantial increase of Vl and compliance, but these lung mechanical changes remained significantly lower at 10 wk of age compared with age-matched +/+ mice. In addition, leptin deficiency attenuated chemical control of breathing, which was sleep state dependent and independent of excessive body weight (33, 46, 47). Again, acute or prolonged leptin replacement ameliorated the regulation of breathing, making the return of function largely comparable to +/+ responses.
Postnatal alveolar development in mice rapidly occurs within the first 14 days. By postnatal (P) day 3 (P3), distinct changes in lung structure are characterized by the formation of primary saccules, subdividing into individual alveoli. This rapid alveolarization continues through P14 in mice, but lung growth persists, since the mouse chest wall epiphysis remains unclosed throughout life (3, 7, 29). Thus it is not known whether the lower Vl in adult ob/ob mice reflects the formation of smaller alveoli, lower number of alveoli postseptation, or both. Furthermore, mechanisms are unknown as to how leptin affects lung postnatal development. Because gas exchange is an essential function of the lung, and leptin deficiency is known to lead to hypometabolism, we question whether decrements in lung development of ob/ob mice parallel alterations in metabolic rate (23, 29). Tenney and Remmers (50) have shown an extraordinary linear relationship between alveolar surface area (Sa) and oxygen consumption (V̇o2) among a range of mammalian species. This same link between metabolic rate and lung development is more recently reviewed by Massaro and Massaro (29). This robust relationship suggests that a highly conserved genetic program is designed to match the events of the developing lung to the overall oxygen needs of a given mammalian organism. This conserved mechanism cannot be neglected in the present study because of the dramatic hypometabolic state known to occur in ob/ob mice. Nevertheless, we hypothesize that leptin deficiency plays a role in alveolar development separate from its effect to attenuate metabolic rate. Alternatively, we postulate that leptin regulates gene expression levels in the lung to augment the development of the extracellular matrix (ECM).
Thus, to test these hypotheses, we performed two complementary studies. In the first study, we examined the effects of leptin deficiency on lung maturation by studying +/+, heterozygous (ob/+), and ob/ob mice at 2, 3, 4, 7, and 10 wk of age. Body weight and V̇o2 were measured to assess changes in obesity and metabolic rate. Quasi-static pressure-volume (PV) curves and respiratory system impedance (Zrs) measurements were also performed to profile group differences in volume and mechanical properties as a function of age. Histological methods were conducted to estimate alveolar size and Sa. In a second series of experiments, we reexamined the changes in respiratory system function and alveolar architecture in ob/ob mice following leptin replacement. We also assessed leptin-induced changes in gene expression levels in lung tissue samples from ob/ob mice to further understand the potential mechanisms by which leptin alters structure and function of the respiratory system. Our results demonstrate a potential interaction of leptin with genes associated with the lung ECM to affect lung remodeling.
METHODS
Animals.
Male and female ob/ob, ob/+, and +/+ mice were generated by mating male and female ob/+ mice. The ob/+ progenitors were purchased from Jackson Laboratory (Bar Harbor, ME), and their offspring were genotyped for ob alleles by PCR and restriction enzyme analysis using tail specimens (19). For each age group, three to five dams were used to breed offspring of the three genotypic groups. Since offspring were randomly selected from different litters at various time points, each dam contributed offspring to multiple age groups. The offspring were weaned at 4 wk of age. All animals were housed in a facility at the Johns Hopkins University under standard conditions; the temperature was maintained at ∼21.5°C, and the light-dark cycle was 12:12 h, beginning at 0700. The animals were fed ad libitum with a pelleted stock diet. Each of the three genotypic groups was represented at 2, 3, 4, 7, and 10 wk of age (n = 5–11 mice per genotype per age). Sex ratios were evenly distributed in all age groups for each genotype. All protocols were approved by the Johns Hopkins University Animal Care and Use Committee and complied with the American Physiological Society Guidelines.
In a second series of studies, 18 female ob/ob mice were purchased from Jackson Laboratories at 4 wk age and were randomly divided into leptin-treated (n = 9 mice) and vehicle-treated groups (n = 9 mice). On a daily basis, each animal was weighed, and food intake was recorded. The leptin-treated group received recombinant murine leptin (r-metmuleptin; Amgen) administered intraperitoneally (ip) at a dose of 5 μg/g body wt daily for 8 days. Vehicle-treated animals were administered physiologically based saline at an equivalent volume as the leptin-treated animals. Six of the nine animals from each treatment group were subjected to the same protocol to assess Vl and respiratory system mechanics (described below) as the mice in the first series of studies. Lung tissue was extracted from the remaining three mice from each group for the analysis of gene expression using microarray and quantitative PCR (qPCR) gene expression techniques.
This dose and timing of leptin administration used in the present study was chosen based on our laboratory's previous work (47) showing the average daily dose regimen required to maintain body weight in ob/ob mice from 4 to 10 wk of age. In this previous study (47), the average daily ip dose of leptin ranged from 5 to 10 μg/g. Subsequently, we performed studies in ob/+ and ob/ob mice to determine which dose (5 or 10 μg/g) was most effective in restoring lung growth. In this dose-ranging study, the total amount of leptin given to the two groups of mice was kept constant by adjusting the number of days (8 or 4 days) that leptin was administered. These studies were accompanied by a second series of studies to determine the serum levels of leptin in ob/ob mice at 1, 3, 6, and 24 h after mice were administered a single ip dose of leptin at 5 μg/g. Serum samples were obtained from individual mice (2 mice per time point) and diluted by 2 to 1,024-fold to achieve a concentration within the detectable range of the mouse leptin RIA kit from Linco Research (St. Charles, MO).
Measurement of V̇o2.
Metabolic rate was estimated by measuring V̇o2 using a commercially available indirect open-circuit calorimetric system (Oxymax Deluxe, Columbus Instruments, Columbus, OH) inline with 200-ml cylindrical Plexiglas chambers. Unhumidified, compressed air was delivered through the chamber, under the control of a calibrated flowmeter. The flow was adjusted to maintain a difference between chamber inflow (compressed air) and outflow oxygen concentrations. The airflow out of the chamber was dried using a column of anhydrous CaSO4 and was sampled for 30 s for fractional concentrations of O2 using a limited diffusion O2 sensor. Sensor output was transmitted to a dedicated computer operated by data-acquisition software (Oxymax version 5.3, Columbus Instruments, Columbus, OH) for online computation and display of metabolic parameters. Gas analyzer calibrations were conducted before each experiment using gas mixtures standardized by the manufacturer (Puritan Bennett, Linthicum Heights, MD). Reference air measurements were obtained intermittently to correct for sensor drift. Finally, V̇o2 data were normalized to standard temperature, pressure, and dry conditions (stpd) and standardized as a function of body weight.
Zrs measurements.
Animals were anesthetized with ip injections of pentobarbital sodium at a dose of 80 mg/kg body wt. After the trachea was cannulated, the mouse was connected to a computer-controlled small-animal ventilator (FlexiVent, Montreal, Canada) while in a supine position. The +/+ and ob/+ mice were mechanically ventilated with 100% O2 at 150 breaths/min and a tidal volume of 10 ml/kg at positive end-expiratory pressures (PEEP) of 2 cmH2O. In ob/ob mice, similar ventilatory settings were used until 4 wk of age. Thereafter, ob/ob mice were ventilated at a tidal volume of 200 μl to avoid hyperinflation of the lung due to volume adjustments calculated with exaggerated body weights. After 10 min of mechanical ventilation, each animal was paralyzed with an ip injection of 0.05 ml succinylcholine (9 mg/ml). Stable breathing in each animal was attained after 3 min, at which time a deep inspiration was applied at an airway pressure of 30 cmH2O. Two minutes after the deep inspiration, the Zrs was measured at a PEEP level of 2 cmH2O and then fitted by Flexivent software to a constant-phase model (18) to provide measures of Raw (airway resistance), G (tissue damping), H (tissue elastance), and η (tissue hysteresivity).
In a separate group of 10-wk-old ob/ob and +/+ mice (7–8 mice per group), impedance measurements were repeated with the chest wall intact and again with the chest wall opened, as previously described (21). These studies were performed to determine the proportional contribution of the chest wall to the overall respiratory system mechanics, as assessed by Raw, G, H, and η.
PV curve.
After the impedance measurements, mice were mechanically ventilated with 100% O2 at a PEEP level of 2 cmH2O for 1 min, and the cannula was sealed with a stopcock for 3 min to degas the lung. Quasi-static PV curves were then performed ex vivo with the respiratory system intact. The rate of inflation and deflation was standardized by a dual-infusion withdraw pump (model 900–610, Harvard Apparatus, Dover, MA), and the airway pressure was measured by using a differential pressure transducer (model 8510B-2, Endevco). The initial inflation was controlled and adjusted to take ∼60 s to ensure that all lung regions opened. All successive PV inflation and deflation limbs were completed in ∼20 s. The pressure limits of the inflation and deflation airway pressures were 40 and −10 cmH2O, respectively. The volumes on deflation at 40 cmH2O (V40) were considered to represent total lung capacity. Compliance of the intact respiratory system was computed from the slopes of the PV relationships between 5 and 0 cmH2O (Crs5-0) on deflation.
Alveolar mean chord length, number, and Sa.
After the PV curve was completed, the tracheal cannula was connected to a 20-cmH2O column of 4% paraformaldehyde with 2% glutaraldehyde for 4 h. Lungs were removed from the thorax, extrapulmonary tissue carefully trimmed, and the whole Vl was determined by the water displacement method (41). We selected left lung sections by a standardized procedure (43) to measure alveolar mean chord length (MCL). Briefly, from the left lung, the top 3-mm section was removed and discarded, and then three serial, 2-mm sections were removed and processed for histology. Blocks were washed in distilled H2O twice, followed by 70% alcohol preservation, and then embedded in paraffin with methacrylate. For the measurement of MCL, 5-μm-thick sections were cut and stained with 0.05% toluidine blue. From each section, seven non-overlapping 676 μm × 505 μm fields were sampled, deliberately avoiding the large airways and blood vessels. Each of these regions was photographed for digital analysis, and conventional morphometric methods were used to determine MCL of alveoli using National Institutes of Health Image software (version 1.62). For consistency with previous studies (43), chord lengths <8 or >250 μm were excluded. Twenty-one histological samples were obtained from each animal and averaged for a given animal.
The lung tissue density (Dti) was computed as the percentage of lung tissue divided by the total area of the field. Like the MCL measurements, 21 histological samples were obtained from each animal and averaged for a given animal. The following formula was used to estimate alveolar number. The average MCL was used to represent the diameter of a spherical alveolus:
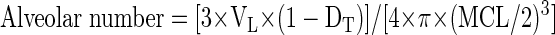 |
Similarly, Sa was estimated as follows:
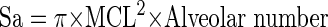 |
Oligonucleotide microarray.
Lungs were isolated, and total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). Extracted RNA was purified using the RNeasy mini kit (Qiagen, Valencia, CA), and the quality of the RNA was assessed using the RNA 6000 nano assay kits (Agilent Technologies, Palo Alto, CA). The isolated RNA was applied to Mouse Genome 430 2.0 GeneChip arrays (Affymetrix, Santa Clara, CA), according to procedures described previously (34). This array contains probes for detecting ∼14,500 well-characterized genes and 4,371 expressed sequence tags.
Scanned output files were analyzed using Affymetrix GeneChip Operating Software, version 1.3, and were independently normalized to an average intensity of 500. To identify the differentially expressed transcripts, pairwise comparison analyses were performed using the DMT 3.0 program (Affymetrix). Only genes that changed in at least six out of nine comparisons (n = 3 mice/group) and that showed a P value ≤0.05 using Mann-Whitney test were considered statistically significant with respect to differential gene expression. An absolute fold change (FC) of ≥1.5 was also used as a criterion to select noteworthy genes. These criteria were consistent with the bioinformatics techniques used in previous mouse studies (11, 14, 31). NetAffx (26) was used to extract gene ontology information for the gene expression profiles. For those genes with multiple probes, the single probe with the highest magnitude FC (positive or negative) was retained, as representing that gene. Microarray data sets were deposited to the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/projects/geo/, with accession no. GSE10915).
mRNA expression.
Total RNA (3 μg/sample) was reverse transcribed to cDNA using random primers and MultiScribe reverse transcriptase (Applied Biosystems, Foster City, CA). Using 100 ng of cDNA as a template, quantification was performed by an ABI Prism 7000 Sequence Detector (Applied Biosystems) using the TaqMan 5′ nuclease activity from the TaqMan Universal PCR Master Mix, fluorogenic probes (Applied Biosystems), and oligonucleotide primers (Invitrogen). TaqMan assays were repeated twice in each lung sample for each of 16 selected genes related to leptin and lung ECM. The mRNA expression levels of all samples were normalized to the levels of glyceraldehyde-3-phosphate dehydrogenase from the same sample and were calculated as FCs using the 2−Δ ΔCT method (42).
Statistical analysis.
Differences between the three genotypic groups with age were analyzed by two-way ANOVA (Graphpad Prism, version 4.03). Mean comparisons between genotypic and age groups were further tested by a Bonferroni posttest. Since all genotypic offspring were randomly selected from different litters at various time points, maternal inheritance was not considered a factor in the statistical analysis. The effects of leptin vs. vehicle treatment in ob/ob mice were evaluated by unpaired t-tests. Statistical significance was established at a P value of 0.05. All results in Tables 1–4 and Figs. 1–8 were expressed as the means ± SE.
Table 1.
Age-dependent volume and mechanical properties of the respiratory system
| Age, wk | Genotype | n | V40, ml | Not Normalized by V40 |
Normalized by V40 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crs5-0, ml/cmH2O | Raw, cmH2O·s·ml−1 | G, cmH2O/ml | H, cmH2O/ml | η | NCrs5-0/V40, cmH2O | Raw × V40, cmH2O/s | G × V40, cmH2O | H × V40, cmH2O | ||||
| 2 | +/+ | 8 | 0.57±0.05 | 0.018±0.001 | 2.28±0.22 | 16.39±1.04 | 117.79±8.94 | 0.14±0.004 | 0.033±0.002 | 1.29±0.16 | 9.11±0.74 | 65.07±5.46 |
| ob/+ | 11 | 0.52±0.03 | 0.018±0.002 | 1.83±0.13 | 18.99±1.11 | 128.76±4.87 | 0.14±0.006 | 0.036±0.002 | 0.99±0.10 | 10.08±0.73 | 68.14±3.68 | |
| ob/ob | 6 | 0.41±0.04* | 0.014±0.001 | 2.85±0.60 | 18.43±1.35 | 133.64±6.53 | 0.15±0.010 | 0.034±0.004 | 1.04±0.15 | 7.85±0.82 | 54.56±5.18 | |
| 3 | +/+ | 10 | 0.67±0.03 | 0.027±0.003 | 1.39±0.14 | 13.01±0.46 | 89.99±4.10 | 0.14±0.006 | 0.040±0.003 | 0.93±0.01 | 8.86±0.67 | 60.48±4.00 |
| ob/+ | 8 | 0.60±0.04 | 0.023±0.001 | 1.43±0.23 | 13.64±1.71 | 91.84±8.03 | 0.15±0.012 | 0.039±0.003 | 0.88±0.10 | 7.87±0.74 | 54.91±6.23 | |
| ob/ob | 6 | 0.44±0.04*§ | 0.017±0.001* | 1.44±0.22 | 15.10±0.70 | 117.17±7.33*§ | 0.13±0.004 | 0.040±0.002 | 0.66±0.13 | 6.65±0.57 | 51.45±4.36 | |
| 4 | +/+ | 11 | 1.02±0.05 | 0.034±0.002 | 1.08±0.10 | 9.66±0.61 | 68.44±5.07 | 0.14±0.005 | 0.035±0.003 | 1.13±0.15 | 9.96±1.02 | 70.86±8.02 |
| ob/+ | 11 | 0.82±0.02† | 0.031±0.002 | 1.13±0.10 | 9.57±0.36 | 71.29±2.68 | 0.13±0.002 | 0.039±0.002 | 0.96±0.10 | 8.15±0.42 | 60.84±3.25 | |
| ob/ob | 9 | 0.60±0.03*§ | 0.021±0.002*§ | 1.27±0.13 | 14.13±0.81*§ | 109.41±3.90*§ | 0.13±0.003 | 0.035±0.002 | 0.76±0.08 | 8.40±0.52 | 65.14±3.08 | |
| 7 | +/+ | 10 | 1.40±0.04 | 0.067±0.003 | 0.80±0.03 | 4.96±0.17 | 33.61±1.07 | 0.15±0.005 | 0.048±0.002 | 1.12±0.06 | 6.89±0.17 | 46.68±1.22 |
| ob/+ | 10 | 1.19±0.02† | 0.057±0.002† | 0.89±0.04 | 5.66±0.22 | 41.98±1.35 | 0.13±0.002 | 0.048±0.001 | 1.06±0.05 | 6.72±0.25 | 49.81±1.31 | |
| ob/ob | 5 | 0.93±0.03*§ | 0.041±0.002*§ | 1.28±0.05 | 8.17±0.38* | 58.19±3.82* | 0.13±0.004 | 0.044±0.001 | 1.28±0.10 | 7.83±0.84 | 56.99±2.57 | |
| 10 | +/+ | 11 | 1.42±0.04 | 0.062±0.001 | 0.89±0.05 | 5.50±0.17 | 35.10±1.02 | 0.16±0.003 | 0.045±0.001 | 1.26±0.09 | 7.73±0.35 | 49.18±1.78 |
| ob/+ | 10 | 1.26±0.02† | 0.055±0.002† | 0.96±0.08 | 6.16±0.12 | 41.47±1.09 | 0.15±0.002 | 0.043±0.001 | 1.22±0.10 | 7.77±0.14 | 52.29±1.23 | |
| ob/ob | 10 | 1.06±0.05*§ | 0.040±0.002*§ | 1.26±0.14 | 9.27±0.58*§ | 59.25±2.47*§ | 0.16±0.004 | 0.038±0.001 | 1.33±0.18 | 9.65±0.48 | 62.14±2.76 | |
Values are means ± SE; n, no. of mice. +/+, Wild-type mice; ob/+, heterozygous mice; ob/ob, leptin-deficient mice; Raw, airway resistance; G, tissue damping; H, tissue elastance; η, hysteresivity; V40, lung volume at 40 cmH2O; Crs5-0, respiratory system compliance between 5 and 0 cmH2O.
P < 0.05, ob/ob vs. +/+ at the same age.
P < 0.05, ob/+ vs. +/+ at the same age.
P < 0.05, ob/ob vs. ob/+ at the same age.
Table 4.
Gene expression profile of selected genes from microarray analysis
| Accession No. | Gene Symbol | Gene Title | Leptin vs. Vehicle Treated |
|---|---|---|---|
| U08020 | Col1a1 | Procollagen, type I, α1 | 3.7±1.0 |
| AW550625 | Col3a1 | Procollagen, type III, α1 | 2.7±0.7 |
| AF064749 | Col6a3 | Procollagen, type VI, α3 | 1.8±0.4 |
| AK011784 | Igfbp2 | Insulin-like growth factor binding protein-2 | 2.1±0.6 |
| BF147716 | Mmp2 | Matrix metalloproteinase-2 | 2.7±0.7 |
| BI220012 | Serpinh1 | Serine (or cysteine) proteinase inhibitor, clade H, member 1 | 2.4±0.7 |
| AW214029 | Stat1 | Signal transducer and activator of transcription 1 | 2.0±0.6 |
| NM_013692 | Tieg1 | Transforming growth factor-β-inducible early growth response 1 | 3.2±1.2 |
Values are average fold change (±SE). Each fold change represents a significant (P < 0.05) upregulation of gene expression in leptin- vs. vehicle-treated ob/ob mice.
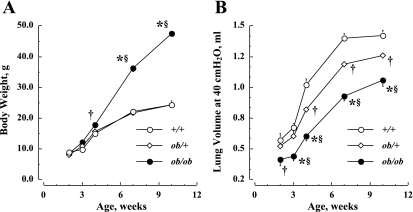
Fig. 1.
A: age-dependent changes in body weight in the three genotypic mice. B: age-dependent changes in lung volume at 40 cmH2O (V40) in the three genotypic mice. +/+, Wild type; ob/+, heterozygous; ob/ob, leptin deficient. Values are means ± SE. †P < 0.05 vs. +/+; *P < 0.01, ob/ob vs. +/+; §P < 0.01, ob/ob vs. ob/+.
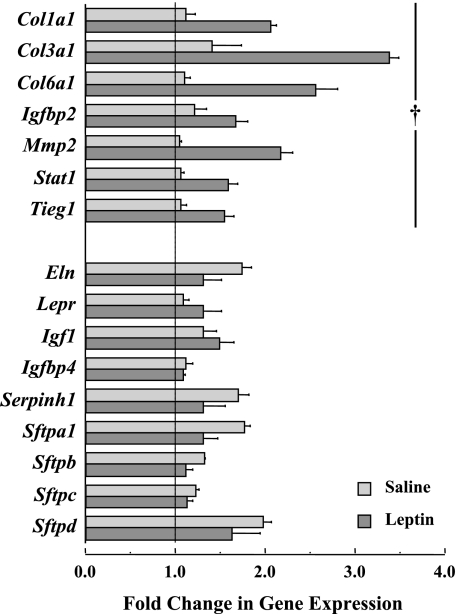
Fig. 8.
Leptin treatment in ob/ob mice significantly upregulated the gene expression of procollagen I (Col1a1), III (Col3a1), and VI (Col6a3), insulin-like growth factor binding protein-2 (Igfbp2), matrix metalloproteinase 2 (Mmp2), signal transducer and activator of transcription 1 (Stat1) in lung tissue, and transforming growth factor-β-inducible early growth response 1 (Tieg1). The gene expression levels of elastin (Eln), leptin receptor (Lepr), insulin-like growth factor I (Igf1), insulin-like growth factor binding protein-2 (Igfbp2) and -4 (Igfbp4), serine peptidase inhibitor h1 (Serpinh1), and the surfactant proteins a1 (Sftpa1), b (Sftpb), c (Sftpc), and d (Sftpd) were not significantly altered by leptin treatment in ob/ob mice. Average values (±SE) are derived from quantitative PCR data referenced to the expression levels of GADPH. The vertical line at a gene expression level of 1 represents no detectable change in expression. †P < 0.05, leptin vs. saline treatment.
RESULTS
Figure 1 shows the changes in body weight and V40 with age for each genotypic group. In Fig. 1A, body weight increases significantly with age in each genotypic group [F(df = 4) = 620.1, where df is degrees of freedom, P < 0.01], and the body weights in ob/ob mice are notably (P < 0.01) greater than those in +/+ mice after 4 wk of age and greater than in ob/+ mice after 7 wk. No difference in body weight is observed between +/+ and ob/+ mice (P > 0.05) for any age group.
In Fig. 1B, changes in V40 with age are shown for each genotypic group. The V40 increases significantly with age in each genotypic group through 7 wk [F(df = 4) = 237.4, P < 0.01], and V40 in ob/ob mice is notably lower than that in +/+ mice (P < 0.05), even at 2 wk, and lower than that in ob/+ mice beginning at 3 wk (P < 0.05). The V40 is also higher in +/+ than in ob/+ mice (P < 0.05) after 4 wk of age. Furthermore, the differences in V40 between +/+ and ob/ob mice are significantly increased from 0.16 ml at 2 wk to 0.47 ml at 7 wk [F(df = 8) = 2.74, P < 0.01].
The mechanical properties of the respiratory system are reported in Table 1. In each genotypic group, the Crs5-0 increases significantly with age through 7 wk [F(df = 4) = 244.6, P < 0.01]. The Crs5-0 in ob/ob mice is significantly lower than that in +/+ mice (P < 0.01), beginning at 3 wk, and lower than that in ob/+ mice (P < 0.05), beginning at 4 wk of age. The Raw, G, and H parameters show progressive declines with age in each genotypic group [F(df = 4) = 37.6, P < 0.01 for Raw; F(df = 4) = 116.0, P < 0.01 for G; and F(df = 4) = 152.9, P < 0.01 for H]. At each age, the Raw, G, and H parameters of ob/ob mice are generally the highest for the three genotypic groups, and those for +/+ mice are lowest. The differences in G and H are significantly higher in ob/ob mice compared with age-matched +/+ mice after 3–4 wk (P < 0.05). The parameter η is not different among the genotypic groups at any age (P > 0.05). However, the differences between genotypic groups for Crs5-0 and each impedance parameter are eliminated after normalizing the results for V40, suggesting that the mechanical differences in ob/ob mice are volume dependent.
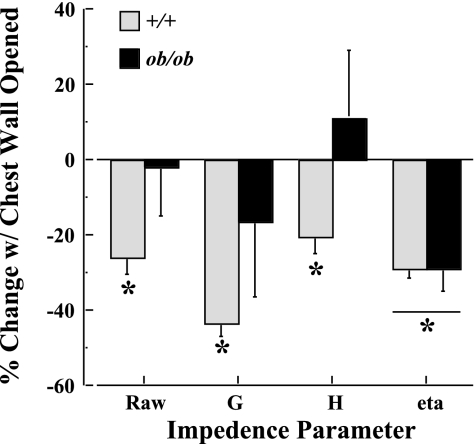
In Fig. 2, the percent changes in Raw, G, H, and η with the chest wall opened relative to the chest wall intact are reported for 10-wk-old ob/ob and +/+ mice. Each of the four parameters is significantly (P < 0.01) reduced with the chest wall opened in +/+ mice. To the contrary, only η is significantly (P < 0.01) reduced in ob/ob mice, leaving Raw, G, and H unchanged (P > 0.05) when the chest wall is opened.
Fig. 2.
Effects of chest wall mechanics on respiratory system impedance parameters expressed as percent change with the chest wall opened relative to the chest wall intact. Impedance parameters are as follows: Raw, airway resistance; G, tissue damping; H, tissue elastance; η, hysteresivity. Measurements were obtained in 10-wk-old +/+ (n = 7) and ob/ob (n = 8) mice. *P < 0.01, chest wall opened vs. intact.
Table 2 shows the age-dependent changes of Vl, MCL, Dti, alveolar number, and Sa in the three genotypic groups. The Vl increases significantly with age in each genotypic group [F(df = 4) = 145.5, P < 0.01]. However, Vl in ob/ob mice is significantly lower after 4 wk relative to that in +/+ mice (P < 0.01) and is significantly lower after 7 wk relative to ob/+ mice (P < 0.01). There is a significant interaction [F(df = 2) = 3.91, P < 0.05] from 2 to 10 wk of age between genotypic groups, suggesting that the MCL in ob/ob mice decreases (−5.9%), whereas the MCL increases for both ob/+ (3.4%) and +/+ mice (5.9%). Generally, alveolar number and Sa in ob/ob mice are notably lower relative to that in +/+ and ob/+ mice, beginning at 2 wk of age [F(df = 2) = 4.60, P < 0.05 for alveolar number; F(df = 2) = 23.2, P < 0.01 for Sa]. In addition, alveolar number and Sa increase significantly with age in each genotypic group through 7 wk [F(df = 4) = 42.82, P < 0.01 for alveolar number; F(df = 4) = 106.6, P < 0.01 for Sa].
Table 2.
Age-dependent changes in mean chord length and alveolar number and surface area
| Age, wk | Genotype | n | Vl, ml | MCL, μ | Dti | Alveolar Number, ×106 | Sa, ×103 mm2 |
|---|---|---|---|---|---|---|---|
| 2 | +/+ | 7 | 0.45±0.01 | 35.8±0.6 | 0.38±0.03 | 11.8±0.8 | 47.2±2.7 |
| ob/+ | 8 | 0.43±0.01 | 35.7±0.9 | 0.36±0.01 | 11.6±0.7 | 45.9±1.7 | |
| ob/ob | 6 | 0.37±0.01 | 37.4±1.6 | 0.42±0.02 | 8.1±1.0 | 34.1±2.0* | |
| 3 | +/+ | 7 | 0.54±0.03 | 34.8±1.2 | 0.33±0.03 | 16.9±1.6 | 62.7±3.7 |
| ob/+ | 7 | 0.53±0.02 | 34.5±0.8 | 0.37±0.04 | 15.6±1.5 | 57.7±4.7 | |
| ob/ob | 6 | 0.48±0.01 | 34.7±0.9 | 0.33±0.02 | 14.9±1.3 | 55.3±2.6 | |
| 4 | +/+ | 6 | 0.67±0.02 | 34.6±0.9 | 0.38±0.02 | 19.2±1.2 | 71.5±2.2 |
| ob/+ | 8 | 0.62±0.02 | 34.5±0.8 | 0.39±0.01 | 16.5±0.5 | 67.2±2.1 | |
| ob/ob | 6 | 0.54±0.02* | 34.5±0.8 | 0.35±0.02 | 16.4±0.7 | 60.8±2.0 | |
| 7 | +/+ | 6 | 0.93±0.03 | 37.1±0.5 | 0.36±0.02 | 22.6±0.7 | 97.1±1.9 |
| ob/+ | 8 | 0.83±0.03† | 36.5±1.1 | 0.36±0.02 | 21.2±1.2 | 88.6±2.3 | |
| ob/ob | 5 | 0.67±0.02*§ | 34.1±0.7 | 0.41±0.03 | 21.2±1.6 | 77.1±6.1* | |
| 10 | +/+ | 6 | 0.94±0.06 | 37.9±0.8 | 0.35±0.01 | 21.5±1.1 | 97.1±5.1 |
| ob/+ | 5 | 0.88±0.04 | 36.9±0.8 | 0.36±0.01 | 21.7±1.1 | 91.4±5.3 | |
| ob/ob | 6 | 0.72±0.03*§ | 34.5±0.6 | 0.38±0.03 | 20.5±0.9 | 76.5±3.5*§ |
Values are mean ± SE; n, no. of mice. Vl, whole lung volume measured by water displacement; MCL, mean chord length of alveoli; Dti, tissue density; Sa, alveolar surface area.
P < 0.05, ob/ob vs. +/+ at the same age.
P < 0.05, ob/+ vs. +/+ at the same age.
P < 0.05, ob/ob vs. ob/+ at the same age.
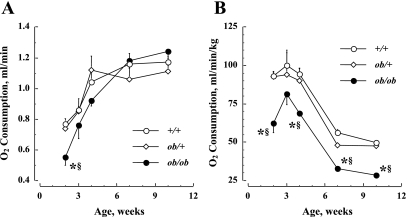
Figure 3 shows the developmental changes in the absolute V̇o2 (Fig. 3A) and the relative V̇o2 corrected for body weight (Fig. 3B) in the three genotypic groups. The absolute V̇o2 is significantly (P < 0.01) lower in ob/ob mice at 2 wk compared with the other two groups. Also, there is a significant increase in the absolute V̇o2 with age in each genotypic group through 7 wk [F(df = 4) = 66.8, P < 0.01]. After 3 wk, there is no significant (P > 0.05) difference among the genotypic groups in the absolute V̇o2. Figure 3B shows the same results but corrected for body weight, and the V̇o2 of the ob/ob mice are consistently lower relative to that in the other two groups at 2, 4, 7, and 10 wk (P < 0.01).
Fig. 3.
Age-dependent changes in O2 consumption (V̇o2) in three genotypic mice. A: absolute V̇o2, expressed as ml/min. B: V̇o2 corrected for body weight, expressed as ml·min−1·kg−1. Values are means ± SE. *P < 0.01, ob/ob vs. +/+; §P < 0.01, ob/ob vs. ob/+.
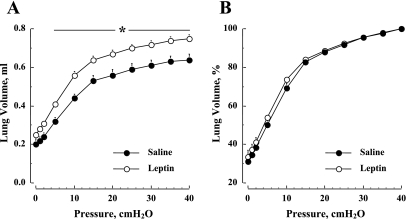
The results from studies to determine the dose of leptin effective in stimulating lung growth in 4-wk-old mice are shown in Fig. 4. In Fig. 4A, daily leptin doses of 5 μg/g for 8 days result in significant (P < 0.05) increases in Vl at 30 cmH2O in both ob/+ and ob/ob mice. There is no change in Vl in either ob/+ and ob/ob mice following doses of 10 μg/g for 4 days. In the same mice, body weight is not different in ob/+ mice receiving 5 or 10 μg/g doses of leptin (data not shown). Also, body weight remains unchanged in ob/ob mice receiving doses of 5 μg/g of leptin, but is significantly (P < 0.05) reduced with 10 μg/g of leptin.
Fig. 4.
A: effects of leptin on lung volume at airway pressures of 30 cmH2O in 4-wk-old mice are dose dependent. An effective dose of 5 μg/g for 8 days significantly increased lung volume in both ob/+ and ob/ob mice, whereas 10 μg/g for 4 days did not significantly alter lung volume. B: time-dependent serum concentration levels of leptin after a single dose at 5 μg/g. †P < 0.01, leptin vs. saline treatment.
As shown in Fig. 4B, the average serum leptin concentrations in 4-wk-old ob/ob mice following a single dose of 5 μg/g are shown as a function of time during a 24-h period. The average serum level of leptin at 1 h after ip injection is approximately three orders of magnitude above plasma levels reported in +/+ mice at a similar age (32). The serum leptin concentration in ob/ob mice after a single dose of 5 μg/g rapidly decays, reaching +/+ levels between 6 and 24 h.
Figure 5 shows the daily changes in body weight (Fig. 5A) and food intake (Fig. 5B) for ob/ob mice during 8 days of saline or leptin treatment. The body weight increases from 27.3 ± 1.0 to 34.8 ± 0.7 g in vehicle-treated ob/ob mice. There is a significantly lower increase in body weight in the leptin-treated group, from 26.7 ± 1.2 to 28.3 ± 1.0 g. The groups differ in body weight after treatment day 3 (P < 0.01). The daily food intake is also decreased after leptin treatment and is significantly (P < 0.01) lower than vehicle after treatment day 2.
Fig. 5.
Effect of leptin treatment in ob/ob mice after an 8-day treatment with saline or leptin. A: changes in body weight. B: changes in food intake. Values are means ± SE. *P < 0.01, leptin vs. saline treatment.
Figure 6 depicts average lung deflation curves for leptin- and vehicle-treated ob/ob mice. In Fig. 6A, the absolute volume is significantly [F(df = 1) = 115.4, P < 0.01] elevated in the leptin-treated group compared with vehicle. However, when the same results are normalized to V40 (Fig. 6B), the differences between the groups are eliminated [F(df = 1) = 3.68, P > 0.05].
Fig. 6.
Effect of leptin treatment on the quasi-static pressure-volume deflation curve in ob/ob mice. A: before normalizing to V40. B: after normalizing to V40. Values are means ± SE. *P < 0.01, leptin vs. saline treatment.
The effects of leptin treatment on respiratory system mechanics in ob/ob mice are reported in Table 3. Compared with vehicle, V40 and Crs5-0 in leptin-treated ob/ob mice are significantly elevated (P < 0.05). However, the V40 of leptin-treated ob/ob mice remains significantly (P < 0.01) attenuated compared with the V40 of +/+ mice at 4 wk (Fig. 1). After normalizing for V40, the difference in Crs5-0 between vehicle- and leptin-treated groups is diminished (P > 0.05). Similarly, there are no detectable differences in the impedance results between leptin- and vehicle-treated ob/ob mice, with or without normalizing for V40 (P > 0.05).
Table 3.
Effect of leptin treatment on ob/ob mice for mechanical parameters of the respiratory system
| V40, ml | Not Normalized by V40 |
Normalized by V40 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crs5-0, ml/cmH2O | Raw, cmH2O·s·ml−1 | G, cmH2O/ml | H, cmH2O/ml | η | NCrs5-0/V40, cmH2O | Raw × V40, cmH2O/s | G × V40, cmH2O | H × V40, cmH2O | ||
| Vehicle treated | 0.64±0.031 | 0.025±0.003 | 1.83±0.37 | 11.46±1.40 | 84.74±4.01 | 0.13±0.015 | 0.039±0.004 | 1.14±0.19 | 7.60±1.12 | 55.53±4.50 |
| Leptin treated | 0.75±0.018* | 0.031±0.001* | 1.87±0.34 | 9.88±0.57 | 81.47±6.10 | 0.12±0.007 | 0.041±0.001 | 1.40±0.23 | 7.43±0.35 | 61.38±4.43 |
Values are means ± SE; n = 6 mice for each group.
P < 0.05, compared with vehicle-treated group.
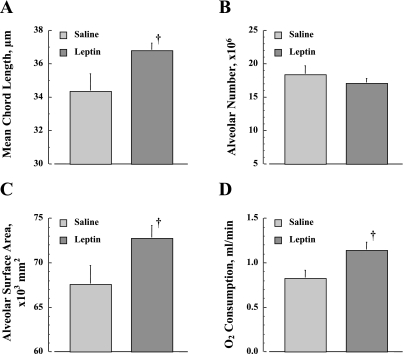
The differences in MCL, alveolar number, Sa, and absolute V̇o2 between the leptin- and vehicle-treated ob/ob mice are shown in Fig. 7. After leptin treatment, the MCL is significantly larger than vehicle (P < 0.05, Fig. 7A). The percent difference in MCL between leptin- and vehicle-treated ob/ob mice is 7.0% (34.4 ± 1.0 and 36.8 ± 0.4 μm, respectively), which is comparable to the change in +/+ mice from 2 to 10 wk of age. The leptin treatment does not significantly affect the alveolar number relative to vehicle (P > 0.05, Fig. 6B), but significantly increases the Sa (P < 0.05, Fig. 7C). Compared with vehicle, leptin treatment also significantly increased the absolute V̇o2 (P < 0.05, Fig. 7D).
Fig. 7.
Effect of leptin treatment in ob/ob mice on lung architecture. A: alveolar mean chord length. B: alveolar number. C: alveolar surface area. D: absolute V̇o2. Values are means ± SE. †P < 0.05, leptin vs. saline treatment.
The results from microarray data analyses show that there are a total of 41 expressed genes that are significantly upregulated (n = 38 genes) or downregulated (n = 3 genes) with leptin treatment. Table 4 shows eight upregulated genes with relevance to leptin and lung parenchymal ECM. These expressed genes included three collagen genes (Col1a1, Col3a1, and Col6a3), and the serine peptidase inhibitor h1 (Serpinh1) gene, which is involved with collagen biosynthesis. Two other expressed genes, including insulin growth factor binding protein-2 (Igfbp2) and matrix metalloproteinase 2 (Mmp2), are also upregulated with leptin treatment. The signal transducer and activator of transcription 1 (Stat1) and transforming growth factor-β-inducible early growth response 1 (Tieg1) genes are also significantly upregulated.
The eight genes shown to be significant by microarray analysis were retested by qPCR, and the average gene expression levels for the leptin- and vehicle-treated groups are also shown in Fig. 8. Seven of the eight genes were validated by both analyses, and only Serpinh1 expression was not confirmed by qPCR. The elastin (Eln) and leptin receptor (Lepr) genes, the surfactant genes (Sftpa1, Sftpb, Sftpc, and Sftpd), as well as the insulin-like growth factor I (Igf1) and Igfbp4 genes, were also examined using qPCR, and the expression levels of these genes in lung tissue were not different (P > 0.05) between leptin- and vehicle-treated groups.
DISCUSSION
The major findings of the present study suggest that adverse effects of leptin deficiency on postnatal lung development are due to altered alveolar formation, which is evident as early as at 2 wk of age. Furthermore, the absence of leptin into adulthood delays incremental increases in alveolar Sa, which is observed in the lung development of +/+ mice (Table 2). Leptin deficiency results in reduced Vl and alveolar Sa by modest, but early, decreases in alveolar number. With further lung development into adulthood, normal alveolar enlargement is also delayed in leptin-deficient ob/ob mice. The effects of leptin deficiency on lung development are supported by the results observed with leptin replacement for 8 days, which include increases in Vl (Table 3) and Sa (Fig. 7). In addition, leptin treatment is accompanied by a notable alveolar enlargement in ob/ob mice.
The reduction in Vl observed in leptin-deficient ob/ob mice is associated with a significantly elevated pulmonary elastance commencing at 3 wk of age (Table 1). Although the MCL is similar at 3 wk among the different genotypic groups, the alveolar number tends to be lower in ob/ob mice by ∼12%. As the alveolar number increases into adulthood in each of the genotypic groups, the elastance decreases accordingly; however, elastance in ob/ob mice is sustained at a higher level relative to the other groups, presumably as a result of a delayed alveolar enlargement. Since the differences in elastance among the genotypic groups are eliminated after normalizing to V40, the variation in lung development among the groups is generally volume dependent. The changes in other mechanical parameters of the respiratory system with development, including compliance and G, are also affected in a volume-dependent manner, although the time course may be modestly altered relative to H. In addition, the developmental changes in H and G for +/+ mice are similar to changes in lung tissue mechanics observed in other inbred mouse strains (7).
With respect to Raw in ob/ob mice, there are no detectable phenotypic differences among the three groups from 2 to 10 wk age. Hence, leptin deficiency appears to affect parenchymal mechanics in the absence of any notable effect on developmental changes in Raw. One mechanism to consider is an imbalance between the parenchymal tethering forces and the elastic recoil of the airway after 3 wk of age in ob/ob mice. That is, one would expect that greater parenchymal elastance would lead to greater airway caliber and lower Raw in ob/ob mice. However, the net effect of this imbalance would be partially offset by the lower V40 in the ob/ob mice. Several other factors may have been important in modulating Raw of ob/ob mice, including the fact that the genotypic groups were ventilated at variable tidal volumes as a percentage of V40. Since tidal stretching of the lung leads to airway smooth muscle relaxation, the bronchomotor tone may have been different among the groups. Nevertheless, leptin deficiency leads to impaired alveolar growth, whereas Raw appears to be left unaffected. Other growth factors beside leptin have been shown to affect airways and alveoli differently (16).
Although ob/ob mice are unable to produce leptin, these mutants are exposed to leptin to some extent through early stages of development by placental blood flow and lactational sources from the ob/+ dams (4, 20, 39, 51). During postnatal development, Mistry and coworkers (32) showed that plasma leptin levels were undetectable (<1 ng/ml) in ob/ob mice from P11 through P36. However, plasma leptin levels in +/+ mice were four- to fivefold greater (∼21 ng/ml) at P11 compared with levels at P36, suggesting an important role for leptin in early postnatal development. In the same study (28), there were no detectable differences in V̇o2 between +/+ and ob/ob mice at P11. Furthermore, in ob/ob mice, three daily ip doses of leptin at 1 μg/g did not lead to detectable differences in V̇o2 between leptin- and vehicle-treated mice at P11. These results led the investigators to conclude that mice are generally unresponsive to leptin in terms of inducing increases in V̇o2 before P11. In the present study, genotypic differences in V̇o2 are observed at P14, with ob/ob mice demonstrating lower V̇o2 than the other two groups. Since alveolar formation is largely complete by P14 in mice (3, 29) before a period of significant lung growth, we postulated that the attenuated lung growth in ob/ob mice was, to some extent, associated with the depression in V̇o2. However, this hypothesis was partially refuted by results showing that the absolute amount of O2 consumed by ob/ob mice between 21 and 70 days of age (Fig. 3A) did not differ from the other groups, while the Vl and Sa remain lower in ob/ob mice. This dissociation is particularly evident in ob/ob mice after 7 wk of age. On the other hand, the same hypothesis was supported by a coincident increase in V̇o2 and Vl in ob/ob mice treated with leptin. These conflicting results between our two studies led us to explore the potential gene expression differences using leptin treatment (ip dose of 5 μg/g) for 8 days. While it is true that this treatment led to plasma levels well above those seen in +/+ mice (32), several physiological responses in treated ob/ob mice, including body weight regulation (Fig. 5) and V̇o2 (Fig. 7D), were modulated at comparable levels to age-matched +/+ mice. We also surmised that the improved Vl phenotype in ob/ob mice justified this leptin dose and duration (Fig. 4A) for gene expression analysis.
Elastin and collagen are ECM proteins that make up the framework of alveolar structure and are most important in determining the mechanical properties of lung parenchyma (44). Several studies have demonstrated that there are close links between elastin expression and lung alveolar formation (28, 54, 55). For example, elastin synthesis in mice is greatest in the postnatal lung during alveolar formation (7, 38). The lung elastic properties develop concurrently with alveolar septation, being that both are essentially stabilized by 7 wk of age, as shown in this (Tables 1 and 2) and other studies (3, 29). In the present study, neither the gene expression of elastin nor alveolar number was altered in ob/ob mice between 4 and 5 wk of age after leptin treatment. These gene expression results suggest that leptin treatment beginning at 4 wk of age merely affected mechanisms of alveolar enlargement without affecting alveolar number or Eln gene expression. Whether or not this phenotypic modification is specific to a role leptin plays in affecting lung development requires future studies. It is possible that leptin treatment in the present study occurred during a developmental stage of the lung involved in alveolar thinning and enlargement (1, 10). Given this possibility, the leptin-induced gene expression profile may reflect the expressed genes essential for these processes to occur.
Collagen is the primary load-bearing component of the lung ECM. During postnatal development, lung tissue is constantly under a preexisting tensile stress, which is a result of lung distension by increased transpulmonary pressure. Additionally, oscillations in tensile stress accompany cyclic breathing. We have shown that prolonged leptin treatment in ob/ob mice leads to decreases in breathing frequency and increases in tidal volume (39). Thus a dynamic remodeling of the lung ECM may have resulted from increased oscillations in breathing pattern through mechanotransducing mechanisms. Accordingly, we observe here that the expression levels of collagen genes are increased in association with alveolar enlargement after leptin treatment. We postulate that increased gene expression levels of various collagens (Col1a1, Col3a1, and Col6a1) reflect the increased tensile stress of greater tidal breaths and the mechanical requirements of enlarged alveoli.
Although potential molecular mechanisms by which leptin-induced lung growth occurs are not clear, these mechanisms are likely a complex interaction of direct and indirect effects of leptin on the lung. For example, indirect mechanotransducing stimuli can induce secretion of various growth factors that accelerate ECM remodeling (8, 45). Tensile force-mediated upregulation of the α1 procollagen gene (Col1a1) was found to depend on the release of transforming growth factor-β (17). In the present study, gene expression changes in Tieg1 after leptin treatment supports the role of a transforming growth factor-β-dependent signal pathway in this remodeling process (22, 52). Our study also found increased expression levels of the Stat1 gene after leptin treatment, suggesting that direct activation of the JAK-STAT signaling pathway mediated through leptin receptors may be important (9, 13, 48). While it is well established that leptin receptors are located in the pulmonary parenchyma and in bronchiolar epithelium between birth and adulthood in the mouse (6, 15, 53), Lepr gene expression was unchanged in ob/ob mice with leptin treatment. Furthermore, the increase in Mmp2 gene expression in the lung following leptin treatment is consistent with another study (25) and implies a role for Mmp2 in the lung ECM remodeling process. Both collagen and elastin fibers in the lung are protease targets known to be the focus of altered Mmp2 activity (2, 30). Taken together, the bioinformatic results from microarray analysis suggest that leptin treatment appears to significantly upregulate collagen (Col1a1) gene expression through leptin-receptor stimulation (40). Lung ECM remodeling processes induced by leptin might also require the upregulation Serpinh1 gene expression. The Serpinh1 protein is one of a family of serine protease inhibitors, but is the only member void of antiprotease activity (36). Instead, Serpinh1 plays an essential role as a chaperone for the normal assembly of collagen synthesis (36, 49). Although it is unclear how this complex interaction commences, there may be an additional influential modification in the activation state of IGF-I signaling through upregulation of Igfbp2 gene expression. To the contrary, the gene expression levels of Igf1 and Igfbp4 were not altered following leptin treatment. Similarly, gene expression levels of the surfactant proteins were unchanged with leptin.
While the conclusions of the present study are supported by previous work (24) showing that antenatal leptin treatment significantly increases fetal lung weight and the number of type II alveolar cells, the present results are inconsistent with other studies. For example, differences in respiratory system mechanics between ob/ob and +/+ mice observed in the present study are somewhat unique relative to the study by Riveria-Sanchez and colleagues (37). These investigators examined pulmonary mechanics in ob/ob and +/+ mice at 8–12 wk of age following filtered air or acute ozone exposure. In their study, mice exposed to filtered air showed comparable mechanical properties of H and G between ob/ob and +/+ mice. Although the reasons for the discrepancies between theirs and the present study remain somewhat elusive, it is well established from this and another study (47) that Vl values in ob/ob mice are substantially reduced by 25–40% relative to those in +/+ mice, given airway pressures ≥30 cmH2O. Given this factor alone, decreased Vl values in ob/ob mice likely lead to increases in lung elastance compared with the other genotypic groups. Although Vl values were not measured in the study by Riveria-Sanchez et al. (37), the absence of significant differences in elastance between ob/ob and +/+ mice is somewhat unexpected.
One principal distinction between the present study and that of Riveria-Sanchez et al. (37) is the difference in the surgical preparation. The surgical technique used by Riveria-Sanchez exposed the lung of each animal studied to atmospheric pressure, presumably eliminating the chest wall effects on lung mechanics. In contrast, the method principally used in the present study left the respiratory system intact, and, therefore, the mechanical influences of the chest wall and lung are inseparable. This approach led from a previous study in our laboratory (47), whereby the effects of the chest wall on Vl values and mechanical properties were examined using only quasi-static PV relationships. The results showed no effect of the chest wall on Vl or static compliance measurements in any of the ob/ob and control groups tested. More recently, Ito and colleagues (21) showed a significant depression on respiratory impedance parameters, including Raw, G, H, and η, with an opened-chest compared with a closed-chest preparation. In studies addressing the same question here, respiratory impedance measurements were repeated in ob/ob and +/+ mice at 10 wk of age with the chest closed and opened, as described previously (21). The results (Fig. 2) from +/+ mice were comparable with those of Ito et al. (21), suggesting that there is a small but significant decline in each impedance parameter with the chest wall opened compared with the chest wall intact. However, there were no detectable differences between the open and closed chest preparation in ob/ob mice, with the exception of η (i.e., hysteresivity). Therefore, it is possible that the physical presence of fat deposition within and around the thoracic cavity affects the mechanical interaction between the chest wall and lung differently in ob/ob compared with +/+ mice. Future studies will be required to determine how this fat deposition plays a role in altering respiratory system mechanics in ob/ob mice.
In conclusion, our results demonstrate the adverse effects of leptin deficiency on postnatal lung development, which are persistent and involve decreased Vl and alveolar Sa during early postnatal development. Leptin deficiency also inhibits the successive alveolar enlargement later in lung development. Moreover, leptin treatment after 4 wk of age increases Vl and alveolar Sa by stimulating alveolar enlargement, but does not ameliorate lung elastic properties. These aspects of lung remodeling are accompanied by a gene expression profile aimed to activate lung ECM turnover. These adverse effects of leptin deficiency on lung development in ob/ob mice are dissociated from reductions in metabolic rate. Since it is clear that Vl and lung architecture differ in ob/ob mice before 2 wk of age, other essential postnatal modifications are affected by leptin deficiency that impair the regulation of alveologenesis.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL10342 and HL081205 (S. Biswal). National Institute of Environmental Health Sciences center microarray core is supported by Grant P30 ES 03819.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ad Hoc Statement Committee, American Thoracic Society. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 170: 319–343, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Ambalavanan N, Nicola T, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res 63: 26–32, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat 124: 131–151, 1977. [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki N, Kawamura M, Matsuda T. Lactation-dependent down regulation of leptin production in mouse mammary gland. Biochim Biophys Acta 1427: 298–306, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Baratta M Leptin–from a signal of adiposity to a hormonal mediator in peripheral tissues. Med Sci Monit 8: RA282–RA292, 2002. [PubMed] [Google Scholar]
- 6.Bergen HT, Cherlet TC, Manuel P, Scott JE. Identification of leptin receptors in lung and isolated fetal type II cells. Am J Respir Cell Mol Biol 27: 71–77, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bozanich EM, Collins RA, Thamrin C, Hantos Z, Sly PD, Turner DJ. Developmental changes in airway and tissue mechanics in mice. J Appl Physiol 99: 108–113, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Breen EC Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J Appl Physiol 88: 203–209, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Bromberg JF Activation of STAT proteins and growth control. Bioessays 23: 161–169, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Burri PH The postnatal growth of the rat lung. 3. Morphology. Anat Rec 180: 77–98, 1974. [DOI] [PubMed] [Google Scholar]
- 11.Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci USA 98: 10630–10635, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes 45: 1455–1462, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Cauzac M, Czuba D, Girard J, Hauguel-de Mouzon S. Transduction of leptin growth signals in placental cells is independent of JAK-STAT activation. Placenta 24: 378–384, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Cobb J, Duboule D. Comparative analysis of genes downstream of the Hoxd cluster in developing digits and external genitalia. Development 132: 3055–3067, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Dal Farra C, Zsurger N, Vincent JP, Cupo A. Binding of a pure 125I-monoiodoleptin analog to mouse tissues: a developmental study. Peptides 21: 577–587, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Galambos C, Demello DE. Regulation of alveologenesis: clinical implications of impaired growth. Pathology 40: 124–140, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez JA, Perr HA. Mechanical stretch modulates TGF-β1 and α1(I) collagen expression in fetal human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 277: G1074–G1080, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa T, Ohara T, Makino S. Genetic typing of the mouse ob mutation by PCR and restriction enzyme analysis. Exp Anim 46: 75–78, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci USA 94: 11073–11078, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito S, Lutchen KR, Suki B. Effects of heterogeneities on the partitioning of airway and tissue properties in normal mice. J Appl Physiol 102: 859–869, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Johnsen SA, Subramaniam M, Janknecht R, Spelsberg TC. TGFbeta inducible early gene enhances TGFbeta/Smad-dependent transcriptional responses. Oncogene 21: 5783–5790, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan ML, Leveille GA. Core temperature, O2 consumption, and early detection of ob-ob genotype in mice. Am J Physiol 227: 912–915, 1974. [DOI] [PubMed] [Google Scholar]
- 24.Kirwin SM, Bhandari V, Dimatteo D, Barone C, Johnson L, Paul S, Spitzer AR, Chander A, Hassink SG, Funanage VL. Leptin enhances lung maturity in the fetal rat. Pediatr Res 60: 200–204, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Lee MP, Madani S, Sekula D, Sweeney G. Leptin increases expression and activity of matrix metalloproteinase-2 and does not alter collagen production in rat glomerular mesangial cells. Endocr Res 31: 27–37, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Loraine AE, Shigeta R, Cline M, Cheng J, Valmeekam V, Sun S, Kulp D, Siani-Rose MA. NetAffx: Affymetrix probesets and annotations. Nucleic Acids Res 31: 82–86, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 26: 1407–1433, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Mariani TJ, Reed JJ, Shapiro SD. Expression profiling of the developing mouse lung: insights into the establishment of the extracellular matrix. Am J Respir Cell Mol Biol 26: 541–548, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Massaro D, Massaro GD. Invited Review: pulmonary alveoli: formation, the “call for oxygen,” and other regulators. Am J Physiol Lung Cell Mol Physiol 282: L345–L358, 2002. [DOI] [PubMed] [Google Scholar]
- 30.McNulty M, Spiers P, McGovern E, Feely J. Aging is associated with increased matrix metalloproteinase-2 activity in the human aorta. Am J Hypertens 18: 504–509, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Misra V, Lee H, Singh A, Huang K, Thimmulappa RK, Mitzner W, Biswal S, Tankersley CG. Global expression profiles from C57BL/6J and DBA/2J mouse lungs to determine aging-related genes. Physiol Genomics 31: 429–440, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Mistry AM, Swick A, Romsos DR. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol Regul Integr Comp Physiol 277: R742–R747, 1999. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 159: 1477–1484, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Osburn WO, Wakabayashi N, Misra V, Nilles T, Biswal S, Trush MA, Kensler TW. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys 454: 7–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Ragg H The role of serpins in the surveillance of the secretory pathway. Cell Mol Life Sci 64: 2763–2770, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol 96: 2200–2206, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Rucker RB, Dubick MA. Elastin metabolism and chemistry: potential roles in lung development and structure. Environ Health Perspect 55: 179–191, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagawa N, Yura S, Itoh H, Kakui K, Takemura M, Nuamah MA, Ogawa Y, Masuzaki H, Nakao K, Fujii S. Possible role of placental leptin in pregnancy: a review. Endocrine 19: 65–71, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Saxena NK, Saliba G, Floyd JJ, Anania FA. Leptin induces increased alpha2(I) collagen gene expression in cultured rat hepatic stellate cells. J Cell Biochem 89: 311–320, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherle W A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26: 57–60, 1970. [PubMed] [Google Scholar]
- 42.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem 285: 194–204, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Soutiere SE, Tankersley CG, Mitzner W. Differences in alveolar size in inbred mouse strains. Respir Physiol Neurobiol 140: 283–291, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol 98: 1892–1899, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka R, Ludwig MS. Changes in viscoelastic properties of rat lung parenchymal strips with maturation. J Appl Physiol 87: 2081–2089, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Tankersley C, Kleeberger S, Russ B, Schwartz A, Smith P. Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol 81: 716–723, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Tankersley CG, O'Donnell C, Daood MJ, Watchko JF, Mitzner W, Schwartz A, Smith P. Leptin attenuates respiratory complications associated with the obese phenotype. J Appl Physiol 85: 2261–2269, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Tartaglia LA The leptin receptor. J Biol Chem 272: 6093–6096, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Tasab M, Batten MR, Bulleid NJ. Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J 19: 2204–2211, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenney SM, Remmers JE. Comparative quantitative morphology of the mammalian lung: diffusing area. Nature 197: 54–56, 1963. [DOI] [PubMed] [Google Scholar]
- 51.Torday JS, Rehan VK. Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am J Physiol Lung Cell Mol Physiol 283: L130–L135, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Tsubone T, Moran SL, Subramaniam M, Amadio PC, Spelsberg TC, An KN. Effect of TGF-beta inducible early gene deficiency on flexor tendon healing. J Orthop Res 24: 569–575, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol 365: 273–279, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Veness-Meehan KA, Pierce RA, Moats-Staats BM, Stiles AD. Retinoic acid attenuates O2-induced inhibition of lung septation. Am J Physiol Lung Cell Mol Physiol 283: L971–L980, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Willet KE, McMenamin P, Pinkerton KE, Ikegami M, Jobe AH, Gurrin L, Sly PD. Lung morphometry and collagen and elastin content: changes during normal development and after prenatal hormone exposure in sheep. Pediatr Res 45: 615–625, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]