Abstract
Spaceflight and bed rest (BR) result in losses of muscle mass and strength. Resistance training (RT) and amino acid (AA) supplementation are potential countermeasures to minimize these losses. However, it is unknown if timing of supplementation with exercise can optimize benefits, particularly with energy deficit. We examined the effect of these countermeasures on body composition, strength, and insulin levels in 31 men (ages 31–55 yr) during BR (28 days) followed by active recovery (14 days). Subjects were randomly assigned to essential AA supplementation (AA group, n = 7); RT with AA given 3 h after training (RT group, n = 12); or RT with AA given 5 min before training (AART group, n = 12). Energy intake was reduced by 8 ± 6%. Midthigh muscle area declined with BR for the AA > RT > AART groups: −11%, −3%, −4% (P = 0.05). Similarly, greatest losses in lower body muscle strength were seen in the AA group (−22%). These were attenuated in the exercising groups [RT (−8%) and AART (−6%; P < 0.05)]. Fat mass and midthigh intramuscular fat increased after BR in the AA group (+3% and +14%, respectively), and decreased in the RT (−5% and −4%) and AART groups (−1 and −5%; P = 0.05). Muscle mass and strength returned toward baseline after recovery, but the AA group showed the lowest regains. Combined resistance training with AA supplementation pre- or postexercise attenuated the losses in muscle mass and strength by approximately two-thirds compared with AA supplement alone during BR and energy deficit. These data support the efficacy of combined AA and RT as a countermeasure against muscle wasting due to low gravity.
Keywords: resistance exercise training, muscle strength, body composition, spaceflight, amino acid supplementation
spaceflight and models of weightlessness and muscle disuse, such as bed rest, result in pronounced losses of skeletal muscle mass, strength, and function (6, 9, 11, 17, 19, 21, 34). During spaceflight this can adversely affect astronauts’ physical performance and their ability to perform extravehicular activities and to respond to emergency situations (31). This creates significant challenges for long-term spaceflight and missions to extraterrestrial gravitational fields such as Mars. It may also have lasting consequences on astronauts’ health on return to Earth, including increased risk of disability, morbidity, and mortality (3). The loss of muscle mass that occurs with weightlessness is primarily the result of reduced protein synthesis (5, 11). Muscle loss may be further exacerbated by inadequate energy intake or anorexia that is seen with spaceflight (31, 33). Insulin sensitivity also appears to be reduced with weightlessness and may also modulate the plasticity of skeletal muscle, although the mechanism by which this occurs is not well understood (37).
Exercise and dietary interventions, particularly essential amino acids, have been shown to attenuate the loss of muscle mass and strength seen with bed rest (1, 2, 4, 12, 20, 26); however, these interventions have not been investigated with energy deficit. In addition, these countermeasures have not been completely successful in preventing the loss of muscle mass and function during bed rest (33). There is conflicting information regarding the effects of timed amino acid supplementation during resistance training. Some studies suggest that an oral protein supplement taken immediately before exercise may provide a greater stimulus for muscle protein synthesis because it increases delivery of amino acids to the exercising muscles (10, 28, 36). In contrast, other investigators have shown no additional benefit to providing an amino acid supplement between 1 and 3 h after exercise (28). To date, there is no information regarding the possibility for synergy between these countermeasures (e.g., resistance training and amino acid supplementation) or what their optimal timing and sequence should be during bed rest. In addition, little is known about the impact of these countermeasures after return to gravity or ambulation. Rehabilitation is important after prolonged episodes of spaceflight and bed rest. In cases of spaceflight, early rehabilitation may help counteract the pronounced losses of muscle mass and function resulting from the adaptive changes of skeletal muscle to a microgravity environment (38).
This study was undertaken to test the combined effects of resistance training and essential amino acid supplementation on body composition, muscle strength, and insulin levels after 28 days of bed rest and 14 days of active recovery in healthy male subjects. During bed rest and recovery, energy intake was decreased by ∼8% in all study subjects in order to simulate anorexia of spaceflight (33). Our working hypotheses were 1) that consumption of an essential amino acid supplement 5 min before resistance exercise training would be more beneficial in maintaining muscle mass and strength during bed rest than an essential amino acid supplement provided 3 h after exercise or an essential amino acid supplement provided alone with no resistance exercise; and 2) that provision of the essential amino acid supplement before exercise would aid in faster recovery after 28 days of bed rest compared with amino acid supplementation 3 h after exercise or amino acid supplementation with no exercise. In addition, we were interested in investigating the association between insulin levels, body composition, and muscle strength in this study.
MATERIALS AND METHODS
Study Design
Thirty one healthy men (aged 30–55 yr old) with a body mass index between 23 and 31 kg/m2, with no contraindications to exercise and no dietary restrictions that prevented consumption of the study diet or amino acid supplement, participated in the study. Potential participants were excluded if they had contraindications to the exercise regimen (myocardial infarction within the last 6 mo, unstable angina, aortic aneurysm, or amputation); diabetes or a glucose tolerance test indicative of impaired glucose tolerance; stroke; chronic inflammatory diseases, such as rheumatoid arthritis or inflammatory bowel disease; arthritis limiting joint mobility or exercise; anemia; abnormal kidney or liver function; or inadequate venous access. People taking anticoagulants, glucocorticoids, insulin, or hypoglycemic agents, beta blockers, and angiotensin-converting enzyme (ACE) inhibitors were also excluded.
Eligible subjects reported to the Jean Mayer USDA Human Nutrition Research Center for Aging (HNRCA) on day 1 of the study and were residents at our Metabolic Research Unit for the duration of the study (49 days). Study phases included baseline (acclimatization and testing for 7 days), bed rest (28 days), and active recovery (14 days). After baseline, before bed rest, subjects were randomized into one of three groups: essential amino acid supplementation alone (AA, n = 7), resistance training with AA supplementation provided 3 h after exercise (RT, n = 12), and resistance training with AA supplementation provided 5 min before exercise (AART, n = 12). The study was approved by the Institutional Review Board at Tufts Medical Center in Boston, MA, and written informed consent was obtained from all subjects.
Bed Rest
All subjects underwent bed rest in the supine position for 28 days at the HNRCA. Bed rest was monitored using video cameras to ensure compliance. Subjects were allowed to sit up straight once a day for a bowel movement. All other activities were performed in the supine position, including eating, bathing, dressing, urinating, reading, and TV/computer viewing.
Energy Intake
During baseline testing, all subjects were provided a weight maintenance diet consisting of 15% energy from protein, 54% from carbohydrates, and 33% from fat. Energy expenditure was estimated from resting metabolic rate. During the bed rest and recovery phases, subjects’ total energy intake was reduced by 8 ± 6% while maintaining the same macronutrient intake that was provided during the baseline phase. This was designed to mimic spaceflight anorexia (33). Calories were adjusted as needed to maintain an approximate weight loss of 4% from baseline. For this, subject's body weight was monitored daily, and weekly dietary adjustments were made as needed if changes in body weight persisted from more than 3 consecutive days. Food was provided by the HNRCA metabolic research kitchen on a 3-day rotating cycle supervised by the research dietitian. Food and dietary analyses were performed using the Minnesota Nutrient Data System (NDS) (Minneapolis, MN).
Essential Amino Acid Supplement
An amino acid supplement drink providing 15 g of essential amino acids in 35 g sucrose (to improve palatability) dissolved in 500 ml water was given once per day, 6 days of the week, during bed rest and recovery, on the same days that the RT and AART groups exercised. The supplement drink schedule was kept the same in the nonexercising AA group, or 6 days/wk. The essential amino acid supplement composition was as follows: 1.4 g l-isoleucine, 1.3 g l-histidine, 2.8 g l-leucine, 2.4 g l-lysine, 1.5 g l-methionine, 2.4 g l-phenylalanine, 2.0 g l-threonine, and 1.2 g l-valine. This supplement provided an additional 200 kcal/day for each subject. The composition of the essential amino acids in the supplement approximated the distribution of amino acids required to increase the intracellular concentrations of essential amino acids in proportion to their respective contributions to the synthesis of muscle protein (26). The composition and amount of the supplement were based on the beneficial effects observed in previous bed rest studies (26). The amino acid supplement was given 5 min before exercise (AART group), 3 h after exercise (RT group), or at the same time as AART in the nonexercising AA group. All exercise sessions were carried out in the morning (starting at 9 AM) and staggered by 1 h to accommodate up to three exercise subjects while having individualized exercise supervision. Exercise was not performed on Sundays, in keeping with current spaceflight schedules. All subjects received breakfast at 7 AM and lunch between 12 and 1 PM. Therefore, RT subjects received the amino acid supplement 3 h postexercise between 12 and 2 PM.
Resistance Training
During bed rest the resistance training protocol was performed by the RT and AART groups only. Subjects were transported to the exercise room on a stretcher and maintained in the supine position at all times. Subjects exercised for ∼1 h/day, 6 days/wk. A total of 24 exercise sessions was performed during the bed rest phase. A progressive, moderate- to high-intensity resistance training protocol was performed as a split routine with alternating days of lower body and upper body exercises. All exercises were performed in the horizontal position using the Shuttle Accel (Contemporary Design, Glacier, WA). Resistance was provided by elastic cords attached from the stationary frame to the sliding carriage of the Shuttle Accel. Resistance for each exercise was quantified as a function of the number of elastic cords and the distance moved by the sliding carriage. For example, an exercise using one elastic cord in which the Shuttle carriage traveled a distance of 20 in. generated a force of 24 lbs. If two elastic cords were used during the same movement, the force generated was equal to 48 lbs. The Shuttle Accel had 10 elastic cords, which provide a maximum resistance of 350 lbs when the sliding carriage moved 36 in. Most exercises in the protocol were performed in the 12- to 18-in. range and therefore had a maximum resistance of ∼250 lbs. The Shuttle Accel was calibrated weekly using a strain gauge to ensure that each cord provided the specified resistance, and cords were replaced as needed to maintain an adequate training stimulus.
The target exercise intensity was between 70 and 80% of one-repetition maximum (1RM) as estimated by the OMNI rating of perceived exercise 10 category scale (29). Seven to eight resistance exercises targeting major muscle groups and joint actions were performed during each exercise session. Upper body exercises included pull-ups, pull-over, triceps press, chest fly, shoulder press, biceps curl, upright row, and lateral arm raise. Lower body exercises included squats, single leg squats, diagonal jump, calf raise, single-leg hip extension, leg curl, and single-leg hip abduction. The order of exercises was specifically choreographed to minimize positional and postural changes by the subject, thereby minimizing the potential effects of gravity on exercise performance. The exercise protocol required significant involvement and supervision by study staff to ensure that the exercises were performed properly, effectively, and safely and that subject compliance with horizontal body positioning was maintained.
Active Recovery
During the recovery phase, all three groups performed 15–30 min of treadmill exercise at intensity between 60 and 85% of age-predicted maximum heart rate, 3 days/wk, with the duration and intensity of exercise increasing gradually over 14 days. This exercise prescription was intended to simulate normal ambulation, which would occur after return to Earth's gravity from spaceflight. In addition, the RT and AART groups performed six resistance training sessions on alternating days using Cybex Selectorized Equipment (Fresno, CA). Three sets of eight repetitions were performed for five exercises: leg press, chest press, knee extension and flexion, and lat pull-down, with intensity increasing over the 14-day recovery phase, constituting active recovery.
Outcome Measures
All measures were performed at baseline (before randomization), after 28 days of bed rest, and after 14 days of active recovery. All testing was performed by investigators blinded to subject's group assignment, with the exception of muscle strength.
Body composition.
Body mass index was calculated from body weight and height (as kg/m2). Whole body and regional lean and fat mass were determined by dual-energy X ray absorptiometry (DXA) using a Hologic QDR2000 (Waltham, MA) scanner operating in array mode with software 5.64A and coefficients of variation of 1.4% and 1.8% for total lean and fat mass, respectively (8). Midthigh muscle and fat areas were determined by image analysis of computerized tomography (CT) scan of the nondominant leg at the Radiology Department at Tufts New England Medical Center with a Siemens Volume Zoom Scanner (Siemens). For all scans a 10-mm CT slice was taken at the precise level of the midpoint of the femur using 100 kV, 110 mA, and 1-s exposure using a standard algorithm (24). The same display field of view (DFOV) was used to take the CT images during consecutive visits, and it was also used for scaling the images during analysis. The images were analyzed by a single blinded observer according to optical densities for quantification of cross-sectional areas of fat and muscle compartments by selecting pixels within a given Hounsfield units (HU) range to the nearest 0.1 cm2 using the Slice-O-Matic Software (Tomovision, Montreal, Canada). Skeletal muscle attenuation was determined by measuring the mean value of all pixels within the range of 0 to 100 HU, while adipose tissue areas were in the range of −150 to −50 HU (23).
Muscle strength.
Dynamic muscle strength was determined by 1RM testing at baseline, and after the bed rest and recovery phases using Cybex Selectorized Equipment (13). A summary score for lower-body 1RM was calculated using values for leg press, leg extension, and knee flexion exercises. Upper-body 1RM was calculated using values for the chest press and lat pull-down exercises.
Insulin levels.
Fasting insulin levels were measured by radioimmunoassay (ICN Biomedical, Costa Mesa, CA) with a coefficient of variation of 5%.
Statistical Analysis
Statistical analysis was performed for subjects who completed the bed rest and recovery phases using SPSS 15.0 for Windows (SPSS, Evanston, IL). Differences in baseline characteristics among groups were assessed by ANOVA. Continuous nonnormally distributed variables were log-transformed for analyses. Data are shown as mean and SD, except for nonnormally distributed variables (insulin), for which group median and interquartile ranges (IQR) are shown. Repeated-measures ANOVA with energy intake as a covariate were used to assess time-by-group interactions in outcomes measured from baseline to the end of bed rest, from the end of bed rest to the end of recovery, and from baseline to the end of recovery. Statistically significant changes over time within group are also shown. Additionally, Tukey's post hoc adjustments for multiple comparisons were performed. Pearson's coefficient of correlation was used to assess univariate associations between insulin levels and outcome measures. Results were considered statistically significant with a two-tailed P value ≤ 0.05.
RESULTS
Baseline Characteristics
Subject characteristics are shown in Table 1. There were no significant differences among groups at baseline in any of the study characteristics.
Table 1.
Baseline subject characteristics
| Characteristic | AA (n = 7) | RT (n = 12) | AART (n = 12) | P Value |
|---|---|---|---|---|
| Age, yr | 44±9 | 42±8 | 44±6 | 0.80 |
| Body weight, kg | 80.4±7.5 | 83.0±8.8 | 83.3±8.3 | 0.74 |
| Height, cm | 178±9 | 179±7 | 181±5 | 0.64 |
| Body mass index, kg/m2 | 25.6±3.5 | 26.3±2.7 | 25.5±2.3 | 0.73 |
| Energy intake, kcal·kg−1·day−1 | 31±3 | 30±2 | 30±2 | 0.57 |
| Protein intake, g·kg−1·day−1 | 1.16±0.11 | 1.13±0.06 | 1.12±0.08 | 0.86 |
Data are means ± SD. Study groups: AA, essential amino acid supplementation alone; RT, resistance exercise training with AA supplement provided 3 h postexercise; AART, resistance exercise training with AA supplementation provided 5 min before exercise. P values represent ANOVA.
Energy Intake
On average, energy intake was reduced by 8 ± 6% in all three study groups during bed rest and recovery (P = 0.89). As shown in Table 2, reductions in energy intake were not different among groups; however, over time, reductions within each group were statistically significant (P < 0.01, time effect).
Table 2.
Energy intake
| Study Groups | Study Phases |
Percent Changes | ||||
|---|---|---|---|---|---|---|
| Baseline (B) | Bed Rest (BR) | Recovery (R) | BR − B | R − BR | R − B | |
| Energy intake, kcal·kg−1·day−1 | ||||||
| AA | 31±3 | 29±4 | 27±3 | −6±7% | −7±9% | −13±9% |
| RT | 30±2 | 28±5 | 28±3 | −7±8% | 0±8% | −7±8% |
| AART | 30±2 | 28±4 | 27±3 | −7±8% | −4±8% | −10±10% |
| P value, time-by-group interactions: | 0.86 | 0.20 | 0.54 | |||
Data are means ± SD; n = 7 for AA group, n = 12 for RT group, and n = 12 for AART group. See Table 1 for description of study groups. P values shown represent time-by-group interactions for comparisons between end of bed rest and baseline, end of recovery and end of bed rest, end of recovery and baseline, as determined by repeated-measures ANOVA.
Body Composition
Body composition measures are shown in Table 3. Body weight decreased from baseline to the end of the bed rest phase in all groups (P < 0.01) and remained significantly lower by the end of the recovery phase (P < 0.01) with no significant time-by-group interactions. Similarly, lean body mass, measured by DXA, also decreased in all groups from baseline to the end of bed rest (each P < 0.01), with no statistically significant differences among groups. After the end of bed rest, lean mass increased in all three groups (P < 0.01). However, lean mass remained significantly lower than baseline values (P < 0.01) in all groups, with no statistically significant differences among groups.
Table 3.
Body composition
| Study Groups | Study Phases |
Percent Changes | ||||
|---|---|---|---|---|---|---|
| Baseline (B) | Bed Rest (BR) | Recovery (R) | BR − B | R − BR | R − B | |
| Body weight, kg | ||||||
| AA | 80.4±2.9 | 76.7±2.5 | 78.5±2.6 | −4.6±0.8% | 2.3±0.9% | −2.4±1.9% |
| RT | 83.0±2.7 | 80.8±2.5 | 81.2±2.5 | −2.7±1.2% | 0.5±1.1% | −2.1±2.1% |
| AART | 83.3±2.4 | 81.3±2.2 | 81.0±2.3 | −2.4±1.1% | −0.4±1.1% | −2.7±0.6% |
| P value, time-by-group interactions: | 0.60 | 0.12 | 0.79 | |||
| Lean body mass by DXA, kg | ||||||
| AA | 59.5±2.5 | 57.7±2.4 | 58.1±2.3 | −3.0±1.8% | 0.7±2.5% | −2.3±2.2% |
| RT | 63.6±1.9 | 62.6±1.9 | 63.0±2.0 | −1.5±1.8% | 0.6±1.8% | −0.9±2.3% |
| AART | 63.7±1.7 | 62.2±1.6 | 63.3±1.8 | −2.3±1.2% | 1.8±1.3% | −0.6±1.2% |
| P value, time-by-group interactions: | 0.34 | 0.75 | 0.69 | |||
| Leg lean mass by DXA, kg | ||||||
| AA | 9.9±0.4 | 9.2±0.4 | 9.5±1.0 | −7.1±1.5% | 3.3±3.5% | −4.0±3.4% |
| RT | 10.8±0.4 | 10.3±0.3 | 10.6±1.3 | −4.6±1.9% | 3.0±1.6% | −1.9±1.8% |
| AART | 10.6±0.3 | 10.1±0.3 | 10.4±1.1 | −4.7±1.7% | 3.0±1.9% | −1.9±1.9% |
| P value, time-by-group interactions: | 0.05 | 0.96 | 0.21 | |||
| Arm lean mass by DXA, kg | ||||||
| AA | 3.8±0.3 | 3.8±0.2 | 3.7±0.3 | 0±3.9% | −2.6±3.3% | −2.6±4.5% |
| RT | 4.1±0.2 | 4.3±0.2 | 4.2±0.2 | 4.9±3.1% | −2.3±3.5% | 2.4±2.9% |
| AART | 4.3±0.2 | 4.3±0.2 | 4.4±0.2 | 0±3.2% | 2.3±3.0% | 2.3±2.3% |
| P value, time-by-group interactions: | 0.09 | 0.67 | 0.06 | |||
| Body fat by DXA, % | ||||||
| AA | 25.0±1.0 | 25.9±1.1 | 25.5±1.2 | 3.6±2.3% | −1.5±3.4% | 2.0±3.7% |
| RT | 23.0±1.3 | 21.8±1.3 | 21.0±1.2 | −5.2±4.0% | −3.7±2.4% | −8.7±3.8% |
| AART | 20.6±1.3 | 20.4±1.3 | 19.7±1.3 | −1.0±3.8% | −3.4±2.8% | −4.4±3.2% |
| P value, time-by-group interactions: | 0.002 | 0.09 | <0.001 | |||
| Midthigh muscle area by CT, cm2 | ||||||
| AA | 153.1±7.1 | 135.8±6.6 | 142.2±8.0 | −11.3±3.9% | 4.7±5.8% | −7.1±3.9% |
| RT | 168.4±6.4 | 163.1±6.8 | 165.8±7.4 | −3.1±2.9% | 1.6±3.2% | −1.5±3.9% |
| AART | 156.2±4.0 | 150.1±4.4 | 150.5±4.4 | −4.0±1.7% | 0.3±2.5% | −3.6±2.0% |
| P value, time-by-group interactions: | <0.001 | 0.13 | <0.001 | |||
| Midthigh intramuscular fat area by CT, cm2 | ||||||
| AA | 2.1±1.2 | 2.4±1.4 | 2.2±1.1 | 14.3±7.2% | −8.3±8.7% | 4.8±9.4% |
| RT | 2.2±1.2 | 2.1±1.1 | 1.9±1.0 | −4.5±9.7% | −9.5±9.7% | −13.6±9.1% |
| AART | 2.0±1.2 | 1.9±1.4 | 2.0±1.5 | −5.0±6.4% | 5.3±8.7% | 0±9.2% |
| P value, time-by-group interactions: | 0.04 | 0.24 | 0.05 | |||
| Midthigh subcutaneous fat area by CT, cm2 | ||||||
| AA | 44.0±18.9 | 47.1±18.8 | 46.4±16.4 | 7.0±11.3% | −1.5±5.2% | 5.4±8.5% |
| RT | 49.7±19.1 | 47.3±17.2 | 45.8±15.5 | −4.8±3.2% | −3.2±7.4% | −7.8±6.2% |
| AART | 42.5±12.5 | 40.1±11.9 | 39.4±12.1 | −5.6±6.6% | −1.7±5.6% | −7.3±7.8% |
| P value, time-by-group interactions: | 0.09 | 0.11 | 0.05 | |||
Data are means ± SD; n = 7 for AA group, n = 12 for group, and n = 12 for AART group. See Table 1 for description of study groups. DXA, dual X-ray absorptiometry, CT, computerized tomography. P values shown represent time-by-group interactions for comparisons between end of bed rest and baseline, end of recovery and end of bed rest, end of recovery and baseline, as determined by repeated measures ANOVA with energy intake as a covariate.
In contrast, there were significant group differences in the reductions in leg lean mass observed from baseline to the end of bed rest (P = 0.05, time-by-group interaction): there was a 7% decrease in leg lean mass in the AA group compared with lesser reductions of ∼5% in the exercise groups undergoing resistance training (RT and AART). The changes seen in the two resistance exercise groups were not statistically different based on post hoc analysis (P = 0.68). Arm lean mass also showed a trend for a time-by-group interaction from baseline to the end of bed rest (P = 0.09). Midthigh muscle area, measured by CT, decreased significantly in all groups from baseline to the end of bed rest (P < 0.001, time-by-group interaction) with greater reductions seen in the AA group (−11%) compared with RT (−3%) and AART (−4%) groups. By the end of recovery, muscle area was regained in all groups by +5%, +2%, and +0.3% for the AA, RT and AART groups, respectively, but it remained lower than baseline values. The changes in muscle area seen in the exercise groups (RT and AART) were not different in post hoc analysis (P = 0.11).
Percent body fat changes were significantly different among groups from baseline to the end of bed rest (P = 0.002, time-by-group interaction) with an increase seen in the AA group (+4%) compared with decreases observed in the RT (−5%) and AART group (−1%). The changes seen in the two resistance exercise groups were not statistically different (P = 0.39, post hoc analysis). Percent body fat changes from the end of the bed rest phase to the end of recovery were −1% in the AA group and −4% and −3% in the RT and AART groups, respectively (P = 0.09, time-by-group interaction), Significant group differences were also observed for the changes in midthigh intramuscular fat from baseline to the end of bed rest with increases seen in the AA group (+14%) compared with reductions in the RT (−4%) and AART groups (−5%; P = 0.04, time-by-group interaction), although there were no differences between the exercise groups (P = 0.73, post hoc analysis). Midthigh subcutaneous fat levels followed a similar trend with increases in the AA group (+7%) and reductions in both RT (−5%) and AART (−6%; P = 0.09, time-by-group interaction).
Muscle Strength
Average resistance training intensity was 45% and 85% of 1RM for the upper and lower body exercises, respectively. As shown in Table 4, lower body strength was significantly different among groups after bed rest (P = 0.01, time-by-group interaction) with greater decreases seen in the AA group (−22%) compared with the exercise groups [RT (−8%) and AART groups (−6%)]. There was no difference between the two resistance exercise groups by post hoc analysis (P = 0.34). There were also significant group differences in upper body strength from baseline to the end of bed rest (P < 0.001, time-by-group interaction). Increases in upper body strength of ∼5% were seen in both the RT and AART groups compared with a decrease in the AA group (−7%), but there were no statistical differences between exercise groups (P = 0.49, post hoc analysis).
Table 4.
Muscle strength
| Study Groups | Study Phases |
Percent Changes | ||||
|---|---|---|---|---|---|---|
| Baseline (B) | Bed Rest (BR) | Recovery (R) | BR − B | R − BR | R − B | |
| Lower body muscle strength, lbs | ||||||
| AA | 214±39 | 166±34 | 182±33 | −22.4±5.8% | 9.6±10.3% | −14.9±11.8% |
| RT | 236±42 | 217±42 | 233±42 | −8.0±11.3% | 7.4±12.6% | −1.4±7.8% |
| AART | 218±37 | 205±37 | 215±39 | −6.0±6.1% | 4.5±5.4% | −1.4±7.5% |
| P value, time-by-group interactions: | 0.01 | 0.49 | 0.04 | |||
| Upper body muscle strength, lbs | ||||||
| AA | 194±69 | 180±68 | 185±58 | −7.1±7.4% | 2.8±6.3% | −4.6±9.3% |
| RT | 233±49 | 245±48 | 245±49 | 5.1±8.8% | 0±7.1% | 5.1±4.8% |
| AART | 219±45 | 230±47 | 227±46 | 5.0±4.0% | −1.3±3.9% | 3.6±5.5% |
| P value, time-by-group interactions: | 0.001 | 0.36 | 0.02 | |||
Data are means ± SD; n = 7 for AA group, n = 12 for RT group, and n = 12 for AART group. See Table 1 for description of study groups. P values shown represent time-by-group interactions for comparisons between end of bed rest and baseline, end of recovery and end of bed rest, end of recovery and baseline, as determined by repeated-measures ANOVA with energy intake as a covariate.
Insulin Levels
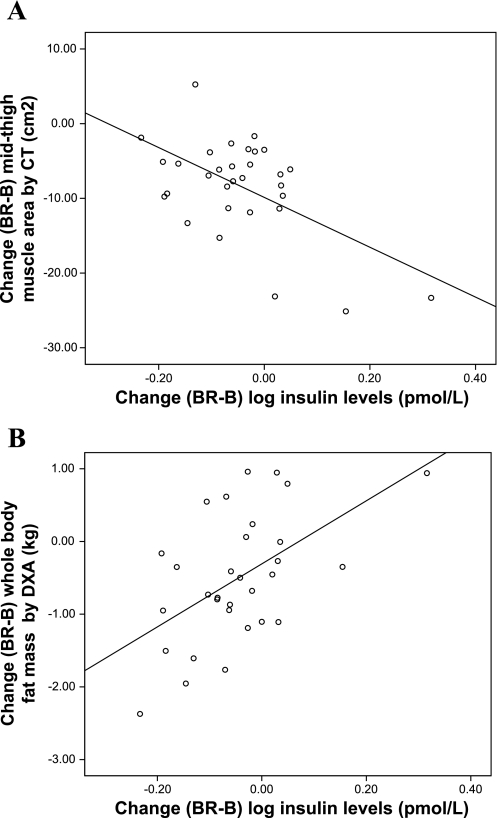
Median (interquartile range) values for plasma insulin are shown in Table 5. Insulin levels increased significantly in the AA group from baseline to the end of bed rest, while they significantly declined in the RT and AART groups (P < 0.001, time-by-group interaction) with no difference between the two exercise groups by post hoc analysis (P = 0.74). All groups showed a decrease in insulin levels from the end of bed rest to recovery (P = 0.01, time-by-group interaction). The changes in insulin levels from baseline to the end of bed rest were inversely associated with the changes in midthigh muscle area by CT (r = −0.55; P = 0.001, Fig. 1A). In contrast, changes in insulin levels from baseline to the end of bed rest were positively associated with whole body fat mass by DXA (r = 0.53; P = 0.002, Fig. 1B), and there was a trend toward a positive association with midthigh intramuscular fat area by CT (r = 0.33; P = 0.06).
Table 5.
Plasma insulin levels
| Study Groups | Study Phases |
Percent Changes | ||||
|---|---|---|---|---|---|---|
| Baseline (B) | Bed Rest (BR) | Recovery (R) | BR − B | R − BR | R − B | |
| Insulin levels, pmol/l | ||||||
| AA | 105 (44) | 120 (63) | 85 (53) | 14±23% | −29±19% | −19±22% |
| RT | 83 (39) | 74 (22) | 65 (27) | −11±18% | −12±21% | −22±23% |
| AART | 77 (26) | 65 (24) | 64 (32) | −16±17% | −1±19% | −17±23% |
| P value, time-by-group interactions: | <0.001 | 0.01 | 0.54 | |||
Insulin levels are group medians with interquartile range in parentheses, while percent changes are given as means ± SD; n = 7 for AA group, n = 12 for RT group, and n = 12 for AART group. See Table 1 for description of study groups. P values shown represent time-by-group interactions for comparisons between end of bed rest and baseline, end of recovery and end of bed rest, end of recovery and baseline, as determined by repeated-measures ANOVA with energy intake as a covariate.
Fig. 1.
Univariate linear association between the absolute changes in insulin levels from baseline (B) to the end of bed rest (BR) with the changes, during the same time period, in midthigh muscle area by computerized tomography (CT; r = −0.55, P = 0.001; A) and whole body fat mass by dual-energy X-ray absorptiometry (DXA; r = 0.53, P = 0.002; B) are shown. Log-transformed insulin levels were plotted and used for analysis.
DISCUSSION
This study was designed to test the combined effects of resistance training and essential amino acid supplementation (15 g daily) with energy deficit on body composition, muscle strength, and insulin levels during 28 days of bed rest followed by 14 days of active recovery. The mean reduction in energy intake achieved in all groups was ∼8%, comparable to the average noted after spaceflight (42). Our findings demonstrated that with negative energy balance, amino acid supplementation alone did not prevent the loss of muscle mass and strength seen with disuse. However, resistance exercise training combined with the essential amino acid supplement provided either 5 min before exercise or 3 h after exercise attenuated these losses to a similar extent during bed rest and resulted in the largest regains toward baseline values during recovery. On average, the RT and AART interventions reduced the impact of bed rest on muscle mass, muscle strength, and fat mass by about two-thirds. To our knowledge, this is the first study to investigate the combined effects of resistance exercise and timing of an essential amino acid supplement as a countermeasure of muscle disuse during bed rest in the setting of an energy deficit similar to that observed during spaceflight. While the timing of the AA provision does not seem to matter, these results suggest that both AA and RT should be considered as part of the countermeasure plans to prevent muscle wasting during spaceflight.
Essential amino acid supplementation at a dose similar to that provided in the present study (∼15 g/day), while consuming isoenergetic diets, has been shown to acutely stimulate muscle protein synthesis during 3-h administration in young and old ambulatory individuals (27), as well as in young adults undergoing acute hypercortisolemia and prolonged inactivity (28 days of bed rest) (25). In contrast, we observed that essential amino acid supplementation alone for a period of 24 of 28 days of bed rest, in subjects consuming a hypocaloric diet, did not prevent the loss of muscle mass and strength seen with inactivity. To our knowledge, only one other study has examined the effect of AA supplementation during bed rest (26). However, our study provided 0.2 g·kg−1·day−1 of AA, or one-third of the dose given to subjects by Paddon-Jones et al. (0.6 g·kg−1·day−1) with an isoenergetic diet (26). We found that, during negative energy balance, provision of essential amino acids for subjects in the AA group did not result in the expected anabolic stimulus capable of reducing the loss of muscle mass and strength. However, the combination of essential amino acid supplementation with resistance training led to more efficient utilization of the amino acid substrate, despite energy deficit and the low AA supplementation level. This was evidenced by the attenuated losses in muscle mass and strength observed when the two countermeasures were combined in the RT and AART groups.
In the present study the energy deficit achieved on all study groups during bed rest was −230 kcal/day, which is similar to the reported −240 kcal/day found in spaceflight (33). During spaceflight such negative energy balance is accompanied by weight loss, body fat loss, and protein loss (i.e., muscle mass loss). We found that subjects in the AA group, who did not exercise during bed rest, lost body weight and lean body mass but gained body fat. By comparison, subjects in the resistance exercise groups lost body weight and body fat but maintained more body protein. Changes in body fat during bed rest have shown conflicting results. Some have shown no change in body fat after 16 days of bed rest in subjects performing endurance exercises (33), while others have reported increases in body fat after 10 days of bed rest when subjects ate all food given to them (18). We found a significant decline in fat mass after 28 days of strict bed rest with a sustained loss of fat after active recovery in the resistance exercise groups, suggesting that resistance exercise prevents unhealthy shifts in body composition (i.e., loss of muscle mass and gain in fat mass) during bed rest. Possible explanations for the shifts in body composition we observed may be that energy intake in the AA group was sufficient (albeit reduced) without the concomitant anabolic effect that resistance exercise training would produce, given that this group did not exercise during bed rest; or may be related to the changes seen in insulin levels during the study period.
Previous studies of spaceflight and bed rest have shown that the body becomes more insulin resistant under weightlessness conditions (37). The mechanisms associated with altered regulation of insulin sensitivity during spaceflight are not known. However, it has been shown that excess triglyceride storage within skeletal muscle is associated with skeletal muscle insulin resistance (7, 16, 30). Insulin-resistant muscle may further contribute to fatty acid accumulation due to reduced mitochondrial enzyme function, defective muscle mitochondria, and reduced fatty acid oxidation (7). In the present study, we found that the change in insulin levels was inversely correlated with the change in midthigh muscle area, while it was positively associated with the changes seen in whole body and midthigh intramuscular fat.
We hypothesized that the ingestion of an essential amino acid supplement immediately before exercise (AART group) would have a synergistic effect, resulting in greater maintenance of muscle mass and strength compared with resistance exercise followed by the amino acid supplement 3 h later (RT group). However, we did not find this to be the case. It is possible that the lack of synergy between timed amino acid supplementation and resistance exercise observed in the AART group was related to the imposed energy deficit, which led to inability of the amino acid substrate to be utilized for muscle protein synthesis (22). Ingestion of essential amino acids immediately before exercise results in increased circulating plasma amino acid levels and greater stimulation of net muscle protein synthesis compared with observations when amino acids are ingested after exercise (35, 36). In contrast, other investigators have found that amino acid supplementation before exercise (without bed rest) provides no greater benefit than ingestion of the supplement after exercise (28). In conditions of prolonged bed rest, the loss of muscle mass has been shown to be associated with alterations in protein turnover (5) due primarily to reduce protein synthesis (11). Taken together, these data suggest that dysregulation in muscle protein turnover, particularly in protein synthesis, may be responsible for the loss of body protein with disuse and weightlessness. Our findings highlight the need for further research into key aspects of these countermeasures such as amino acid supplementation type and timing, and exercise modality and intensity that will be sufficient to maintain muscle mass and strength with the energy deficit seen during spaceflight.
An important strength of this study was the 14-day period of active recovery following bed rest. Previous studies have focused primarily on the in-flight bed rest or immediate, but not active, recovery period (32). Full recovery from spaceflight has been reported to take several months (14, 15). Our findings showed that after a short period of active recovery, all study groups regained muscle mass and strength, albeit levels remained below baseline values. However, since Stein et al. (32) reported that amino acid supplementation during bed rest may not affect protein kinetics during immediate (∼4 days) recovery, and our subjects did not return fully to baseline values, longer follow-up and recovery periods may be needed to fully recover after bed rest and spaceflight.
Some limitations of this study include the following. We focused on the effect of restricted energy intake in combination with dietary manipulation and resistance training as countermeasures to the loss of muscle mass and strength during bed rest. Therefore, by design this study did not have a control group receiving an adequate energy intake. Findings related to countermeasures of disuse and weightlessness, without the stressor of a low energy intake, have been extensively published (1, 2, 4, 10, 12, 20, 26, 28, 36) and thus were not addressed in the present study. Perhaps more importantly, we did not have an RT-only control group without any AA supplementation. This was because our chief aim was to determine whether timing of the AA supplement just before exercise was important to the ultimate effect. Our results clearly demonstrate the timing of supplementation is not important in this setting. The comparable results seen with RT and AART regardless of timing allow us to conclude that this combination is a useful countermeasure against bed rest-induced muscle atrophy, preventing or reversing about two-thirds of the effect compared with AA alone. It is an unanswered question whether RT alone could have done as well.
In conclusion, we demonstrated that the combination of resistance exercise training and essential amino acid supplementation conferred greater protection against the reductions in muscle mass and strength than an amino acid supplement alone, after 28 days of strict bed rest and 14 days of active recovery while consuming a hypocaloric diet. Shunting of amino acids away from muscle maintenance with negative energy balance may have contributed to an attenuation of the potential benefits reported for these countermeasures. Future studies are needed to test different combinations of these countermeasures, such as type and timing of supplementation and variety of exercise modalities and intensities, to understand the effects of negative energy balance on muscle mass and function during weightlessness and disuse.
GRANTS
This work was supported by the National Space Biomedical Research Institute (NSBRI) through NCC 9-58, by USDA Agriculture Research Service agreement 58-1950-9-001, and by National Institutes of Health GCRC Grant M01-RR-000054.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily represent the views of the USDA or any of the funding sources.
Acknowledgments
We are especially grateful for the kind and valuable cooperation of the volunteers who made this study possible. We like to thank the General Clinical Research Center (GCRC) and the Jean Mayer U.S. Department of Agriculture (USDA) Human Nutrition Research Center for Aging (HNRCA) staff for their help, and Shuttle 2000 Sports Health Equipment, for their assistance with the resistance training equipment. We are indebted to undergraduate and graduate students from Tufts University, the Universities of Massachusetts, and Northeastern University for their assistance with training supervision and data collection.
The results of this study have been presented in part at the Experimental Biology Meeting in San Francisco, CA, in April 2006.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akima H, Kubo K, Imai M, Kanehisa H, Suzuki Y, Gunji A, Fukunaga T. Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol Scand 172: 269–278, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Akima H, Ushiyama J, Kubo J, Tonosaki S, Itoh M, Kawakami Y, Fukuoka H, Kanehisa H, Fukunaga T. Resistance training during unweighting maintains muscle size and function in human calf. Med Sci Sports Exerc 35: 655–662, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin KM, Haddad F. Skeletal muscle plasticity: cellular and molecular responses to altered physical activity paradigms. Am J Phys Med Rehabil 81: S40–S51, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 84: 157–163, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bettany GE, Ang BC, Georgiannos SN, Halliday D, Powell-Tuck J. Bed rest decreases whole-body protein turnover in post-absorptive man. Clin Sci (Lond) 90: 73–75, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Bloomfield SA Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 29: 197–206, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18: 1144–1146, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Clasey JL, Hartman ML, Kanaley J, Wideman L, Teates CD, CB, Weltman A. Body composition by DEXA in older adults: accuracy and influence of scan mode. Med Sci Sports Exerc 29: 560–567, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Edgerton VR, Zhou MY, Ohira Y, Klitgaard H, Jiang B, Bell G, Harris B, Saltin B, Gollnick PD, Roy RR, Day MK, Greenisen M. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J Appl Physiol 78: 1733–1739, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol 535: 301–311, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Ferrando AA, Tipton KD, Bamman MM, Wolfe RR. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol 82: 807–810, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263: 3029–3034, 1990. [PubMed] [Google Scholar]
- 14.Gazenko OG, Grigor'ev AI, Egorov AD. [Medical studies concerning the program of long-term manned space flights on “Saliut-7”-“Soiuz-T” orbital complex]. Kosm Biol Aviakosm Med 24: 9–15, 1990. [PubMed] [Google Scholar]
- 15.Gazenko OG, Shul'zhenko EB, Grigor'ev AI, At'kov O, Egorov AD. [Medical studies during an 8-month flight on the orbital complex “Saliut-7”–“Soiuz-T”]. Kosm Biol Aviakosm Med 24: 9–14, 1990. [PubMed] [Google Scholar]
- 16.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52: 2191–2197, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Greenleaf JE, Kozlowski S. Physiological consequences of reduced physical activity during bed rest. Exerc Sport Sci Rev 10: 84–119, 1982. [PubMed] [Google Scholar]
- 18.Gretebeck RJ, Schoeller DA, Gibson EK, Lane HW. Energy expenditure during antiorthostatic bed rest (simulated microgravity). J Appl Physiol 78: 2207–2211, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Hikida RS, Gollnick PD, Dudley GA, Convertino VA, Buchanan P. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviat Space Environ Med 60: 664–670, 1989. [PubMed] [Google Scholar]
- 20.Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, Imai M, Suzuki Y, Gunji A, Kanehisa H, Fukunaga T. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol 84: 7–12, 2001. [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol 73: 2172–2178, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Millward DJ Macronutrient intakes as determinants of dietary protein and amino acid adequacy. J Nutr 134: 1588S-1596S, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85: 115–122, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Nelson M, Fiatarone M, Layne J, Trice I, CDE, Fielding R, Ma R, Pierson R, Evans W. Analysis of body-composition techniques and models for detecting change in soft tissue with strength training. Am J Clin Nutr 63: 678–686, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Aarsland A, Wolfe RR, Ferrando AA. The catabolic effects of prolonged inactivity and acute hypercortisolemia are offset by dietary supplementation. J Clin Endocrinol Metab 90: 1453–1459, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 89: 4351–4358, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286: E321–E328, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88: 386–392, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Robertson RJ, Goss FL, Rutkowski J, Lenz B, Dixon C, Timmer J, Frazee K, Dube J, Andreacci J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc 35: 333–341, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Shulman GI Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein TP Nutrition and muscle loss in humans during spaceflight. Adv Space Biol Med 7: 49–97, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Stein TP, Donaldson MR, Leskiw MJ, Schluter MD, Baggett DW, Boden G. Branched-chain amino acid supplementation during bed rest: effect on recovery. J Appl Physiol 94: 1345–1352, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Stein TP, Leskiw MJ, Schluter MD, Hoyt RW, Lane HW, Gretebeck RE, LeBlanc AD. Energy expenditure and balance during spaceflight on the space shuttle. Am J Physiol Regul Integr Comp Physiol 276: R1739–R1748, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki Y, Murakami T, Kawakubo K, Haruna Y, Takenaka K, Goto S, Makita Y, Ikawa S, Gunji A. Regional changes in muscle mass and strength following 20 days of bed rest, and the effects on orthostatic tolerance capacity in young subjects. J Gravit Physiol 1: P57–P58, 1994. [PubMed] [Google Scholar]
- 35.Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol Endocrinol Metab 276: E628–E634, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab 281: E197–E206, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Tobin BW, Uchakin PN, Leeper-Woodford SK. Insulin secretion and sensitivity in space flight: diabetogenic effects. Nutrition 18: 842–848, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Trappe S Effects of spaceflight, simulated spaceflight and countermeasures on single muscle fiber physiology. J Gravit Physiol 9: P323–P326, 2002. [PubMed] [Google Scholar]