Abstract
Noninvasive techniques for assessing cardiopulmonary function in small animals are limited. We previously developed a rebreathing technique for measuring lung volume, pulmonary blood flow, diffusing capacity for carbon monoxide (DlCO) and its components, membrane diffusing capacity (DmCO) and pulmonary capillary blood volume (Vc), and septal volume, in conscious nonsedated guinea pigs at rest. Now we have extended this technique to study guinea pigs during voluntary treadmill exercise with a sealed respiratory mask attached to a body vest and a test gas mixture containing 0.5% SF6 or Ne, 0.3% CO, and 0.8% C2H2 in 40% or 98% O2. From rest to exercise, O2 uptake increased from 12.7 to 25.5 ml·min−1·kg−1 while pulmonary blood flow increased from 123 to 239 ml/kg. The measured DlCO, DmCO, and Vc increased linearly with respect to pulmonary blood flow as expected from alveolar microvascular recruitment; body mass-specific relationships were consistent with those in healthy human subjects and dogs studied with a similar technique. The results show that 1) cardiopulmonary interactions from rest to exercise can be measured noninvasively in guinea pigs, 2) guinea pigs exhibit patterns of exercise response and alveolar microvascular recruitment similar to those of larger species, and 3) the rebreathing technique is widely applicable to human (∼70 kg), dog (20–30 kg), and guinea pig (1–1.5 kg). In theory, this technique can be extended to even smaller animals provided that species-specific technical hurdles can be overcome.
Keywords: lung volume, pulmonary capillary blood volume, cardiac output, exercise, membrane diffusing capacity
the techniques available for noninvasive assessment of cardiopulmonary function in small animals are limited. While ventilation, O2 uptake, and CO2 output can be measured noninvasively (9, 13), other parameters such as lung volume, diffusing capacity, and cardiac output generally require invasive methodology such as vascular catheterization and tracheostomy. These invasive techniques are usually performed as terminal procedures under anesthesia. As survival procedures, invasive instrumentation incurs various complications (e.g., anemia, infection) that increase animal wastage, shorten the chronicity of the animal preparation, and limit exercise performance and can potentially confound data interpretation. Given the increasing use of small animal models for cardiopulmonary research, there is a need for the development of noninvasive methodology in conscious nonsedated small animals in order to allow longitudinal characterization of functional capacity at rest and during exercise and to facilitate the study of chronic animal preparations.
Our laboratory has long established a rebreathing technique for noninvasively measuring multiple parameters, including lung volume, pulmonary blood flow, diffusing capacity of the lung and membrane (DlCO and DmCO, respectively), pulmonary capillary blood volume (Vc), and septal volume, at rest and during exercise in human (1, 11) and canine (8) subjects. These measurements have not been extended to smaller animals until recently. In 2005, we developed a rebreathing technique for measuring these parameters in conscious, spontaneously breathing guinea pigs at rest (17). The animal was placed inside a Plexiglas chamber with its head enclosed in a snug, leak-free mask. Open-circuit measurements included O2 uptake, CO2 output, ventilation, respiratory rate, and tidal volume; these were followed by the rebreathing maneuver. Using this technique, we demonstrated significant increases in lung volume and lung and membrane diffusing capacities at rest in weanling guinea pigs that were raised to maturity at high altitude (3,800 m) compared with those raised at lower altitudes (18). Since DlCO and its components (DmCO and Vc) index alveolar microvascular recruitment, simultaneous measurement of pulmonary blood flow and DlCO permits comparison of the components of diffusing capacity at comparable levels of perfusion as well as the estimation of their recruitment as blood flow increases from rest to exercise.
We now aim to extend this noninvasive rebreathing technique for measuring normal and deranged cardiopulmonary function in exercising small animals. The objective of the present study was to establish whether guinea pigs exhibit exercise-induced responses in pulmonary gas exchange, diffusion, and perfusion similar to those observed in larger species. To address this objective we have overcome not only the technical challenges associated with miniaturization of the apparatus but also the species-specific issues associated with exercise training and measurement.
METHODS
The Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center approved all animal protocols. The protocol for human subjects was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, and all subjects gave informed consent.
Animals.
Male Hartley guinea pigs (n = 17, 9–20 mo of age at time of measurement, body wt 1,228 ± 100 g, mean ± SD; Harlan Industries, Indianapolis, IN) were trained to exercise on a motorized treadmill beginning at 4 mo of age. Body weight was measured each week.
Exercise training.
A small animal treadmill was constructed (Echols Machining, Dallas, TX) with adjustable speeds and inclines and with multiple lanes that fit the body width of the animals. The animals were first trained to stand on the stationary treadmill; then the treadmill speed was gradually increased. The animals were motivated to run by placing a drape over the front half of the treadmill; guinea pigs prefer darkness and will voluntarily run to keep themselves in the darker front part. If necessary, we prodded their hindquarter with a finger. When the animal tolerated walking on the treadmill at 12–14 m/min for 5 min, the speed was gradually increased as tolerated every 2 min by 2 m/min up to 22 m/min. Further increases in workload were accomplished by raising the treadmill elevation to 5% or 10%. Exercise sessions lasted 20 min per day, 3 days per week. After exercise, the animal walked on the treadmill (12–14 m/min) to cool down. Rectal temperature was measured after each session. Animals were rewarded with petting, play, and treats after exercise.
Apparatus.
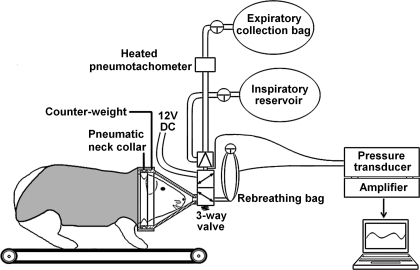
The apparatus is shown in Fig. 1. The animal was trained to wear a cone-shaped respiratory mask made of transparent Plexiglas with a pneumatic sealing cuff around the neck. The mask was attached to a body vest made of stretchable fabric and fastened by Velcro. Masks of different sizes were available to ensure a snug fit over the animal's head. The neck cuff was pressurized (15 psi) to prevent leak. Before being placed on the animal, the cuff diaphragm was tested for leaks. After placement on the animal, cuff pressure was monitored continuously. The orifice of the mask was attached via a three-way solenoid valve (model B3317, Precision Dynamics, New Britain, CT) to a two-way non-rebreathing valve (model 2384A, Hans Rudolph, Kansas City, MO) and a small (∼15 ml) double-layer latex rebreathing bag (apparatus dead space 1.2 ml). During open-circuit breathing the inspiratory port opened to room air or a reservoir. The expiratory port connected to a heated pneumotachometer (Hans Rudolph, series 8311) and a collection bag for measuring expired ventilation. Tidal respiratory pressures were detected at the mouth during open-circuit breathing and between the two layers of the latex bag during rebreathing maneuvers. Pneumotachometer signals were amplified (Hans Rudolph Amplifier 1, series 1100) and acquired by a data acquisition card (PCM-DAS08, Computer Boards, Middleboro, MA) on a PC laptop (Pentium III, Dell Inspiron 8000) running LabVIEW 5.0 (National Instruments, Austin, TX) and Universal Library (ComputerBoards) acquisition software for real-time measurement of respiratory rate and minute ventilation. The entire mask-valve assembly was counterweighted.
Fig. 1.
Schematic diagram of the experimental apparatus.
Expired gas collected in the anesthetic bag and in the rebreathing bag was sampled, and the concentrations of O2, CO2, N2, CO, C2H2, and SF6 or Ne were measured by a gas chromatograph (CP-4900 Micro GC, Varian) with two columns (M5AHIBF and PPUHI) and a thermal conductivity detector using 100% high-purity helium as a carrier gas. Linearity of the gas chromatograph was checked under dry as well as humidified conditions by generating calibration curves with gases of known concentrations. Water vapor was eliminated with a moisture trap (model MT120-2, Agilent Technologies, Palo Alto, CA) at the insertion port of the gas chromatograph. The pneumotachometer was calibrated before each study by delivering 60 strokes of room air or 100% O2 at different flow rates with a 20-ml syringe. Because 100% O2 generated 11% higher voltage than room air at the same flow rate, the pneumotachometer was calibrated with the same inspired O2 concentration as that used during each study. Ventilatory measurements were verified by timed collection of expired air in an anesthetic bag. The volume of the collected gas was measured with a calibrated syringe, and the gas concentrations were measured by either gas chromatograph or mass spectrometer.
Protocol.
Each animal wearing the mask-vest assembly and the necessary respiratory attachments was placed on the treadmill for several minutes. The pneumatic neck cuff was inflated. Baseline measurements were made with the animal standing at rest. The treadmill was then started at a speed of 10–12 m/min and gradually increased to the desired workload (up to 22 m/min) as tolerated for 3 min. Ventilation, respiratory rate, and tidal volume were recorded continuously, and the expired air was collected to verify expired volume as well as mean expired gas concentrations. After exercise at a constant workload for 3 min, the rebreathing maneuver was performed.
Immediately before the commencement of exercise, the double-layer rebreathing bag was prefilled with a known volume (10–12 ml or 5 ml larger than the average tidal volume) of a rebreathing gas mixture (0.3% CO, 0.5% SF6 or Ne, 0.8% C2H2, in either 40% or 98% O2 in balance of N2). When using the gas mixture containing 98% O2, the animal prebreathed 100% O2 from the inspiratory reservoir for ∼30 s before performing the rebreathing maneuver. At a selected end expiration, approximated from continuous recordings of mouth pressure, the solenoid valve automatically switched from the open-circuit breathing port to the rebreathing port, so that the animal breathed in and out of the latex bag for a measured interval (5, 7, or 9 s in random order). At the end of this period, the solenoid valve switched again at end exhalation to allow the animal to breathe ambient air or from the inspiratory reservoir. The treadmill was stopped. The volume remaining in the latex rebreathing bag was measured immediately with a glass syringe, and the gas concentrations were measured by a gas chromatograph. The precise rebreathing duration was determined from changes in mouth pressure coincident with valve switching. Measurements at a given workload were repeated at two levels of O2 tension. The rest interval between successive measurements was at least 5 min. Rectal temperature was recorded before and immediately after exercise. On separate days before and after exercise, a toenail was clipped in order to obtain blood for measurement of capillary hematocrit.
Analysis of rebreathing data.
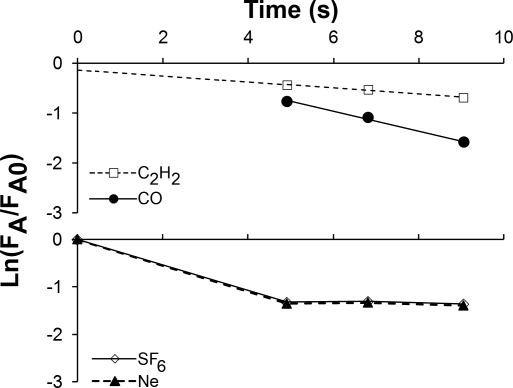
The measured volumes were expressed at BTPS conditions, and rectal temperature was measured at the end of each run. System volume was calculated from SF6 or Ne dilution, from which the apparatus dead space was subtracted to obtain mean end-inspiratory lung volume. Pulmonary blood flow and DlCO were calculated from the simultaneous logarithmic disappearance of C2H2 and CO, respectively, with respect to SF6 or Ne obtained at 5, 7, and 9 s of rebreathing in separate runs at a given workload. Owing to a rapid respiratory rate during exercise, mixing efficiency was achieved rapidly and the concentrations of SF6 and Ne stabilized well within 6 s while the concentrations of C2H2 and CO declined in a log-linear fashion. Representative disappearance curves are shown in Fig. 2. The rebreathing bag selectively absorbed C2H2 at a slow, constant rate (7.5 × 10−6 ml/s); the small loss was measured and accounted for in the calculation of pulmonary blood flow and septal volume.
Fig. 2.
Representative disappearance curves show the exponential disappearance of C2H2 and CO and the stabilization of SF6 and Ne dilution during rebreathing. FA, fractional gas concentration during rebreathing; FA0, initial fractional gas concentration.
DmCO and Vc were calculated from DlCO estimated at two alveolar O2 tensions (PaO2) with the Roughton-Forster relationship (14):
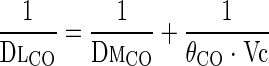 |
(1) |
where θCO is the empirical rate of CO uptake by whole blood at 37°C in milliliters of CO per milliliter of blood per minute per Torr estimated from mean PaO2 (in Torr) during rebreathing. Since the relationship between θCO and O2 tension is not available for guinea pig blood, we used the values obtained for dog whole blood expressed at 39°C as well as for human blood (4) :
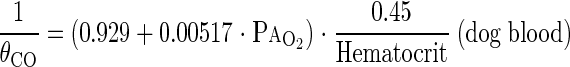 |
(2) |
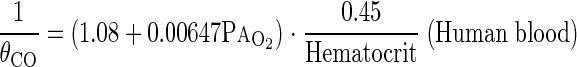 |
(3) |
Estimates of DmCO and Vc were used to standardize DlCO (DlCO-std) to a constant PaO2 of 120 Torr and hematocrit of 0.45. Estimates of DlCO, DmCO, and Vc were analyzed with respect to the simultaneously measured pulmonary blood flow. Septal volume, which includes the volume of tissue as well as blood in the gas exchange region, was estimated from the extrapolated intercept of the acetylene disappearance curve back to time 0.
Statistical analysis.
Ventilation and CO2 output were plotted with respect to O2 uptake. DlCO, DmCO, and Vc were plotted against pulmonary blood flow. The relationship for DlCO-std in guinea pigs was plotted together with the corresponding data obtained in normal adult dogs (n = 8) and healthy nonsmoking human subjects (n = 17) studied from rest to exercise in our laboratory with a rebreathing technique. The slope and intercept of linear regression relationships between standardized body mass-specific DlCO and pulmonary blood flow were compared among species by the method of Zar (19).
RESULTS
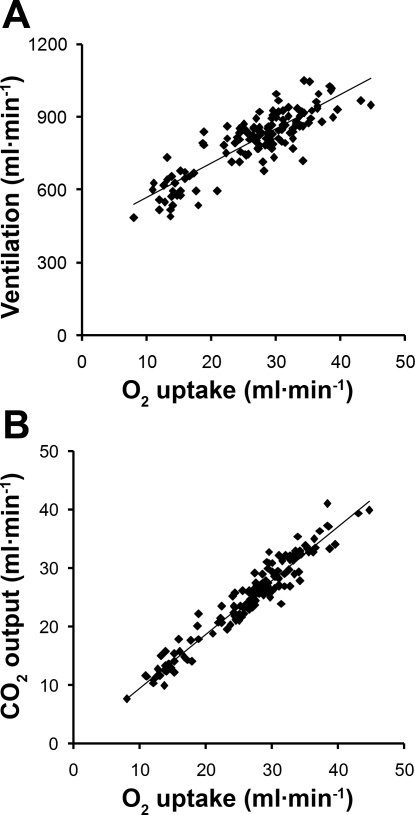
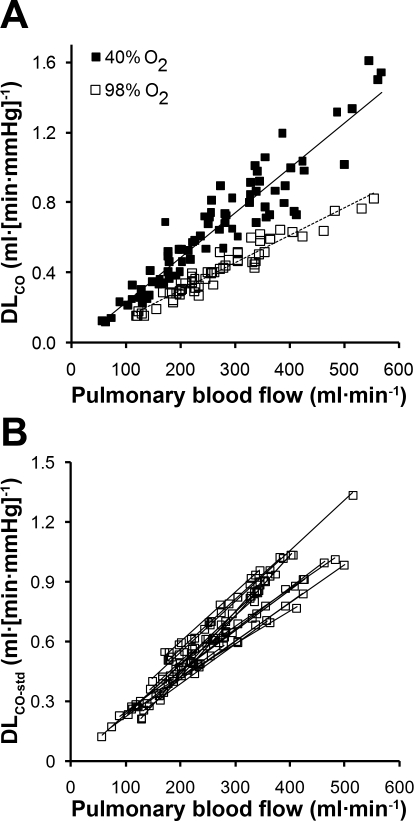
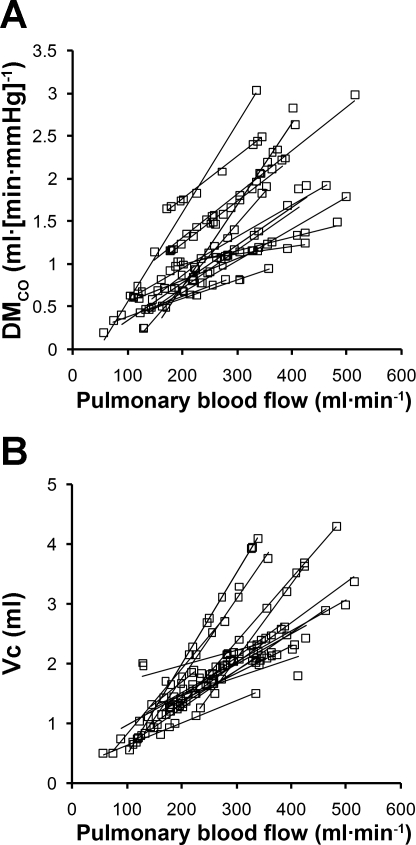
On average, it required 2–3 mo of training before an animal was ready for measurements during exercise. Mean results, normalized by body weight, are shown in Table 1. Average rectal temperature increased from 38.6°C at rest to 39.8°C immediately after exercise while wearing the respiratory mask and attachments, and to 39.5°C when animals exercised without the mask and attachments (Table 1). From rest to exercise, O2 uptake and CO2 output approximately doubled. Ventilation and CO2 output increased linearly with O2 uptake (Fig. 3). Minute ventilation increased via an increase in respiratory rate, while tidal volume measured during either open-circuit breathing or rebreathing did not change significantly. Lung volumes measured during rebreathing increased 67–85% from rest to exercise. Mean pulmonary blood flow also doubled from rest to exercise. As expected, DlCO was lower at a given blood flow while breathing 98% O2 compared with breathing 40% O2. (Fig. 4A). Regardless of the inspired O2 concentration or after standardization to constant PaO2 and hemoglobin concentration (Fig. 4B), DlCO increased in a linear relationship with respect to pulmonary blood flow. Similarly, DmCO and Vc increased linearly with respect to pulmonary blood flow (Fig. 5, A and B, respectively). From rest to exercise DlCO increased 89%, associated with 70% and 100% increase in DmCO and Vc, respectively. Mean septal volume also increased proportionately from rest to exercise (Table 1). We calculated DmCO and Vc with published θCO values for both dog and human blood. Compared with human blood, using θCO for dog blood resulted in <2% lower estimates of DmCO and 11% higher estimates of Vc at rest and during exercise (Table 1).
Table 1.
Open-circuit and rebreathing data at rest and exercise
| Rest | Exercise | |
|---|---|---|
| Rectal temperature, °C | ||
| With mask and attachments | 38.6±0.4 | 39.8±0.4 |
| Without mask and attachments | 38.6±0.5 | 39.5±0.5 |
| Hematocrit, % | 47.5±2.2 | 47.1±2.0 |
| Ventilation, ml·min−1·kg−1 | 523±84 | 729±79 |
| Tidal volume, ml/kg | 5.5±0.6 | 5.2±0.7 |
| O2 uptake, ml·min−1·kg−1 | 12.7±3.1 | 25.5±3.5 |
| CO2 output, ml·min−1·kg−1 | 12.1±3.2 | 24.3±3.7 |
| Respiratory exchange ratio | 0.95±0.11 | 0.96±0.06 |
| Respiratory rate, breaths/min | 96±14 | 142±14 |
| Rebreathing measurements | ||
| Mean alveolar Po2, Torr | ||
| Rebreathing 98% O2 | 556±28 | 544±13 |
| Rebreathing 40% O2 | 114±28 | 93±8 |
| End-inspiratory lung volume, ml/kg | 24.4±4.9 | 40.7±6.7 |
| End-expiratory lung volume, ml/kg | 16.5±4.6 | 30.5±6.4 |
| Pulmonary blood flow, ml·min−1·kg−1 | 127±45 | 239±50 |
| DlCO, ml·min−1·Torr−1·kg−1 | ||
| Rebreathing 98% O2 | 0.188±0.073 | 0.376±0.071 |
| Rebreathing 40% O2 | 0.248±0.093 | 0.591±0.142 |
| DlCO-std, ml·min−1·Torr−1·kg−1 | 0.290±0.108 | 0.553±0.105 |
| DmCO, ml·min−1·Torr−1·kg−1 | ||
| θCO of dog blood | 0.614±0.313 | 1.105±0.392 |
| θCO of human blood | 0.606±0.315 | 1.084±0.380 |
| Vc, ml/kg | ||
| θCO of dog blood | 0.9±0.4 | 1.8±0.5 |
| θCO of human blood | 1.0±0.4 | 2.0±0.7 |
| Septal volume, ml/kg | 2.2±1.2 | 4.9±2.0 |
Values are means ± SD. DlCO, lung diffusing capacity for CO; DmCO, membrane diffusing capacity for CO; DlCO-std, DlCO standardized to hematocrit = 0.45 and alveolar Po2 = 120 Torr; θCO, rate of CO uptake; Vc, pulmonary capillary blood volume.
Fig. 3.
Relationship between minute ventilation (V̇e) and O2 uptake (V̇o2) (A) and between CO2 output (V̇co2) and V̇o2 (B). Linear regression equations through pooled data: V̇e = 14.15V̇o2 + 426.0, R2 = 0.74, and V̇co2 = 0.920V̇o2 + 0.383, R2 = 0.93.
Fig. 4.
A: relationships of lung diffusing capacity for CO (DlCO) with respect to pulmonary blood flow (Q̇c) were measured while animals were rebreathing 40% or 98% O2. Linear regression equations through pooled data: DlCO = 0.0026Q̇c − 0.0246, R2 = 0.89, and DlCO = 0.0016 Q̇c − 0.0080, R2 = 0.93. B: DlCO was standardized (DlCO-std) to a constant hematocrit (0.45) and alveolar O2 tension (120 Torr) and plotted with respect to Q̇c for individual animals. Mean linear regression equation: DlCO-std = 0.0023Q̇c + 0.0211, R2 =0.88.
Fig. 5.
A: membrane diffusing capacity (DmCO) plotted with respect to Q̇c. Each regression line belongs to an individual animal. Mean regression equation: DmCO = 0.0046Q̇c + 0.0695, R2 = 0.53. B: pulmonary capillary blood volume (Vc) plotted with respect to Q̇c. Each regression line belongs to an individual animal. Mean regression equation: Vc = 0.0068Q̇c + 0.154, R2 = 0.65. Both DmCO and Vc data were estimated with the rate of CO uptake (θCO) value for dog blood.
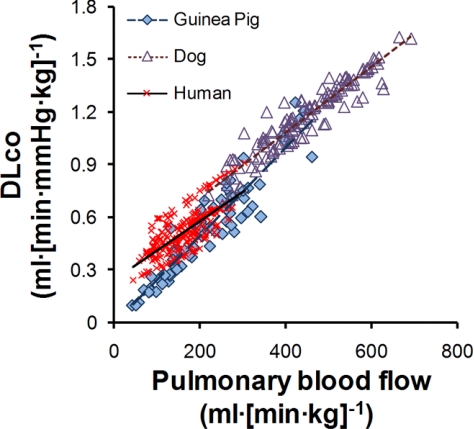
Increase in body mass-specific DlCO expressed at standardized conditions with respect to pulmonary blood flow for different species is shown in Fig. 6. Guinea pig and untrained human subjects share a comparable range of body mass-specific pulmonary blood flow from rest to exercise; both were significantly below the range seen in dogs. In guinea pigs the baseline DlCO standing at rest was significantly lower than in dogs or human subjects, while the slope of exercise-induced increase in DlCO was significantly higher (both P < 0.05) so that at moderately heavy work intensities the data from guinea pigs converged upon those from the other two species.
Fig. 6.
Body mass-specific relationships of standardized DlCO (hematocrit 0.45 and alveolar O2 tension 120 Torr) to Q̇c for guinea pigs (present data), 8 normal dogs, and 17 healthy human subjects studied by a similar rebreathing technique in our laboratory. Linear regression equations through pooled data: guinea pig, DlCO = 0.0023Q̇c + 0.0224, R2 = 0.88; dog, DlCO = 0.0019Q̇c + 0.3294, R2 = 0.89; human, DlCO = 0.0017Q̇c + 0.2412, R2 = 0.52. Slope of the relationship for guinea pig is significantly higher while the intercept was significantly lower than that in canine and humans subjects (both P < 0.05).
DISCUSSION
This is the first report of the noninvasive measurement of multiple cardiopulmonary parameters and alveolar microvascular recruitment in small animals during exercise. By adapting a rebreathing technique that has been well established in larger animals and human subjects to exercising guinea pigs, we found linear increases in DlCO, DmCO, and Vc with respect to pulmonary blood flow that are consistent with the relationships previously reported in larger species. These results, by establishing the feasibility of this technique and allowing serial noninvasive studies to be carried out on the same animals without the complications associated with chronic instrumentation, significantly advance the use of small animal models in cardiopulmonary research.
Guinea pigs are docile and sedentary animals. With training, most animals tolerated the respiratory assembly and achieved a level of exercise intensity analogous to that seen in sedentary human subjects. In contrast to earlier studies that used electrical stimulation to motivate the animals, we motivated the animals to run voluntarily by exploiting their natural preference for darkness. The pressurized neck cuff, attached body vest, and counterweight effectively sealed the respiratory mask and prevented air leaks during rebreathing. Air leaks may develop if the animal struggles against the mask. If this occurred, the treadmill was stopped, the mask was removed, and the animal was allowed to rest before attempting the procedure again. If the animal refused to cooperate, the session was terminated for that day. The animals did not reach maximal O2 uptake, judged by a respiratory exchange ratio below 1.0. However, they were clearly exercise stressed and demonstrated the expected physiological relationships during exercise, including rectal temperature, gas exchange, ventilation, diffusing capacities, pulmonary blood flow, and pulmonary capillary blood volume.
Limitations of the technique.
Electronic switching of the solenoid respiratory valve was synchronized to end-expiration detected from mouth pressure. Errors in synchronization could occur as respiratory rate increases upon exercise, which would cause overestimation of the true end-expiratory lung volume. This potential error would not affect the estimation of system or end-inspiratory lung volume. The double-layered latex rebreathing bag selectively absorbs acetylene at a slow constant rate; this loss, if uncorrected, contributed to no more than 1.7% error in the estimated pulmonary blood flow and no more than 12–19% error in the estimated septal volume at rest and during exercise. We measured and accounted for the rate of loss in the calculation of these parameters. In addition, we minimized the loss by filling the latex bag immediately before exercise. In theory, Mylar is a better material than latex for a rebreathing bag, but Mylar is difficult to construct and use in a small volume and tended to distract the exercising animal.
The respiratory mask was constructed to enclose the animal's head snugly without being overly restrictive, with an estimated geometric dead space of 1 ml. Resistance of the breathing circuit was 0.08 and 0.1 cmH2O·s·ml−1 at ventilatory rates corresponding to rest and exercise, respectively, which contributed a 0.8- to 1.0-cmH2O increase in airway pressure during exercise. Wearing the mask increased rectal temperature slightly (0.3°C) immediately after exercise. These data suggest that the apparatus did not load the respiratory system excessively in guinea pigs compared with the analogous rebreathing technique used in humans and dogs.
Comparison with previous resting studies.
The mean end-expiratory lung volume in guinea pigs standing at rest on the treadmill (16.5 ml/kg) is slightly higher than that found in an earlier resting study (13.1 ml/kg) where the conscious spontaneously breathing guinea pig was confined inside a body chamber (17, 18), but close to the 17.0 ml/kg reported by Paré et al. (10) in guinea pigs by body plethysmography. The present estimate of end-inspiratory lung volume at rest (24.4 ml/kg) agrees well with that reported in our earlier study (17) using a body chamber (21.0 ml), but it is lower than postmortem estimates of lung volume in guinea pigs of a similar size (∼30 ml/kg for 2 lungs) after instillation of liquid fixatives at a tracheal pressure of 25 cmH2O (7). Chamber confinement in the previous study (17) may have altered the breathing pattern and reduced resting lung volume compared with that while standing on the treadmill.
The present estimate of resting pulmonary blood flow (127 ml·min−1·kg−1) in guinea pigs standing on a treadmill is lower than that previously measured in conscious guinea pigs confined within a body chamber (163 ml·min−1·kg−1; Ref. 17). Both values are significantly lower compared with the cardiac output measured by other methods in unanesthetized chronically instrumented guinea pigs (273 ml·min−1·kg−1; Refs. 15, 16) and instrumented rats (265–307 ml·min−1·kg−1; Ref. 3) but similar to that measured in conscious catheterized rabbits at rest (∼100–130 ml·min−1·kg−1; Ref. 2). Clearly, there is wide fluctuation in the baseline state of cardiac output in different studies. The present estimate of resting DlCO standardized to constant hematocrit and PaO2 is similar to that previously reported in guinea pigs confined within a body chamber (0.29 and 0.32 ml·min−1·Torr−1·kg−1, respectively; Ref. 17).
Response to exercise.
In these guinea pigs, the increase in ventilation from rest to exercise was entirely attributed to a higher respiratory rate; tidal volume measured during open-circuit breathing or during rebreathing did not increase. The animal may have adopted this pattern of breathing while wearing the respiratory mask assembly, or perhaps the high breathing frequency prevented tidal volume from increasing during exercise. Consistent with the rapid shallow breathing pattern, system volume and end-expiratory lung volume during rebreathing increased by 67–85% from rest to exercise. Pulmonary blood flow increased by 93% from rest to exercise in proportion to the increase in O2 uptake; the relative increase is similar to that in catheterized rats, where cardiac output also nearly doubled from rest to exercise (3).
At a given pulmonary blood flow, DlCO normally declines as inspired O2 concentration increases. This pattern was observed in guinea pigs as in other species (Fig. 4A). Also similar to other species, the slope of increase in DlCO is strikingly constrained (Fig. 4B). As expected, the derived parameters DmCO and Vc showed greater variance among animals but both increased consistently from rest to exercise, similar to the findings in human subjects (6) and dogs (5) with the rebreathing technique.
Alveolar microvascular recruitment.
In cumulative studies we have demonstrated functional recruitment of alveolar microvasculature in three species: human, dog, and guinea pig (Fig. 6). While the absolute baseline DlCO and pulmonary blood flow differ among species and under different “resting” conditions, upon exercise the relationship of DlCO with respect to pulmonary blood flow becomes highly consistent. The comparative data underscore the key concept of pulmonary diffusion-perfusion (Dl/Q̇) interactions in sustaining oxygen uptake. Recruitment of diffusing capacity via proportional increases in conductance of the membrane and blood components upon exercise ensures an adequate Dl-to-Q̇ ratio, which is a major determinant of the oxygen saturation of end-capillary blood leaving the lung (12). These data also highlight the practical need for interpreting DlCO, DmCO, and Vc with respect to the simultaneously measured pulmonary blood flow. An example in which diffusion-perfusion interactions altered data interpretation is seen in our study of guinea pigs raised at high altitude (18), where the absolute DlCO measured at rest was similar to that in control animals raised at a lower altitude. However, high-altitude residence caused a simultaneous reduction in resting pulmonary blood flow. After the measurements were adjusted to a given blood flow, DlCO was significantly higher in animals raised at high altitude than in control animals raised at low altitude.
We conclude that cardiopulmonary interactions from rest to exercise can be measured noninvasively in guinea pigs and that guinea pigs exhibit patterns of exercise response and alveolar microvascular recruitment similar to those of larger species. This study establishes a wide range of applicability for the rebreathing technique, from human (∼70 kg) to dog (20–30 kg) to guinea pig (1–1.5 kg). In theory, this technique can be extended to study even smaller animals provided that species-specific technical hurdles can be overcome.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant R01-HL-045716.
Acknowledgments
The authors thank Jennifer Fehmel for exercise training, animal care, and technical assistance. The authors also thank the staff of the Animal Resources Center at the University of Texas Southwestern Medical Center for veterinary assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barazanji KW, Ramanathan M, Johnson RL Jr, Hsia CCW. A modified rebreathing technique using an infrared gas analyzer. J Appl Physiol 80: 1258–1262, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Boerboom LE, Boelkins JN. Dye-dilution cardiac output without blood withdrawal in the conscious rabbit. Am J Physiol Heart Circ Physiol 235: H258–H261, 1978. [DOI] [PubMed] [Google Scholar]
- 3.Favret F, Henderson KK, Allen J, Richalet JP, Gonzalez NC. Exercise training improves lung gas exchange and attenuates acute hypoxic pulmonary hypertension but does not prevent pulmonary hypertension of prolonged hypoxia. J Appl Physiol 100: 20–25, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Holland RAB Rate at which CO replaces O2 from O2Hb in red cells of different species. Respir Physiol 7: 43–63, 1969. [DOI] [PubMed] [Google Scholar]
- 5.Hsia CCW, Herazo LF, Ramanathan M, Johnson RL Jr. Cardiopulmonary adaptations to pneumonectomy in dogs. IV. Membrane diffusing capacity and capillary blood volume. J Appl Physiol 77: 998–1005, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Hsia CCW, McBrayer DG, Ramanathan M. Reference values of pulmonary diffusing capacity during exercise by a rebreathing technique. Am J Respir Crit Care Med 152: 658–665, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Hsia CCW, Carbayo JJ, Yan X, Bellotto DJ. Enhanced alveolar growth and remodeling in guinea pigs raised at high altitude. Respir Physiol Neurobiol 147: 105–115, 2005. [DOI] [PubMed] [Google Scholar]
- 8.McDonough P, Dane DM, Hsia CC, Yilmaz C, Johnson RL Jr. Long-term enhancement of pulmonary gas exchange after high-altitude residence during maturation. J Appl Physiol 100: 474–481, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol 65: 964–970, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Paré PD, Michoud MC, Boucher RC, Hogg JC. Pulmonary effects of acute and chronic antigen exposure of immunized guinea pigs. J Appl Physiol 46: 346–353, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Phansalkar AR, Hanson CM, Shakir AR, Johnson RL Jr, Hsia CC. Nitric oxide diffusing capacity and alveolar microvascular recruitment in sarcoidosis. Am J Respir Crit Care Med 169: 1034–1040, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Piiper J Alveolar-capillary gas transfer in lungs: development of concepts and current state. Adv Exp Med Biol 345: 7–14, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Rezende EL, Gomes FR, Malisch JL, Chappell MA, Garland T Jr. Maximal oxygen consumption in relation to subordinate traits in lines of house mice selectively bred for high voluntary wheel running. J Appl Physiol 101: 477–485, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Roughton FJW, Forster RE. Relative importance of diffusion and chemical reaction rates in determining the rate of exchange of gases in the human lung, with special reference to true diffusing capacity of the pulmonary membrane and volume of blood in lung capillaries. J Appl Physiol 11: 290–302, 1957. [DOI] [PubMed] [Google Scholar]
- 15.Spence CR, Thompson BT, Janssens SP, Steigman DM, Hales CA. Effect of aerosol heparin on the development of hypoxic pulmonary hypertension in the guinea pig. Am Rev Respir Dis 148: 241–244, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Thompson BT, Hassoun PM, Kradin RL, Hales CA. Acute and chronic hypoxic pulmonary hypertension in guinea pigs. J Appl Physiol 66: 920–928, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz C, Johnson RL Jr, Hsia CC. A rebreathing method for measuring lung volume, diffusing capacity and cardiac output in conscious small animals. Respir Physiol Neurobiol 146: 215–223, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz C, Dane DM, Hsia CC. Alveolar diffusion-perfusion interactions during high-altitude residence in guinea pigs. J Appl Physiol 102: 2179–2185, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Zar JH Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall, 1999.