Abstract
The metabolic syndrome is defined as the coexistence of multiple cardiovascular and diabetes risk factors, the prevalence of which has increased dramatically in adult populations in the last decades. More recently, the same cluster of metabolic risk factors has also been recognized in children and adolescents. Epidemiological evidence suggests that high levels of cardiorespiratory fitness (CRF) and physical activity are associated with a favorable metabolic risk profile in adults. However, in youth the role of these factors is less clear. Therefore, the purpose of this mini-review is to examine the recent evidence between objectively measured habitual physical activity and CRF with clustered metabolic risk in youth. In general, it appears that both physical activity and CRF are separately and independently associated with metabolic risk factors in youth, possibly through different causal pathways. Further research is necessary to quantify how much physical activity is needed to prevent the metabolic syndrome and the diseases with which it is associated. Public health approaches that encourage increased physical activity and reduce sedentary behaviors may prove useful in reducing the population burden associated with metabolic risk.
Keywords: children, adolescents, objective measurement, sedentary behavior
the prevalence of the metabolic syndrome has dramatically increased over the last few decades and has become a major health challenge worldwide, increasing the risk of cardiovascular disease (CVD), Type 2 diabetes (T2D), nonalcoholic liver disease, renal disease, and some forms of cancer in adults (73, 76). The metabolic syndrome may be defined as the clustering of three or more of the following risk factors: adiposity, hypertension, hyperglycemia, low high-density lipoprotein (HDL)-cholesterol, and high triglycerides. Until recently, metabolic risk factor clustering had only been reported in adults. However, observations of single risk factors as well as multiple-clustered risk factors have now been observed in children and adolescents (44, 64). Although definitions of clustered metabolic risk in adults vary across different health advisory boards (2, 3, 39), a recent International Diabetes Federation consensus report defines the syndrome in children and adolescents as central obesity plus any two of a raised triglyceride level, a reduced HDL-cholesterol level, hypertension, and elevated fasting plasma glucose (75).
The emergence of the metabolic syndrome parallels the rising rates of overweight and obesity observed in youth worldwide (57, 64). Prevalence figures of the metabolic syndrome in young people vary between 0 and 60% depending on the definition of the metabolic syndrome and the population examined, with the highest prevalence figures observed in obese youth (44, 64). However, only a few studies have assessed the prevalence of the metabolic syndrome in representative population-based samples of children and adolescents. Data from these studies suggest that the prevalence of the metabolic syndrome varies between 3 and 12% in representative samples of youth (17, 19, 22, 49, 59). However, lack of a universal definition of metabolic syndrome in these studies limits the generalizability, diagnosis, and clinical utility for therapeutic intervention, as well as the ability to compare prevalence rates across countries and studies.
Sedentary behavior, persistent low levels of physical activity, and poor cardiorespiratory fitness (CRF) are known to predict progression toward CVD, T2D, and metabolic syndrome in adults (9, 21, 33, 53, 62). A recent review by Ford and Li (33), outlined the evidence from a diverse range of cross-sectional and prospective studies, identifying the influence of physical activity and CRF on the metabolic syndrome in adults. Physical activity and CRF may also be associated with metabolic risk factors in children and adolescents (24, 35, 37). Evidence also suggests that sedentary behavior, low levels of physical activity, and CRF in youth track into adulthood, (35, 54, 74); likewise metabolic risk factors also appear to track over time (16, 52), and this may predispose young people to disease in later in life (42).
In this review, we describe the recent epidemiological evidence of the relationship between habitual physical activity and CRF with clustered metabolic risk with emphasis on recent studies (from 2004 onward) that have employed objective measures of physical activity and CRF. As a consequence, a few large studies using self-reported physical activity (51, 56, 60) are excluded. In addition, our focus here is on habitual physical activity and not specifically exercise, so we have also excluded exercise training studies, most of which have been conducted in nonrepresentative populations such as overweight or obese children. A thorough discussion of the biological plausibility is beyond the scope of this review, so we restrict our focus to describing the direction and magnitude of association. We begin this review with the operational definitions of physical activity and CRF and a brief overview of the merits and limitations of using nondichotomized scores for defining metabolic health in youth. We then review the association between physical activity, sedentary behavior, CRF, and clustered metabolic risk, and we discuss the mediating role of obesity. Finally, we provide a summary of current physical activity recommendations for children and adolescents.
DEFINITIONS OF PHYSICAL ACTIVITY AND CARDIORESPIRATORY FITNESS
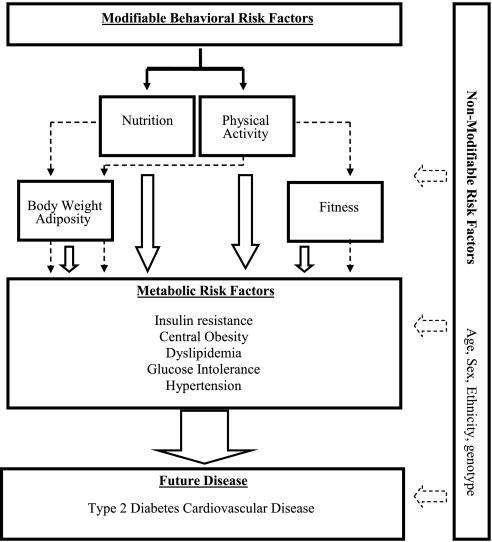
The term “physical activity” is often used interchangeably with energy expenditure, exercise, and physical fitness. These terms have been defined by Caspersen et al. (15) with “physical activity” as “any bodily movement produced by skeletal muscles that results in energy expenditure” and “physical fitness” as a “set of attributes either health- or skill related” that is not synonymous with physical activity. “Exercise” on the other hand is “a subset of physical activity that is planned, structured, and repetitive” leading to the improvement or maintenance of physical fitness. In this review, we refer to fitness almost exclusively as CRF or endurance-related fitness, which relates very closely to maximal capacity for oxygen consumption. An important distinction between physical activity and CRF is the intraindividual day-to-day variability; physical activity will undoubtedly vary on a daily basis, whereas CRF will remain relatively static, taking time to change. This variability will impact on the ability to measure these two quantities and consequently will influence the ability to demonstrate their relationship with metabolic outcomes. Figure 1 conceptualizes the role of physical activity and CRF in the etiology of metabolic syndrome and CVD risk.
Fig. 1.
Conceptual model illustrating physical activity, cardiorespiratory fitness, and its associations with metabolic risk and future disease progression. Dashed lines represent the hypothesized mediating effect between phenotypes and metabolic risk, solid lines represent direct effect.
IS THE METABOLIC SYNDROME CONCEPT APPLICABLE TO YOUTH?
Opinions differ as to whether the clustering of metabolic risk factors is truly a syndrome (50) and whether reducing a complex cluster to a binary category has either clinical utility in altering therapy or etiological value in producing enhanced understanding about causation. In adult populations, some have argued that insulin resistance is the central feature (55), whereas others state that central or upper body obesity are key features (41). Insulin resistance, central obesity, and systemic inflammation are interrelated and generally coexist in individuals with increased risk of CVD. The link between obesity, insulin resistance, and metabolic risk has been described in children and adolescents (67). Despite some inconsistencies in the definition and lack of a universal acceptance of the metabolic syndrome, the central message is that the presence of one or multiple risk factors of any type in youth should be cause for concern. In fact, one may broaden this concern to include any worsening of multiple features of the metabolic syndrome, even if they do not exceed established clinical thresholds.
The dichotomization of the metabolic syndrome may be useful in clinical diagnosis. However, given the relatively low prevalence of metabolic syndrome in youth, and the fact that these risk factors are correlated with each other both in terms of their level and rate of change over time (16) and therefore may manifest as disease later in life, it may be more relevant to view the metabolic syndrome as a continuously distributed risk score. Moreover, the risk of CVD and T2D increases progressively with higher levels of each individual risk factor and dichotomization reduces the predictive and statistical power to observe associations between exposures and outcomes (58). We and others have therefore focused on using a clustered risk score (summarized Z score) for investigating associations between physical activity, CRF, and metabolic risk (e.g., Refs. 4, 25, 28, 61). A Z score is computed as the number of standard deviation (SD) units from the sample mean after normalization of the variable [i.e., Z = (value − mean)/SD]. This is done for each risk factor and then summed up to denote clustered metabolic risk. Other scoring techniques include principle component analysis, which may provide added methodological utility (71).
Limitations to the use of a summarized metabolic risk score include 1) that it may in fact obscure the true associations between physical activity, CRF, and individual risk factors, 2) that it is specific to the sample populations from which it was derived, and 3) that it is based on the assumption that each component is equally weighted in predicting future disease progression, although this limitation could be addressed with appropriate optimization techniques (34).
IS OBJECTIVELY MEASURED PHYSICAL ACTIVITY ASSOCIATED WITH CLUSTERED METABOLIC RISK IN YOUTH?
Studies in adults have shown that higher levels of physical activity predict slower progression toward the metabolic syndrome in apparently healthy men and women (29, 53), an association that is independent of change in body fatness and CRF (31). Population studies focusing on these relationships in children and adolescents are limited, and the use of self-reported activity, which is more imprecise in children, tends to attenuate the associations observed. More precise methods of measuring physical activity utilizing objective measurement (e.g., accelerometry) are necessary to accurately establish the dose-response relationships between physical activity and its subdimensions (e.g., time spent at different intensity levels of physical activity) with metabolic health outcomes in youth.
In recent years, only a handful of studies have examined the association between physical activity and clustered metabolic risk using objective measures of physical activity (Table 1). Although most of the studies published have used a standardized risk score, the absolute definition varies across studies. For example, one study included the homeostasis model assessment score (the product of fasting glucose and insulin divided by a constant) and CRF in the clustered risk score (4), whereas others have used individual measures of insulin and glucose (e.g., Refs. 12, 28). Different measures of obesity (e.g., waist circumference or sum of skinfolds) have been included, and the risk score has also been defined with and without an obesity measure (e.g., Refs. 30, 63).
Table 1.
Associations between objectively measured physical activity and clustered metabolic risk factors in children and adolescents
| Reference | Objective of Study | Participants' Characteristics | Measure of Physical Activity | Measure of Clustered Metabolic Risk | Confounders Adjusted for | Findings |
|---|---|---|---|---|---|---|
| Andersen et al. (4) (2006) | To examine the associations between PA and metabolic risk | n (male/female): 817/915 Age: 9 and 15 yr Population Danish, Portuguese, and Estonian | Accelerometer (hip mounted, uniaxial) >3 days; TPA (average intensity, counts/min), and number of 5- and 10- min activity bouts >2,000 counts/min) | SBP, TG, TC:HDL-C, HOMA, sum of 4 skinfolds, CRF (maximal bike test) Dichotomized summary score zMS (>1 SD) | Age, sex, country | Odds ratios for having clustered risk for ascending quintiles of TPA were 3.29 (95% CI 1.96–5.52), 3.13 (95% CI 1.87 to 5.25), 2.51 (95% CI 1.47–4.26), and 2.03 (95% CI 1.18 to 3.50), respectively, compared with the most active quintile Similar odds ratios for quintiles of number of activity bouts |
| Brage et al. (12) (2004) | To examine the associations between PA and metabolic risk | n (male/female): 279/310 Age: 9 and 15 yr Population: Danish | Accelerometer (hip mounted, uniaxial) >3 days; TPA (average intensity, counts/min), normalizes by square root | BP, sum of 4 skinfolds, insulin, GLU, TG, and HDL-C zMS, non-Ob zMS | Age, sex, sexual maturation, ethnicity, parental smoking, SES, accelerometer unit, (+adiposity when outcome non-Ob zMS) |
TPA inversely related to zMS; −0.020 (95% CI −0.035 to −0.006), P = 0.008, and non-Ob zMS; −0.025 (95% CI −0.043 to −0.008), P = 0.005 |
| As above plus CRF | No significant association for zMS; −0.012 (95% CI −0.027 to 0.004), P = 0.127, inverse association for non-Ob zMS −0.020 (95% CI −0.039 to −0.00), P = 0.045 | |||||
| Butte et al. (14) (2007) | To examine the associations between PA and metabolic risk | n (male/female): 441/456 Age: 4–19 yr Population: Hispanic | Accelerometer (wrist-worn, uniaxial) >3 days; TPA (counts/day) and bouts of 5 and 10 min MVPA | WC, HDL-C, TG, BP, GLU Number of components of the MS (0–5) | Sex, age, BMI | Ordinal odds ratio 0.98 (95% CI 0.96 to 0.99), P = 0.03 for TPA and 0.94 (95% CI 0.89 to 0.99), P = 0.02 for 5-min bouts MVPA |
| Ekelund et al. (30) (2006) | To examine associations between PA, television viewing, and metabolic risk | n (male/female): 911/1010 Age: 9 and 15 yr Population: Danish, Portuguese, and Estonian | PA: Accelerometer (hip-mounted, uniaxial) >3 days; TPA (average intensity, counts/min) Sedentarism: time spent television viewing (hours/day) | BMI, sum of skin-folds, BP, GLU, HDL-C, TG, insulin zMS, non-Ob zMS | Sex, age group, study location, birth weight, maturity, smoking, parental SES, CRF (+adiposity when outcome non-Ob zMS) | Inverse association between TPA and zMS; −0.08 (95% CI −0.11 to −0.05), P < 0.001 and non-Ob zMS −0.9 (95% CI −0.23 to −0.06) P < 0.001 Associations between television viewing and zMS; 0.026 (95% CI −0.0003 to 0.052), P = 0.053, and non-Ob zMS; 0.01 (95% CI −0.017 to 0.038), P = 0.46 |
| Ekelund et al. (28) (2007) | To examine associations between PA with metabolic risk | n (male/female): 838/908 Age: 9 and 15 yr Population: Danish, Portuguese, and Estonian | Accelerometer (hip-mounted, uniaxial) ≥3 days; TPA (average intensity, counts/min); Time spent Sedentary, LPA, MPA, and VPA (min/day) | WC, BP, GLU, insulin; HDL-C, TG zMS zMS non-Ob | Sex, age, study location, birth weight, sexual maturity, smoking, maternal BMI, parental SES, and CRF (+adiposity when non-Ob zMS outcome) | Significant associations for ZMS and; TPA −0.08 (95% CI −0.12 to −0.06), P < 0.001, Sedentary 0.05 (95% CI 0.03–0.08), P < 0.001; LPA −0.03 (95% CI −0.06 to −0.005), P < 0.001; VPA −0.05 (95% CI −0.07 to −0.02), P < 0.001. Significant associations for non-Ob zMS and TPA −.008 (95% CI −0.11 to −0.06), P < 0.01; Sedentary 0.08 (95% CI 0.05–0.1), P < 0.001; LPA −0.06 (95% CI −0.08 to −0.03), P < 0.001; MPA −0.07 (95% CI −0.10 to −0.05), P < 0.001; VPA −0.06 (95% CI −0.08 to −0.03), P < 0.001 |
| Rizzo et al. (61) (2007) | To examine the associations of PA and metabolic risk | n (male/female) = 264/265 Age: 9 and 15 yr Population: Swedish | Accelerometer (hip mounted, uniaxial) ≥3 days; TPA (average intensity, counts/min), Time spent MPA, VPA, and MVPA (min/day) | Insulin, GLU, TG, TC, HDL-C, BP, sum of skinfolds zMS, non-Ob zMS | Pubertal status, body height, SES, and parental smoking Age and sex stratified |
TPA inversely associated with zMS and non-Ob zMS in 15- yr-old girls; −0.214 (p = 0.018) and −0.207 (p = 0.018), respectively. No significant associations between TPA and zMS or non-Ob zMS for all other age-sex groups. MPA, VPA, and MVPA all associated with non-Ob zMS in 15-yr-old adolescents only (results for zMS not reported). |
| As above plus CRF | TPA not associated with zMS or non-Ob zMS in any group; MPA, VPA, and MVPA not associated with non-Ob zMS (results for zMS not reported). |
BMI, body mass index; BF, body fat; BP, blood pressure; BW, body weight; CRF, cardioreparatory fitness; DBP, diastolic blood pressure; FM, fat mass; FFM, fat-free mass; HDL-C, high-density lipoprotein cholesterol; HOMA, homeostasis model assessment; GLU, glucose, LPA, light physical activity; MAP, mean arterial pressure; PWC, physical work capacity; MPA, Moderate vigorous activity; MS, Metabolic Syndrome; MVPA, moderate plus vigorous physical activity; PA, physical activity; SBP, systolic blood pressure; SES, socioeconomic status; TG, triglycerides; TC, total cholesterol; TPA, total physical activity (average intensity, volume); VPA, vigorous physical activity; WC, waist circumference; zMS, standardized clustered metabolic risk score (higher zMS represents a less favorable metabolic profile); non-Ob zMS, standardized clustered metabolic risk score excluding adiposity.
In a sample of overweight and nonoverweight Hispanic children, Butte et al. (14) showed that the number of metabolic risk factors was inversely associated with total physical activity as well as the number of physical activity bouts of at least 5 min of moderate to vigorous-intensity physical activity performed per day as measured by wrist accelerometry. These associations were independent of age, sex, and standardized body mass index (BMI) score. In the European Youth Heart Study (EYHS) cohort, Andersen et al. (4) showed a graded inverse association between physical activity quintiles measured by hip accelerometry, and clustered metabolic risk. The highest risk was observed in the lowest three quintiles of physical activity. Time spent in moderate and vigorous physical activity [≥2,000 counts/min, approximately equal to walking at 4 km/h (13)], was between 34 and 38 min in the least active quintile and increased to 167–131 min/day in the most active quintile. These observations did not differ by obesity level (i.e., across BMI categories).
In an earlier EYHS study using only the Danish cohort of 9- to 10-yr-old children, physical activity was also shown to be inversely associated with clustered metabolic risk (11). The influence of varying intensity levels of physical activity [i.e., time (min/day) spent at light-, moderate-, and vigorous-intensity physical activity] may in fact be differentially associated with metabolic risk. Ekelund et al. (28) observed inverse associations between total physical activity, as well as each subdimension of physical activity including time spent in light, moderate, and vigorous physical activity, in 9- and 15-yr-old youth for clustered metabolic risk, independent of CRF. The strength of the association was stronger for total physical activity compared with time spent at moderate- to-vigorous-intensity physical activity. In a similar but smaller study in Sweden, Rizzo et al. (61) found that vigorous physical activity was associated with nonobesity clustered metabolic risk in 15-yr-old girls. However, the association was attenuated after further adjustment for adiposity and CRF. Unfortunately, the magnitude of the association was not reported, and comparison across intensity domains could not be determined. Further research investigating the influence of physical activity intensity on clustered metabolic risk is warranted.
IS SEDENTARY BEHAVIOR ASSOCIATED WITH CLUSTERED METABOLIC RISK?
The association between sedentary behavior and metabolic health has also been explored. In adult studies where sedentary behavior has been defined by sitting time or general screen time (i.e., time spent television viewing and/or computer, video game use), associations with individual components of metabolic risk and the metabolic syndrome have been observed (1, 23, 32). The associations of television viewing and objectively measured physical activity by hip accelerometry, with individual and clustered metabolic risk factors, were examined in a large population-based sample from EYHS (30). The positive association between television viewing and clustered metabolic risk was only of borderline significance, after adjustment for physical activity and other confounding factors. However, these associations were explained by a positive association between television viewing and adiposity. In contrast, physical activity was independently and inversely associated with systolic and diastolic blood pressure, fasting glucose, insulin and triglycerides, and nonobesity clustered risk score, independent of obesity and other confounders. Similarly, objectively measured time spent in sedentary behavior (28) has also been shown to be positively and independently associated with clustered metabolic risk, in the same cohort.
Television viewing may not be a good marker of sedentary behavior in children, because associations observed between television viewing, obesity, and metabolic risk may be confounded or mediated by other behaviors, such as snacking while watching television.
The introduction of new generation technology games (i.e., Dance Dance Revolution, Konmami Computer Entertainment; Nintendo Wii) may result in higher energy expenditure compared with traditional sedentary-based computer games, although it appears the intensity of these games may not necessarily contribute toward recommendations for daily physical activity (38). Furthermore, the amount of energy expended during these games may decrease with increasing skills. However, with increasing interest in the inactivity physiology paradigm (physiological, medical, and public health impact of too much sedentary time), more research examining the effect of reducing overall sedentary time, breaking bouts of sedentary time, and increasing nonexercise activity on the metabolic profile of inactive populations is warranted (40).
Based on the limited number of studies available examining the dose-response association between subcomponents of physical activity and metabolic risk in youth, it appears that both reducing time spent sedentary and increasing overall physical activity and time spent at moderate and vigorous intensity may have beneficial effects on metabolic risk in healthy youth.
IS THE ASSOCIATION BETWEEN PHYSICAL ACTIVITY AND MARKERS OF METABOLIC HEALTH MEDIATED OR CONFOUNDED BY ADIPOSITY?
The degree to which adjustment for adiposity attenuates or modifies the association between physical activity and metabolic risk varies across studies. In general, physical activity is independently associated with clustered metabolic risk after adjusting for adiposity, and it appears that adiposity neither completely mediates nor confounds the associations between physical activity and nonobesity clustered risk. For example, when level of adiposity is excluded from the risk score and adjusted for as a covariate, the magnitude of the association between physical activity and the nonobesity metabolic risk tends to remain unchanged in some studies (11, 28), but it is attenuated in others (61).
Attenuation may be interpreted as evidence of confounding, although it is equally possible that this may suggest a mediating effect of adiposity, because this factor may lie on the causal pathway between physical activity and metabolic health. The different stability of the variables is relevant to an evaluation of potential confounding and mediation. For example, physical activity may detectably impact on variables such as fasting glucose and blood lipids, whereas no effect would be observed for adiposity, which is likely to be the result of long-term balance or imbalance between energy expenditure and intake. However, long-term effects of physical activity on other risk factors could plausibly exist. Moreover, different degrees of measurement precision in the assessment of adiposity and physical activity may also explain attenuation of results and variation between studies.
IS CARDIORESPIRATORY FITNESS ASSOCIATED WITH CLUSTERED METABOLIC RISK IN YOUTH?
In general, studies examining the association between CRF and clustered metabolic risk in youth report an inverse relationship, such that as CRF increases, the risk of an unfavorable metabolic risk profile is reduced. A more diverse range of populations have been investigated compared with studies that have only assessed physical activity, and measures of CRF have included both maximal and submaximal tests, bike and treadmill tests, and various field running tests (Table 2).
Table 2.
Associations between cardiorespiratory fitness and clustered metabolic risk factors in children and adolescents
| Reference | Objective of Study | Participants Characteristics | Measure of CRF | Measure of Clustered Metabolic Risk | Confounders Adjusted for | Findings |
|---|---|---|---|---|---|---|
| Anderssen et al. (5) (2007) | To examine associations between CRF and metabolic risk | n (male/female) = 1391/1454 Age: 9 and 15 yr Population: Danish, Portuguese, and Estonian | Maximal bike test (maximal W/kg) | TC:HDL-C, sum 4 skinfolds, SBP, TG, HOMA, zMS, non-Ob zMS | Puberty, SES, family history of CVD, and diabetes, country, age, and sex | Odds ratios (ascending quartiles of fitness): 13.0 (95% CI 8.8 to 19.1); 4.8 (95% CI 3.2 to 7.1) and 2.5 (95% CI 1.6 to 3.8). Stratified analyses by age group, sex, and country showed similar associations |
| Dubose et al. (20) (2007) | To examine the influence of CRF and BMI on metabolic risk | n (male/female) = 182/193 Age: 7–9 yr Population: American | Submaximal bike test based on PWC170 (peak W/kg) | WC, BP, HOMA, HDL-C, TG zMS, non-Ob zMS | Age, sex, race | In low vs. high CRF, mean (SE) zMS and non-Ob zMS were 1.21(3.3) vs −1.21 (2.0) and 0.88 (2.6) vs. −0.88 (1.7), respectively (all P < 0.001). |
| Ekelund et al. (28) (2007) | To examine associations between CRF and metabolic risk | n (male/female): 838/908 Age: 9 and 15 yr Population: Danish, Portuguese, Estonian | Maximal bike test (W/per unit FFM) | WC, BP, GLU, insulin, HDL-C, TG zMS non-Ob zMS | Sex, age, study location, birth weight, sexual maturity, smoking, maternal BMI, parental SES, PA, WC (except when zMS outcome) | Significant associations between zMS and CRF −0.09 (95% CI −0.12 to −0.06); Significant associations for non-Ob zMS and CRF −0.05 (95% CI −0.08 to −0.02) |
| Eisenmann et al. (25) (2005) | To examine the association of BMI and CRF with metabolic risk | n (male/female): 416/345 Age: 9–18 yr Population: French Canadian | PWC150 (W at a heart rate of 150 beats/min) | GLU, MAP, LDL-C, TG, TC:HDL-C zMS | Age-adjusted BMI | High-fit/low-BMI group had better risk profile while the low-fit/high-BMI showed a poorer profile. Significant main effects for BMI and PWC (P < 0.05) in male and female subjects, interaction BMI by PWC significant in female subjects (P < 0.05) |
| Eisenmann et al. (26) (2007) | To examine differences in metabolic risk across four cross-tabulated groups of CRF and BF | n (male/female): 860/755 Age: 9–15 yr Population: Australian | Maximal 1.6-k run (min) | WC, MAP, HDL-C, TG, zMS | Age standardized, sex stratified | Significant trend for risk score across groups (low-fat/high-fit; low-fat/low-fit; high-fat/high-fit, high-fat/low-fit) F = 60.6 P < 0.001 in male and F = 57.3, P < 0.001 in female subjects |
| Eisenmann et al. (27) (2007) | To examine differences in metabolic risk across 4 cross-tabulated groups of CRF and BMI | n (male/female): 296/188 Age: 8–18 yr Population: American | Maximal treadmill (min) | GLU, MAP, HDL-C, TG, WC zMS | Age standardized BMI and CRF | Trend for risk score across groups (low-fat/high-fit; low-fat/low-fit; high-fat/high-fit, high-fat/low-fit) F = 6.84, p = 0.001 in male but not in female subjects, F = 1.78, P = 0.15 |
| Garcia-Artero et al. (36) (2007) | To determine whether the level of physical fitness (CRF and muscle strength) influences lipid metabolic profiles | n (male/female) 248/212 Age: 13–18 yr Population: Spanish | 20-m shuttle run Muscle strength (standing long jump, handgrip, beat-arm hang test) | TG, LDL-C, HDL-C, GLU Standardized lipid-metabolic index | Age, sex, and maturation | CRF inversely associated with lipid metabolic risk in male (P = 0.001) but not female subjects (adjusted for PA and muscle strength). Inverse association between muscle strength and the lipid metabolic index in female subjects (P = 0.048) (adjusted for CRF) |
| Janssen et al. (47) (2007) | To examine associations between CRF and metabolic risk | n (male/female) = 829/732 Age: 12–19 yr Population: American | Submaximal treadmill test (ml O2·kg−1·min−1) | WC, TG, HDL-C, GLU, BP ≥ 3 Components of MS | Age, ethnicity, poverty-to-income ratio, smoking, carbohydrate and fat intake | Odds ratios for MS by CRF tertiles (low CRF referent were) 0.18 (0.07 to 0.48), P < 0.05 for moderate CRF, and 0.01 (0.00 to 0.07), P < 0.05 for highest CRF |
| Rizzo et al. (61) (2007) | To examine the associations between CRF and metabolic risk | n (male/female) = 264/265 Age: 9 and 15 yr Population: Swedish | Maximal bike test (ml O2·kg−1·min−1) | Insulin, GLU, TG, TC, HDL-C, BP, sum of skinfolds zMS, non-Ob zMS | Pubertal status, body height, SES, and parental smoking and PA | CRF inversely associated with zMS in: 9-yr-old girls −0.220 (95% CI −0.037 to −0.005, P = 0.011), 15-yr-old girls −0.322 (95% CI −0.042 to −0.012, P = 0.001), 9-yr-old boys −0.27 (95% CI −0.032 to −0.007), P = 0.002; 15-yr-old boys −0.447 (95% CI −0.062 to −0.028), P < 0.001. Non-Ob zMS nonsignificant in 9-yr-old boys and girls, inverse associations 15-yr-old girls −0.231 (95% CI −0.038 to −0.004), P = 0.015; 15-yr-old boys −0.354 (95% CI −0.056 to −0.019), P < 0:001. |
| Ruiz et al. (63) (2007) | To examine the associations between CRF and metabolic risk | n (male/female) = 429/444 Age: 9–10 yr Population: Swedish and Estonian | Maximal bike, test (ml O2·kg−1·min−1) | Insulin, GLU, HDL-C, TG, skinfolds, BP zMS <75th percentile = lower risk | Inverse associations of CRF quartiles with zMS. Girls CRF>37 ml O2·kg−1·min−1 increased OR of a low zMS score (3.09; 95% CI 1.98 to 4.82), P < 0.001) compared with girls ≤ 37 ml O2 · kg−1·min−1; Boys CRF>42.1 ml O2·kg−1·min−1 increased OR of a low zMS score (2.42; 95% CI 1.57 to 3.76), P < 0.001) compared with boys < 42.1 ml O2·kg−1·min−1 | |
| Shaibi et al. (66) (2005) | To determine the association of CRF with number of MS risk factors | n (male/female) = 91/72 Age: 11 yr Population: Latino | Maximal treadmill (l/min, l·min−1·BW−1, l·min−1·FFM−1) | WC, TG, HDL-C, BP, FG Number of components of the MS (0, 1, 2, or ≥3) | Sex, age, FM, and FFM | No association between V̇o2max with number of MS risk factors. V̇o2max/BW (P < 0.001) and V̇o2max/FFM inversely associated with number of risk factors |
V̇o2max, maximal oxygen consumption; PWC170, physical working capacity at 170 W.
In a large sample of 9- and 15-yr olds, CRF was inversely associated with clustered metabolic risk (5). Odds ratios for the relationship between age- and sex-specific quartiles of CRF assessed by a maximal bike test and the clustering of metabolic risk factors was 10.4 for girls and 15.8 for boys in lowest quartile of CRF compared with those in the highest quartile. Expressing CRF from a maximal bike test as watts per unit of fat-free mass (FFM), Ekelund et al. (28) showed significant inverse associations between CRF and clustered metabolic risk with and without adiposity. Similarly after normalization for body weight or FFM, Shaibi et al. (66) also showed that CRF was inversely associated with the number of risk factors present in a Latino population. Ruiz et al. (63) showed an inverse association between CRF (ml·kg−1·min−1) and clustered metabolic risk in 9- and 10-yr old Swedish and Estonian children. Similarly, in Swedish 9- and 15-yr olds, an inverse association of CRF with clustered metabolic risk was observed across age-sex groups (61). However, excluding obesity from the outcome and controlling for body fat as a covariate attenuated the association.
IS THE ASSOCIATION BETWEEN CARDIORESPIRATORY FITNESS AND METABOLIC HEALTH MEDIATED BY ADIPOSITY?
It is difficult to determine whether adiposity confounds, mediates, or modifies the association between CRF and metabolic health. Interpretation of the magnitude of association between CRF and metabolic risk depends on how the exposures and outcome are measured and expressed, and most studies have expressed CRF in relation to body weight, which is strongly related to adiposity. Normalization by body weight may “overcorrect” for differences in body size between individuals and test duration for indirect CRF tests involving weight-bearing activity may underestimate maximal oxygen consumption in heavier children (6), whereas the converse may be true for a bicycle test. When adiposity is also a part of the outcome, there is a risk of creating statistical artifacts in the subsequent adjustment for adiposity. One way to overcome this is to remove adiposity from the outcome and normalize CRF by FFM. Indeed, Ekelund et al. (28) found that the association between CRF per kilogram of FFM and metabolic risk was still apparent, although attenuated, after adjustment for adiposity. This then suggests that confounding is unlikely to explain all of the association, or alternatively that only some of the effect of CRF on clustered metabolic risk is mediated by adiposity.
Another way to examine effect mediation is by stratification. Building on previous studies, Eisenmann et al. (26) showed that children and adolescents with high percent body fat and high CRF have better metabolic risk profiles than those classified as high fat but low CRF. A linear relationship was evident in each of the four cross-tabulated groups of CRF and fatness with the metabolic risk score. In another study, Eisenmann et al. (27) reported that in 8- to 18-yr olds, there was a significant difference in metabolic risk scores across four fitness-fatness groups in male subjects and a trend for significance in females. Although high CRF did not completely remove the risk associated with high BMI on clustered metabolic risk, the study did show an attenuation of the association between BMI and clustered risk after adjustment for CRF. DuBose et al. (20) also showed that BMI was positively and CRF negatively associated with clustered metabolic risk. Clustered metabolic risk varied across fitness-fatness groups, where high CRF appeared to attenuate the metabolic risk score within BMI categories. These studies support the protective role of CRF across levels of obesity, therefore also in obese youth, although the magnitude of the associations appears to be small to moderate.
ARE PHYSICAL ACTIVITY AND CARDIORESPIRATORY FITNESS INDEPENDENTLY ASSOCIATED WITH CLUSTERED METABOLIC RISK IN YOUTH?
Earlier studies examining the cross-sectional and prospective effects of both CRF and physical activity on metabolic risk in adulthood have relied on self-report measures of physical activity and a comparably stronger objective measure of CRF. Such studies tend to show associations for metabolic risk with CRF but not physical activity. For example, the Amsterdam Growth and Health Longitudinal study showed that CRF but not self-reported physical activity in adolescence was inversely related to CVD risk factors in adulthood (32 yr), (70). Similarly, the Muscatine Study showed an inverse association between changes in CRF and CVD risk factors from childhood to adolescence; however, no associations were found for self-reported physical activity (48). This is in line with findings in 460 Spanish adolescents, in whom no association was found between self-reported physical activity and a standardized lipid-metabolic risk score (triglyceride, low-density lipoprotein-cholesterol, HDL-cholesterol, and glucose) but an inverse relationship for CRF and metabolic risk was observed for males, independent of physical activity (36). Studies using objective measures of both physical activity and CRF, however, tend to demonstrate independent roles of both physical activity and CRF with metabolic health.
In 301 Danish 9-yr olds (11), CRF and accelerometry-measured physical activity were both inversely related to clustered metabolic risk but only independently so for physical activity when excluding adiposity from the standardized clustered risk score and adjusting for it as a confounder. Furthermore, a significant interaction between physical activity and CRF was observed, suggesting that CRF may modify the association between physical activity and metabolic health in such a way that the effect of physical activity may be stronger amongst children with low CRF. In a larger sample, Ekelund et al. (28) found independent inverse associations of physical activity and CRF with clustered metabolic risk. The relationship between physical activity and clustered risk was similar in magnitude as that observed for CRF after adjustment for body fatness. The association between CRF with clustered risk was partly mediated by adiposity, whereas the association between physical activity and clustered risk was independent of adiposity. Conversely, a smaller Swedish study showed that physical activity did not exert an independent effect on clustered metabolic risk after controlling for CRF (61). Differences between studies may be explained by different sample sizes, which affect the power to detect associations, and different ways of expressing CRF as discussed previously.
CRF is partly influenced by genetic factors (10) and by regular exercise. However, the association between CRF and physical activity appears to be weak in population-based studies in children (28, 45). This may then suggest that physical activity and CRF influence metabolic risk through separate pathways or that CRF is a marker for specific muscle characteristics, for example, muscle fiber-type composition, which may affect metabolic health. Indeed, insulin-stimulated glucose transport (18) and expression of the insulin-regulated glucose transporter GLUT4 maybe muscle fiber specific (18), and others have suggested that slow-twitch muscle fibers are associated with increased lipid oxidation (69).
For the most part, there are a limited number of studies using sufficiently precise measures of physical activity to determine whether CRF or physical activity is a more important determinant of metabolic disease risk. Physical activity is difficult to measure accurately, and limitations are often amplified in youth, partly due to the intermittent nature of their physical activity compared with adults and problems with the accurate self-reporting of physical activity by youth (7, 65). Consequently, weak associations between physical activity and metabolic risk previously observed are likely to be at least partly due to the use of self-report methods to assess physical activity. In general, inaccurate measurement with nondifferential error leads to an underestimation of the true association between an exposure and an outcome in epidemiological studies (72). Regardless of different levels of measurement precision in assessing the two exposures, both physical activity and CRF are associated with metabolic disease risk. Some could regard the statistically significant but relatively weak associations between physical activity, CRF and metabolic disease risk summarized in this review as clinically insignificant. However, all observed associations are from studies including healthy children, and because metabolic risk factors track over time, it would appear that regular participation in physical activity and improvements in CRF may result in substantial reductions of disease occurrence in later in life (8).
CONCLUSION
Recent studies using more precise quantification of the exposures suggest that both physical activity and CRF are separately and independently associated with metabolic risk factors in children, but possibly through different pathways. Further research using accurate measurements of both exposure and outcome are necessary to further investigate these relationships in youth. Evidence that physical activity and CRF develop early in life and track into adulthood suggests that reducing sedentary behavior and increasing overall physical activity in youth is a potential strategy to overcoming the emerging population burden associated with metabolic cardiovascular disease risk.
From a public health perspective, associations between increasing levels of physical activity and metabolic risk factors that are independent of CRF and fatness are important. First, encouraging small incremental increases in physical activity may be more feasible at a population level, but this may not necessarily improve CRF. Second, increasing levels of habitual physical activity may confer benefits even if fat mass is not changed. For example in adults, physical activity does not appear to significantly contribute to weight loss, but it has an important role in the maintenance of weight loss (43). The minimal and optimal amount of physical activity required to prevent the clustering of metabolic risk in children are unknown. With the absence of direct evidence due to the lack of longitudinal population-based studies using objective measures of physical activity and large-scale randomized controlled trials, current guidelines of at least 60 min/day of moderate- to vigorous-intensity physical activity may be reasonable. These are consistent with current evidence of the effects of physical activity on general health and behavioral outcomes (46, 68). However, higher levels of physical activity may be required (4). Further research is warranted to quantify how much physical activity is needed to prevent the metabolic syndrome and how much is required to reduce the risk in those who already have unfavorable but subclinical levels in one or more features of the syndrome.
REFERENCES
- 1.Aadahl M, Kjaer M, Jorgensen T. Influence of time spent on TV viewing and vigorous intensity physical activity on cardiovascular biomarkers. The Inter 99 study. Eur J Cardiovasc Prev Rehabil 14: 660–665, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469–480, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–553, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, Anderssen SA. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study). Lancet 368: 299–304, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Anderssen SA, Cooper AR, Riddoch C, Sardinha LB, Harro M, Brage S, Andersen LB. Low cardiorespiratory fitness is a strong predictor for clustering of cardiovascular disease risk factors in children independent of country, age and sex. Eur J Cardiovasc Prev Rehabil 14: 526–531, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong N, Welsman JR. Aerobic fitness: what are we measuring? Med Sport Sci 50: 5–25, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Bailey RC, Olson J, Pepper SL, Porszasz J, Barstow TJ, Cooper DM. The level and tempo of children's physical activities: an observational study. Med Sci Sports Exerc 27: 1033–1041, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med 154: 1842–1847, 1994. [PubMed] [Google Scholar]
- 9.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol 99: 1193–1204, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc 33: S446–S451; discussion S452–S453, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Brage S, Wedderkopp N, Ekelund U, Franks PW, Wareham NJ, Andersen LB, Froberg K. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS). Diabetes Care 27: 2141–2148, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Brage S, Wedderkopp N, Ekelund U, Franks PW, Wareham NJ, Andersen LB, Froberg K. Objectively measured physical activity correlates with indices of insulin resistance in Danish children. The European Youth Heart Study (EYHS). Int J Obes Relat Metab Disord 28: 1503–1508, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Brage S, Wedderkopp N, Franks PW, Andersen LB, Froberg K. Reexamination of validity and reliability of the CSA monitor in walking and running. Med Sci Sports Exerc 35: 1447–1454, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Butte NF, Puyau MR, Adolph AL, Vohra FA, Zakeri I. Physical activity in nonoverweight and overweight Hispanic children and adolescents. Med Sci Sports Exerc 39: 1257–1266, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 100: 126–131, 1985. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in Blacks and Whites: the Bogalusa Heart Study. Am J Epidemiol 166: 527–533, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 157: 821–827, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA. Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes 49: 1092–1095, 2000. [DOI] [PubMed] [Google Scholar]
- 19.de Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, Rifai N. Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clin Chem 52: 1325–1330, 2006. [DOI] [PubMed] [Google Scholar]
- 20.DuBose KD, Eisenmann JC, Donnelly JE. Aerobic fitness attenuates the metabolic syndrome score in normal-weight, at-risk-for-overweight, and overweight children. Pediatrics 120: e1262–1268, 2007. [DOI] [PubMed]
- 21.Duncan GE Exercise, fitness, and cardiovascular disease risk in type 2 diabetes and the metabolic syndrome. Curr Diab Rep 6: 29–35, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999–2000. Diabetes Care 27: 2438–2443, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Dunstan DW, Salmon J, Owen N, Armstrong T, Zimmet PZ, Welborn TA, Cameron AJ, Dwyer T, Jolley D, Shaw JE. Associations of TV viewing and physical activity with the metabolic syndrome in Australian adults. Diabetologia 48: 2254–2261, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Eisenmann JC Aerobic fitness, fatness and the metabolic syndrome in children and adolescents. Acta Paediatr 96: 1723–1727, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Eisenmann JC, Katzmarzyk PT, Perusse L, Tremblay A, Despres JP, Bouchard C. Aerobic fitness, body mass index, and CVD risk factors among adolescents: the Quebec family study. Int J Obes (Lond) 29: 1077–1083, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 39: 1251–1256, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Eisenmann JC, Welk GJ, Wickel EE, Blair SN. Combined influence of cardiorespiratory fitness and body mass index on cardiovascular disease risk factors among 8–18 year old youth: The Aerobics Center Longitudinal Study. Int J Pediatr Obes 2: 66–72, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Ekelund U, Anderssen SA, Froberg K, Sardinha LB, Andersen LB, Brage S. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: the European youth heart study. Diabetologia 50: 1832–1840, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care 28: 1195–1200, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Ekelund U, Brage S, Froberg K, Harro M, Anderssen SA, Sardinha LB, Riddoch C, Andersen LB. TV viewing and physical activity are independently associated with metabolic risk in children: the European Youth Heart Study. PLoS Med 3: e488, 2006. [DOI] [PMC free article] [PubMed]
- 31.Ekelund U, Franks PW, Sharp S, Brage S, Wareham NJ. Increase in physical activity energy expenditure is associated with reduced metabolic risk independent of change in fatness and fitness. Diabetes Care 30: 2101–2106, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES, Kohl HW 3rd, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among US adults Obes Res 13: 608–614, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Ford ES, Li C. Physical activity or fitness and the metabolic syndrome. Expert Rev Cardiovasc Ther 4: 897–915, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, Infante AM, Krakoff J, Looker HC. Childhood predictors of young-onset type 2 diabetes. Diabetes 56: 2964–2972, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Froberg K, Andersen LB. Mini review: physical activity and fitness and its relations to cardiovascular disease risk factors in children. Int J Obes (Lond) 29, Suppl 2: S34–S39, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Artero E, Ortega FB, Ruiz JR, Mesa JL, Delgado M, Gonzalez-Gross M, Garcia-Fuentes M, Vicente-Rodriguez G, Gutierrez A, Castillo MJ. [Lipid and metabolic profiles in adolescents are affected more by physical fitness than physical activity (AVENA study)]. Rev Esp Cardiol 60: 581–588, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab 88: 1417–1427, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Graves L, Stratton G, Ridgers ND, Cable NT. Comparison of energy expenditure in adolescents when playing new generation and sedentary computer games: cross sectional study. Br Med J (Clin Res Ed) 335: 1282–1284, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grundy SM Metabolic syndrome scientific statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol 25: 2243–2244, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Hanley AJ, Festa A, D'Agostino RB Jr, Wagenknecht LE, Savage PJ, Tracy RP, Saad MF, Haffner SM. Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes 53: 1773–1781, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Hasselstrom H, Hansen SE, Froberg K, Andersen LB. Physical fitness and physical activity during adolescence as predictors of cardiovascular disease risk in young adulthood. Danish Youth and Sports Study. An eight-year follow-up study. Int J Sports Med 23, Suppl 1: S27–S31, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Hill JO, Wyatt HR. Role of physical activity in preventing and treating obesity. J Appl Physiol 99: 765–770, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Huang TT, Ball GD, Franks PW. Metabolic syndrome in youth: current issues and challenges. Appl Physiol Nutr Metab 32: 13–22, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Imperatore G, Cheng YJ, Williams DE, Fulton J, Gregg EW. Physical activity, cardiovascular fitness, and insulin sensitivity among U.S. adolescents: the National Health and Nutrition Examination Survey, 1999–2002. Diabetes Care 29: 1567–1572, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Janssen I Physical activity guidelines for children and youth. Appl Physiol Nutr Metab 32: S109–S121, 2007. [PubMed] [Google Scholar]
- 47.Janssen I, Cramp WC. Cardiorespiratory fitness is strongly related to the metabolic syndrome in adolescents. Diabetes Care 30: 2143–2144, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Janz KF, Dawson JD, Mahoney LT. Increases in physical fitness during childhood improve cardiovascular health during adolescence: the Muscatine Study. Int J Sports Med 23, Suppl 1: S15–S21, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Jolliffe CJ, Janssen I. Development of age-specific adolescent metabolic syndrome criteria that are linked to the Adult Treatment Panel III and International Diabetes Federation criteria. J Am Coll Cardiol 49: 891–898, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 28: 2289–2304, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Kelishadi R, Razaghi EM, Gouya MM, Ardalan G, Gheiratmand R, Delavari A, Motaghian M, Ziaee V, Siadat ZD, Majdzadeh R, Heshmat R, Barekati H, Arabi MS, Heidarzadeh A, Shariatinejad K. Association of physical activity and the metabolic syndrome in children and adolescents: CASPIAN Study. Horm Res 67: 46–52, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Kristensen PL, Wedderkopp N, Moller NC, Andersen LB, Bai CN, Froberg K. Tracking and prevalence of cardiovascular disease risk factors across socio-economic classes: a longitudinal substudy of the European Youth Heart Study. BMC Public Health 6: 20, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 25: 1612–1618, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Malina RM Tracking of physical activity and physical fitness across the lifespan. Res Q Exerc Sport 67: S48–S57, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 163: 427–436, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia 49: 2078–2085, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord 28, Suppl 3: S2–9, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Ragland DR Dichotomizing continuous outcome variables: dependence of the magnitude of association and statistical power on the cutpoint. Epidemiology 3: 434–440, 1992. [DOI] [PubMed] [Google Scholar]
- 59.Raitakari OT, Porkka KV, Viikari JS, Ronnemaa T, Akerblom HK. Clustering of risk factors for coronary heart disease in children and adolescents. The Cardiovascular Risk in Young Finns Study. Acta Paediatr 83: 935–940, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Ribeiro JC, Guerra S, Oliveira J, Teixeira-Pinto A, Twisk JW, Duarte JA, Mota J. Physical activity and biological risk factors clustering in pediatric population. Prev Med 39: 596–601, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Rizzo NS, Ruiz JR, Hurtig-Wennlof A, Ortega FB, Sjostrom M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: the European youth heart study. J Pediatr 150: 388–394, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Roberts CK, Barnard RJ. Effects of exercise and diet on chronic disease. J Appl Physiol 98: 3–30, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz JR, Ortega FB, Rizzo NS, Villa I, Hurtig-Wennlof A, Oja L, Sjostrom M. High cardiovascular fitness is associated with low metabolic risk score in children: the European Youth Heart Study. Pediatr Res 61: 350–355, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Saland JM Update on the metabolic syndrome in children. Curr Opin Pediatr 19: 183–191, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Sallis JF Self-report measures of children's physical activity. J Sch Health 61: 215–219, 1991. [DOI] [PubMed] [Google Scholar]
- 66.Shaibi GQ, Cruz ML, Ball GD, Weigensberg MJ, Kobaissi HA, Salem GJ, Goran MI. Cardiovascular fitness and the metabolic syndrome in overweight latino youths. Med Sci Sports Exerc 37: 922–928, 2005. [PubMed] [Google Scholar]
- 67.Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation 107: 1448–1453, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, Gutin B, Hergenroeder AC, Must A, Nixon PA, Pivarnik JM, Rowland T, Trost S, Trudeau F. Evidence based physical activity for school-age youth. J Pediatr 146: 732–737, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Turpeinen JP, Leppavuori J, Heinonen OJ, Kaila K, Salo J, Lilja M, Kesaniemi YA. Muscle fiber type I influences lipid oxidation during low-intensity exercise in moderately active middle-aged men. Scand J Med Sci Sports 16: 134–140, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Twisk JW, Kemper HC, van Mechelen W. The relationship between physical fitness and physical activity during adolescence and cardiovascular disease risk factors at adult age. The Amsterdam Growth and Health Longitudinal Study. Int J Sports Med 23, Suppl 1: S8–S14, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Wijndaele K, Beunen G, Duvigneaud N, Matton L, Duquet W, Thomis M, Lefevre J, Philippaerts RM. A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care 29: 2329, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Wong MY, Day NE, Luan JA, Chan KP, Wareham NJ. The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? Int J Epidemiol 32: 51–57, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Yach D, Hawkes C, Gould CL, Hofman KJ. The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA 291: 2616–2622, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Yang X, Telama R, Leskinen E, Mansikkaniemi K, Viikari J, Raitakari OT. Testing a model of physical activity and obesity tracking from youth to adulthood: the cardiovascular risk in young Finns study. Int J Obes (Lond) 31: 521–527, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes 8: 299–306, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 414: 782–787, 2001. [DOI] [PubMed] [Google Scholar]