Abstract
Effects of intrathecally administered pituitary adenylate cyclase activating polypeptide-38 (PACAP-38, 0.1–30 µg) on lower urinary tract function were examined in unanesthetized, decerebrate rats with an intact spinal cord and after chronic spinal cord transection (SCT). PACAP-38 was also studied in rats with intact or bilaterally transected hypogastric nerves (HGNs), to determine if sympathetic pathways to the bladder influenced responses. In SCT rats with intact HGNs under isovolumetric conditions, 30 µg of PACAP-38 but not lower doses (0.1–10 µg) increased (mean 194 %) bladder contraction amplitude (BCA). In SCT rats with sectioned HGNs, 10 µg and 30 µg of PACAP-38 increased BCA by 62 % and 195 %, respectively. On the other hand, during continuous infusion cystometrograms (CMGs) in SCT rats with intact or sectioned HGNs, PACAP-38 (10 µg and 30 µg) markedly reduced or completely suppressed BCA (60 % and 90 %, respectively) and reduced external urethral sphincter (EUS) EMG activity (58 % and 91 %, respectively). During CMGs in spinal cord intact rats, with intact HGNs PACAP-38 30 µg increased BCA (26 %) but after HGN section PACAP-38 10 µg and 30 µg increased BCA by 21 % and 35 %. These results suggest that after SCT, PACAP-38 activates spinal circuitry to facilitate the parasympathetic outflow to the urinary bladder and that the elimination of sympathetic pathways enhances this effect. The decrease in BCA by PACAP-38 during CMGs in SCT rats is most reasonably attributed to a reduction in urethral outlet resistance due to suppression of excitatory EUS reflexes.
Keywords: external urethral sphincter, hypogastric nerves, micturition reflex, neuropeptide, parasympathetic, sympathetic, decerebration, spinal cord injury
INTRODUCTION
Pituitary adenylate cyclase activating polypeptide (PACAP) is a brain-gut 38 amino acid peptide which was isolated from ovine hypothalamic tissue extracts (Miyata et al., 1989). Identical primary structures of PACAP were identified in sheep, rat and human. PACAP is a member of the secretin/glucagon/vasoactive intestinal polypeptide (VIP) family and has a 68 % homology to VIP (Arimura, 1992). PACAP can act on three types of receptors including VPAC1 and VPAC2 which respond to PACAP and VIP, and PAC1 which is a specific receptor for PACAP (Laburthe et al., 2002).
PACAP is not only present in the central nervous system (Arimura et al., 1991) but also in the peripheral nervous system in primary afferent neurons (Dun et al., 1996) innervating various peripheral organs such as the pancreas (Fridolf et al., 1992), gastrointestinal tract (Sundler et al., 1992), the eye (Wang et al., 1995) and the urogenital tract (Fahrenkrug and Hannibal, 1998; Hernández et al., 2006a,b; Reubi, 2000).
Immunohistochemical studies identified PACAP-containing or VIP-containing nerve fibers projecting to thoracolumbar sympathetic and lumbosacral parasympathetic nuclei (de Groat et al., 1990; Dun et al., 1996; Zvarova et al., 2005). In the rat intrathecal (i.t.) administration of PACAP caused an elevation of blood pressure and when tested in vitro induced a long-lasting excitatory effect on sympathetic preganglionic neurons (Lai et al., 1997). I.t. injection of PACAP-27 also facilitated bladder contractions in normal, conscious rats, indicating that the peptide has an excitatory effect on parasympathetic pathways in lumbosacral spinal cord (Ishizuka et al., 1995). Electrophysiological studies using the whole-cell patch clamp techniques revealed that PACAP had an excitatory action on lumbosacral parasympathetic preganglionic neurons in the neonatal rat (Miura et al, 2001). On the contrary, i.t. administration of VIP did not change micturition pressure in rats, but did reduce the bladder volume for inducing micturition and facilitated voiding (Igawa et al., 1993). Interestingly, in normal cats, large doses of VIP (1–10 µg i.t.) inhibited bladder activity, whereas in chronic spinal cats, smaller doses of VIP (0.1–1 µg i.t.) facilitated bladder activity (de Groat et al, 1990). Thus, the spinal actions of VIP were altered by spinal cord injury. In addition, after spinal cord injury the density and/or pattern of VIP-containing afferent projections to the sacral parasympathetic nucleus in cats (Thor et al., 1986) and PACAP-containing afferent projections to the lumbosacral parasympathetic nucleus in rats were increased (Zvarova et al., 2005), suggesting that spinal cord injury induces plasticity in VIP/PACAP afferent pathways. The i.t. administration of a PAC1 receptor antagonist in chronic spinal cord transected rats reduced bladder hyperactivity, suggesting that PACAP plays a role in bladder dysfunction after spinal cord injury (Zvara et al., 2006).
The present experiments were conducted to determine whether the effects of PACAP on bladder activity in the rat were also altered after spinal cord injury and whether sympathetic pathways to the bladder traveling in the hypogastric nerves (HGNs) affected the responses induced by i.t. administration of PACAP. In these studies, effects of a range of doses of PACAP-38 (0.1–30 µg i.t.) on lower urinary tract functions were evaluated in decerebrate, unanesthetized chronically-spinal transected as well as spinal intact rats with the HGNs intact or transected.
A preliminary report of this study has been presented in an abstract (Yoshiyama and de Groat, 1997).
MATERIALS AND METHODS
Animal Preparation
Forty-three female Sprague-Dawley rats (Charles River Laboratories, Boston, MA) weighing 235–340 g (mean=271 ± 3 g) were used in this study. The animals were housed under a 12 h light/dark cycle with controlled humidity and temperature. Standard pellet diet and water were available ad libitum. All animal procedures were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee and complied with guidelines outlined in the National Institute of Health Guide for the Care and Use of Laboratory Animals. The animals were anesthetized with halothane (2 %) in oxygen (flow rate: 1.0 l/min) during all surgical procedures. The trachea was cannulated with a polyethylene tube (PE-240) to facilitate respiration. An indwelling i.t. catheter was inserted on the day of an experiment, according to the technique of Yaksh and Rudy (1976). A midline incision on the dorsal side of neck was made with a scalpel and the occipital crest of the skull was exposed with fine scissors. The atlanto-occipital membrane was then incised at the midline using the tip of an 18-gauge needle as a cutting edge. After identifying the T13-vertebrae at the most caudal rib, the distance between the occipital crest of the skull and the L1-vertabrae, where the L6-spinal segment is located, was measured in each rat. A catheter (PE-10) was inserted through the opening in atlanto-occipital membrane and passed caudally to the L6-level of the spinal cord. At the end of the experiment, a laminectomy was performed to verify the catheter tip located at the L6-segmental level.
Spinal cord transection was performed in 24 rats by sectioning at the T8–9 level under halothane anesthesia. After the laminectomy, the dura and spinal cord were cut with fine scissors, and a sterile sponge (Gelform, The Upjohn Company, Kalamazoo, MI) was placed between the cut ends. The bladders of spinalized rats were manually expressed two or three times daily by applying pressure over the lower abdomen to release retained urine, and perigenital stimulation with cotton swab was performed to encourage reflex bladder emptying (Mallory et al., 1989). The experiments on spinalized rats were performed 3 to 4 weeks post-spinalization. In spinalized rats on the day of cystometric recording, the T11–12 spinal vertebrae were removed and the dura mater was incised using the tip of a 30-gauge needle as a cutting edge to insert an i.t. catheter (PE-10) caudally to the L6-level of the spinal cord.
Precollicular decerebration was performed according to a published method (Sapru and Krieger, 1978) that included ligating both carotid arteries followed by removal of the forebrain using a blunt spatula. Halothane was then discontinued. Cotton and Avitene (MedChem Products Inc., Woburn, MA) were placed in the intracranial cavity and covered with agar. Experiments were started 2 h after the decerebration and conducted under unanesthetized conditions (Yoshiyama et al., 1997).
A transurethral bladder catheter connected to a pressure transducer was used to record the bladder pressure isovolumetrically with the urethral outlet ligated at the urethral orifice or to record the pressure during a cystometrogram (CMG) when the bladder was filled with a constant infusion of physiological saline and allowed to void around the catheter. To evoke isovolumetric contractions the bladder was filled to a volume that evoked a large amplitude (>20 cm H2O) contraction and then infusion was stopped. Continuous CMGs were performed using a constant, rapid infusion (0.21 ml/min) of saline into the bladder to elicit repetitive voidings, which allowed collection of data for a large number of voiding cycles (Maggi et al., 1986). PE-90 and PE-50 cannulae were used for isovolumetric recording and constant infusion CMGs, respectively. In all animals during isovolumetric recording, the ureters were tied distally, cut and the proximal ends cannulated (PE-10) and drained externally. This procedure prevented the bladder from filling with urine during the experiment.
In some CMG experiments, epoxy-coated stainless steel wire (50 µm, M.T Giken Co., Ltd., Tokyo, Japan) EMG electrodes were placed percutaneously in the external urethral sphincter (EUS) to examine synergy between bladder and EUS. This was performed using a 30-gauge needle with a hooked EMG electrode positioned at the tip. The needle was inserted into the sphincter approximately 5 mm lateral to the urethra and then withdrawn leaving the EMG wires embedded in the muscle (Kruse et al., 1990). The EMG activity was passed through a discriminator/ratemeter, and the output was recorded on a chart recorder. The peak activity (in pulses per second) during each micturition contraction was measured.
Data Analysis and Statistics
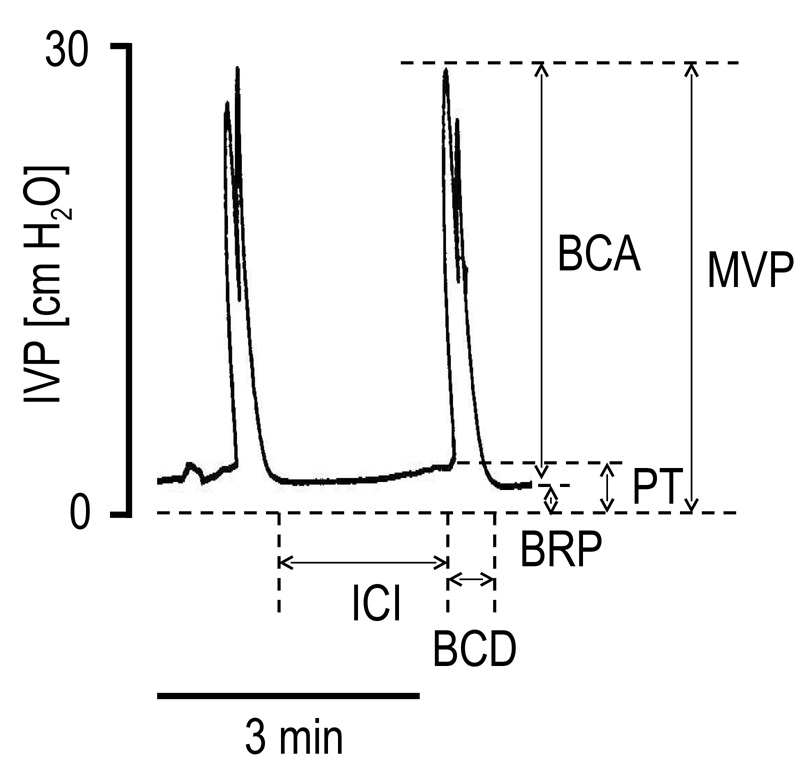
The effects of PACAP-38 on reflex bladder contraction amplitude (BCA), duration (BCD) and frequency (BCF) were recorded under isovolumetric conditions or with the urethra open allowing the bladder to empty. Maximal voiding pressure (MVP), pressure threshold for inducing voiding (PT), baseline resting intravesical pressure (BRP) and intercontraction interval (ICI) were also measured during continuous CMGs (Figure 1). Before PACAP-38 dosing, data were collected for at least 1h until the CMG activity stabilized. Graded doses of PACAP-38 were injected in each animal at 30–50 min intervals, depending on recovery time from the previous dose, to examine dose-response relationships. To analyze the data, values of the CMG parameters and EUS EMG activity after injection of each dose were averaged at 5 min intervals for up to 30 min post-injection and then compared with baseline values obtained for a period of 7–8 min before each dosing. All values are expressed as mean ± S.E.M. Repeated measures analysis of variance (ANOVA) and paired t test were used for statistical analysis and P<0.05 was considered significant.
FIG. 1.
Representative cystometrograms during continuous saline infusion (0.21 ml/min) obtained in a decerebrate, unanesthetized rat. BCA, bladder contraction amplitude; MVP, maximal voiding pressure; PT, pressure threshold; BRP, baseline resting pressure; BCD, bladder contraction duration; ICI, intercontraction interval.
Drugs
Drugs used include: halothane (Ayerst Lab. Inc., Philadelphia, PA) and PACAP-38 (Peninsula Laboratories, Inc., Belmont, CA). PACAP-38 was dissolved in 0.01 M phosphate buffered saline to make concentrations of 0.01 mM, 0.1 mM, 1 mM, which were adjusted to pH 7.4. The volume of fluid within the i.t. catheter was kept constant at 6 µl in all animals. Single doses of PACAP-38 were administered in a volume of 2.2 to 6.6 µl followed by 7 µl flush with artificial cerebrospinal fluid (CSF) (Feldberg and Fleischhauer, 1960).
RESULTS
Effects of PACAP-38 in spinal cord transected rats with intact hypogastric nerves, under isovolumetric conditions
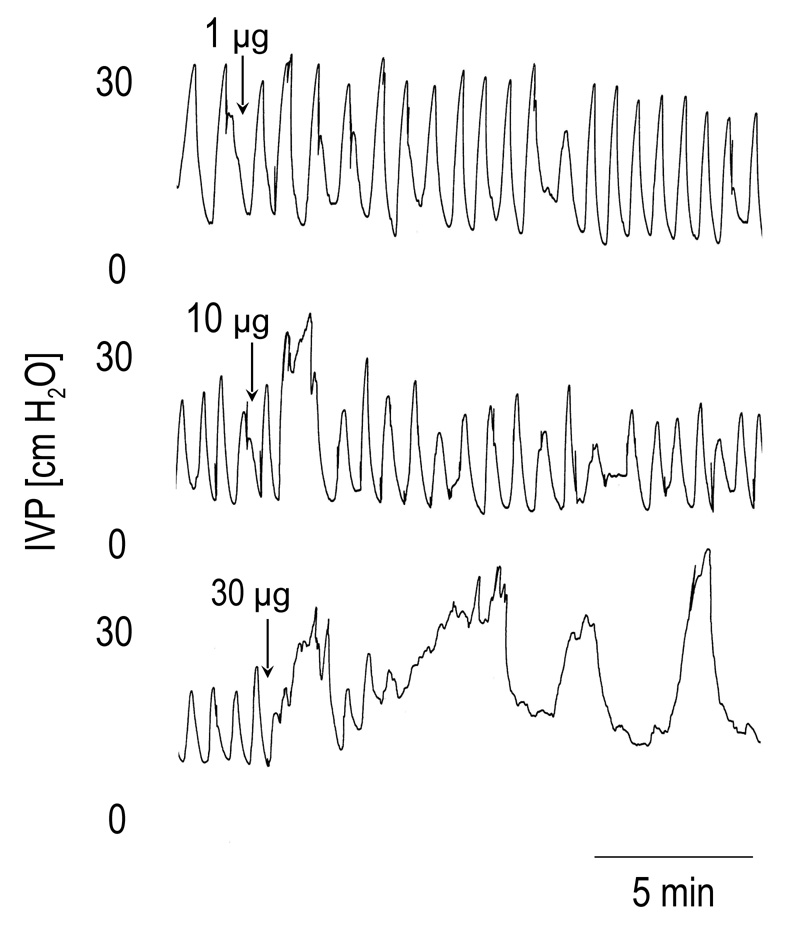
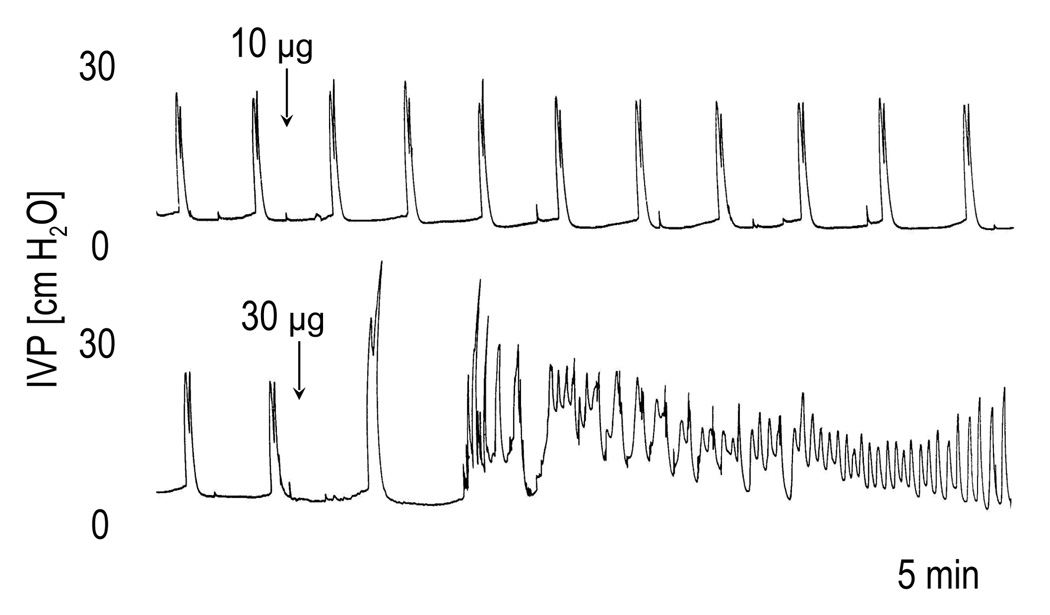
When the bladder of SCT rats was filled with saline, low amplitude bladder contractions (<15 cm H2O) appeared at low bladder volumes. These small amplitude contractions represent non-voiding reflexes (Cheng et al., 1997). In the present experiments the bladder was filled until the contractions exceeded 15 cm H2O and then the infusion was stopped. The large amplitude contractions (average amplitude 26 cm H2O) recorded under isovolumetric conditions usually occurred at a regular frequency (Fig. 2) that persisted for several hours and were not affected by injection of vehicle (6.6 µl). I.t. injection of PACAP-38 in doses between 0.1 µg and 10 µg did not significantly change the amplitude (BCA) or duration (BCD) of bladder contractions. However the frequency (BCF) of the contractions was significantly reduced by PACAP 10 µg; and this same dose increased, although not significantly, the amplitude by 54 % and the duration by 104% (Figure 2 and Table 1). The highest dose of PACAP (30 µg) significantly increased the BCA (by 196 %) and decreased the BCF (by 78 %). The excitatory effects of PACAP appeared within 5–10 min after injection and in some cases persisted for the duration of the recording although on average the effects disappeared after 20–25 min. In 3 of the 5 rats which received PACAP 30 µg, a depression of BCA occurred 10–30 min after the initial excitation.
FIG. 2.
The effects of PACAP-38 (1–30 µg i.t.) on the reflex bladder contractions under isovolumetric conditions in a spinal cord transected rat with intact hypogastric nerves. Note that larger doses (10 µg and 30 µg) of PACAP-38 increased the bladder contraction amplitude and duration and decreased the frequency of contractions, whereas smaller dose (1 µg) had little effect on these parameters.
TABLE 1.
Effects of Intrathecal Administration of PACAP on Reflex Bladder Activity of Spinal Cord Transected Rats with Intact or Bilaterally Sectioned Hypogastric Nerves, under Isovolumetric Conditions
| BCA | BCD | BCF | |
|---|---|---|---|
| (cm H2O) | (s) | (contractions/min) | |
| Transected SC + Intact HGN |
|||
| PACAP (10 µg) (n=7) | |||
| before | 24.5 ± 7.7 | 50 ± 7 | 1.08 ± 0.24 |
| after | 37.7 ± 9.8 | 102 ± 25 | 0.76 ± 0.23* |
| PACAP (30 µg) (n=5) | |||
| before | 16.7 ± 3.0 | 47 ± 10 | 1.49 ± 0.30 |
| after | 49.1 ± 10.6* | 141 ± 56 | 0.38 ± 0.21** |
| Transected SC + Sectioned HGN |
|||
| PACAP (10 µg) (n=7) | |||
| before | 22.4 ± 3.2 | 53 ± 6 | 0.94 ± 0.08 |
| after | 36.2 ± 2.7** | 90 ± 19* | 0.59 ± 0.12* |
| PACAP (30 µg) (n=4) | |||
| before | 22.9 ± 5.2 | 93 ± 37 | 0.71 ± 0.18 |
| after | 67.6 ± 15.1* | 276 ± 66 | 0.18 ± 0.04* |
Note. BCA, bladder contraction amplitude; BCD, bladder contraction duration; BCF, bladder contraction frequency; SC, spinal cord; HGN, hypogastric nerves.
P<0.05
P<0.01 (paired t test), compared “after” to “before” the i.t. injection of PACAP.
Effects of PACAP-38 in spinal cord transected rats under isovolumetric conditions after bilateral transaction of the hypogastric nerves
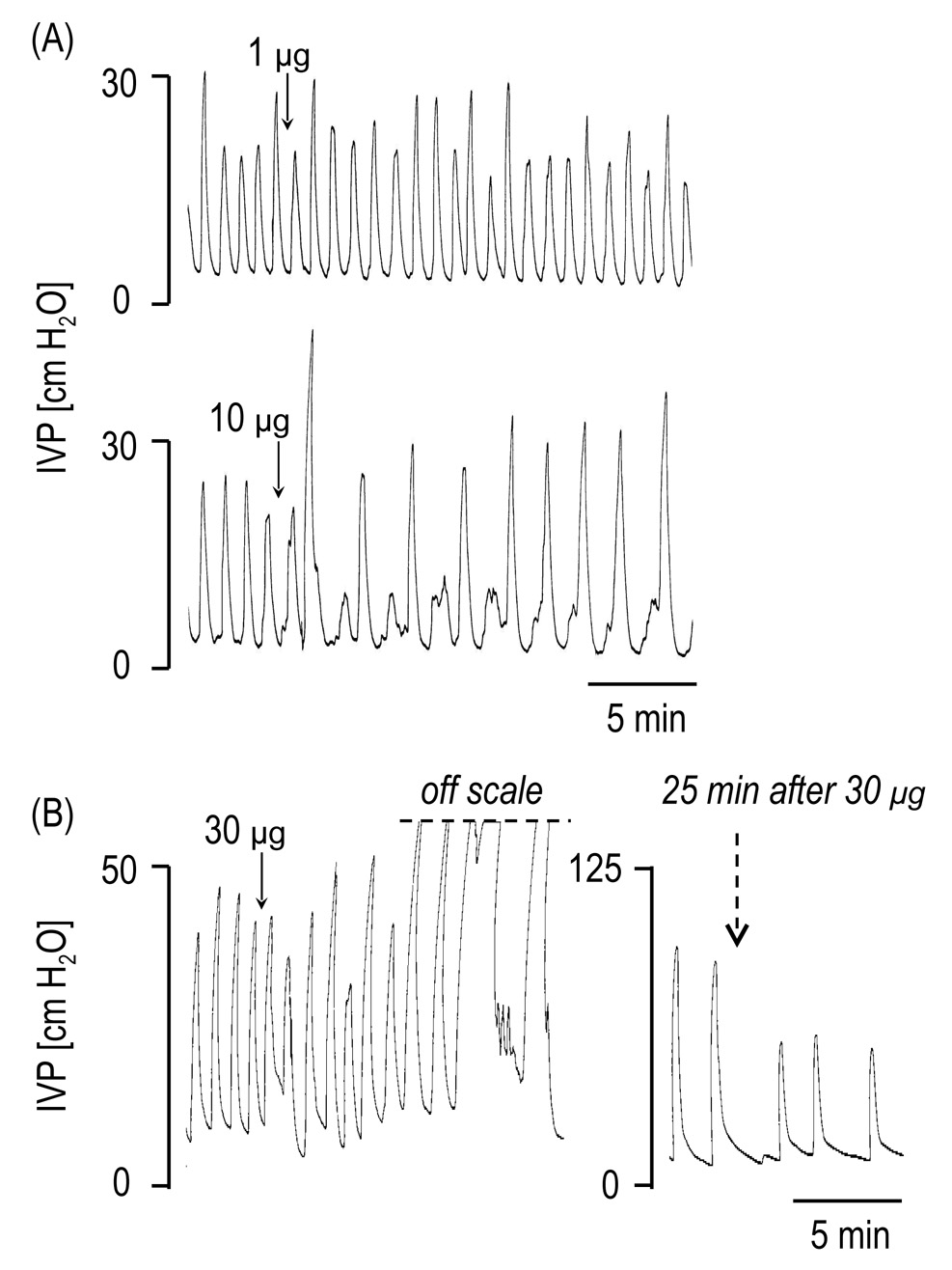
As observed in rats with intact HGNs, low doses of PACAP (0.1 µg and 1 µg) did not significantly alter bladder activity. However, the 10 µg dose of PACAP (n=7) significantly increased BCA (by 80 %) and BCD (by 74 %), and decreased BCF (by 34 %) (Figure 3). PACAP 30 µg (n=4) also increased the BCA (by 277 %) and decreased BCF (by 67 %) (Table 1). The effect of the 30 µg dose on the BCA was sustained for 30–40 min in 3 of 4 rats. In the other rat, bladder activity was completely abolished for at least 90 min after a large amplitude (BCA, 77 cm H2O), long duration (BCD, 438 sec) bladder contraction.
FIG. 3.
The effects of PACAP-38 (1–30 µg i.t.) on the reflex bladder contractions under isovolumetric conditions in spinal cord transected rats with transected hypogastric nerves. Note that the 10 µg increased the bladder contraction amplitude and duration and decreased the frequency of contractions, whereas smaller dose (1 µg) had no effect on these parameters (A). In other rat (B), the 30 µg produced marked increases of the bladder contraction amplitude and duration and decreased bladder contraction frequency.
Effects of PACAP-38 in spinal cord transected rats with intact hypogastric nerves, during continuous CMGs
Continuous infusion of saline into the bladder with the urethra open elicited repeated voiding reflexes at pressures (MVP) ranging from 40–49 cm H2O and at a mean interval of 18 sec. The baseline intravesical pressure during the CMG was in the range of 6–34 cm H2O. In contrast to the increase in BCA elicited under isovolumetric conditions, the large doses (10 µg and 30 µg) of PACAP-38 significantly decreased the BCA (19–100 %), MVP (5–85 %) and BCD (11–23 %) during CMGs (Table 2). The 30 µg dose also significantly increased BRP (16–295 %) (Table 2). In the 5 rats continuous dribbling of fluid from the bladder occurred in 3 and 4 rats after 10 µg and 30 µg, respectively.
TABLE 2.
Effects of Intrathecal Administration of PACAP on Micturition Reflexes of Spinal Cord Transected Rats with Intact or Bilaterally Sectioned Hypogastric Nerves, during Continuous Infusion Cystometrograms
| BCA | MVP | PT | BRP | BCD | ICI | |
|---|---|---|---|---|---|---|
| (cm H2O) | (cm H2O) | (cm H2O) | (cm H2O) | (s) | (s) | |
| Transected SC + Intact HGN |
||||||
| PACAP (10 µg) (n=4–5) | ||||||
| before | 33.9 ± 1.9 | 42.0 ± 2.5 | 9.0 ± 1.8 | 8.6 ± 1.3 | 50 ± 9 | 18 ± 5 |
| after | 12.2 ± 7.9* | 27.3 ± 5.8* | 12.5 ± 1.3* | 17.0 ± 3.3 | 43 ± 8* | 10 ± 4 |
| PACAP (30 µg) (n=4–5) | ||||||
| before | 33.5 ± 1.8 | 40.6 ± 1.7 | 8.6 ± 1.7 | 7.2 ± 1.5 | 64 ± 4 | 32 ± 3 |
| after | 3.1 ± 3.1** | 18.2 ± 5.1* | 12.6 ± 2.6* | 17.7 ± 4.1* | 53 ± 4** | 21 ± 7 |
| Transected SC + Sectioned HGN |
||||||
| PACAP (10 µg) (n=5) | ||||||
| before | 27.8 ± 5.1 | 38.5 ± 7.1 | 10.7 ± 2.5 | 10.3 ± 2.3 | 52 ± 5 | 14 ± 6 |
| after | 12.2 ± 2.2** | 27.4 ± 4.4 | 16.4 ± 3.3* | 16.8 ± 2.1* | 43 ± 4* | 19 ± 5 |
| PACAP (30 µg) (n=5) | ||||||
| before | 23.9 ± 4.6 | 34.6 ± 6.3 | 11.4 ± 2.9 | 9.9 ± 2.5 | 54 ± 7 | 39 ± 25 |
| after | 2.5 ± 1.7** | 18.3 ± 4.4* | 17.9 ± 3.1** | 16.2 ± 2.2* | 41 ± 7** | 29 ± 14 |
Note. BCA, bladder contraction amplitude; MVP, maximal voiding pressure; PT, pressure threshold; BRP, baseline resting pressure; BCD, bladder contraction duration; ICI, intercontraction interval; SC, spinal cord; HGN, hypogastric nerves.
P<0.05
P<0.01 (paired t test), compared “after” to “before” the i.t. injection of PACAP.
Effects of PACAP-38 in spinal cord transected rats with bilaterally sectioned hypogastric nerves, during continuous CMGs
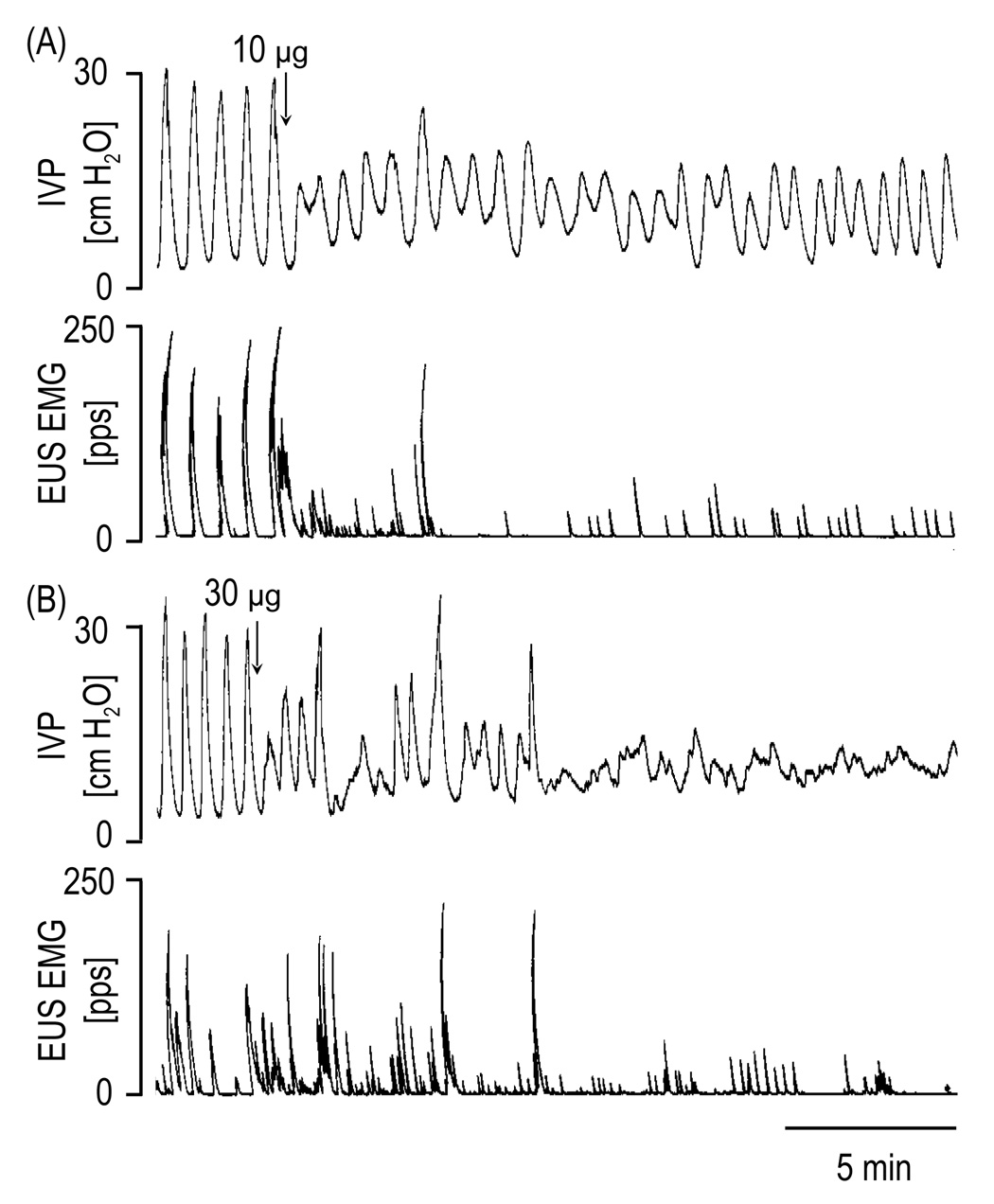
PACAP-38 at doses of 10 µg and 30 µg significantly decreased the BCA (24–100 %) and the BCD (6–33 %), and increased the PT (7–147 %) and the BRP (2–359 %) (Figure 4 and Table 2). Only the 30 µg dose reduced the MVP (30–78 %). In a total of 5 rats that were tested, overflow incontinence occurred in 1 rat at 10 µg and in 5 rats at 30 µg dose. The incontinence lasted for 8–30 min. PACAP-38 in a range of doses 0.1–30 µg did not affect the ICI prior to or after the period of overflow continence (Table 2).
FIG. 4.
The effects of PACAP-38 (10 µg and 30 µg i.t.) on the micturition reflexes during continuous filling (0.21 ml/min) cystometrograms in a spinal cord transected rat with transected hypogastric nerves. Note that the 10 µg and 30 µg markedly suppressed the bladder and EUS EMG activity.
Effects of PACAP-38 in spinal cord intact rats with intact hypogastric nerves, during CMGs
During continuous infusion CMGs in spinal intact rats repeated voiding reflexes occurred on average at longer intervals (92 sec) than in SCT rats (20 sec) and at lower intravesical pressures (32 cm H2O versus 45 cm H2O). Low doses (0.1–10 µg) of PACAP did no alter any CMG parameters, whereas the highest dose (30 µg) significantly increased BCA, MVP and BCD without changing PT or BRP (Table 3). In 2 of 4 rats the 30 µg dose produced a large increase in baseline bladder pressure and increased the frequency of bladder contractions. This was followed by a gradual decrease in the amplitude of contractions (Figure 5). In one of these rats the 30 µg dose induced overflow incontinence lasting for 80 min, after the facilitation of bladder contractions.
TABLE 3.
Effects of Intrathecal Administration of PACAP on Micturition Reflex of Spinal Cord Intact Rats with Intact or Bilaterally Sectioned Hypogastric Nerves, during Continuous Infusion Cystrometrograms.
| BCA | MVP | PT | BRP | BCD | ICI | |
|---|---|---|---|---|---|---|
| (cm H2O) | (cm H2O) | (cm H2O) | (cm H2O) | (s) | (s) | |
| Intact SC + Intact HGN |
||||||
| PACAP (10 µg) (n=5) | ||||||
| before | 28.8 ± 5.7 | 32.6 ± 6.8 | 6.8 ± 1.6 | 5.8 ± 1.8 | 36 ± 5 | 96 ± 32 |
| after | 29.6 ± 4.3 | 34.6 ± 6.6 | 8.2 ± 2.6 | 7.0 ± 2.4 | 39 ± 3 | 85 ± 37 |
| PACAP (30 µg) (n=4) | ||||||
| before | 26.8 ± 3.5 | 31.9 ± 5.0 | 7.2 ± 2.0 | 6.6 ± 2.2 | 36 ± 5 | 107 ± 38 |
| after | 33.8 ± 3.7* | 41.0 ± 6.8* | 9.9 ± 2.7 | 15.2 ± 6.6 | 45 ± 5** | 88 ± 39 |
| Intact SC + Sectioned HGN |
||||||
| PACAP (10 µg) (n=5) | ||||||
| before | 25.3 ± 2.9 | 32.9 ± 3.2 | 9.8 ± 2.2 | 5.9 ± 1.9 | 53 ± 10 | 103 ± 33 |
| after | 30.5 ± 1.9* | 39.2 ± 3.3* | 11.3 ± 2.0* | 7.9 ± 2.9 | 63 ± 10 | 126 ± 23 |
| PACAP (30 µg) (n=5) | ||||||
| before | 23.2 ± 3.3 | 30.7 ± 3.2 | 9.9 ± 2.0 | 6.1 ± 1.8 | 41 ± 11 | 106 ± 35 |
| after | 31.3 ± 1.8* | 40.0 ± 3.0** | 13.5 ± 2.8* | 17.5 ± 2.7** | 58 ± 15* | 75 ± 41 |
Note. BCA, bladder contraction amplitude; MVP, maximal voiding pressure; PT, pressure threshold; BRP, baseline resting pressure; BCD, bladder contraction duration; ICI, intercontraction interval; SC, spinal cord; HGN, hypogastric nerves.
P<0.05
P<0.01 (paired t-test), compared “after” to “before” the i.t. injection of PACAP.
FIG. 5.
The effects of PACAP-38 (10 µg and 30 µg i.t.) on the micturition reflexes during continuous filling (0.21 ml/min) cystometrograms in a spinal cord intact rat with intact hypogastric nerves. Note that the 30 µg increased the bladder contraction pressure followed by a suppression of bladder activity, whereas the 10 µg had no effect.
Effects of PACAP-38 administered during CMGs in spinal cord intact rats with hypogastric nerves transected bilaterally
In HGN transected rats PACAP 10 µg as well as 30 µg increased the BCA, MVP and the PT (Table 3). A late occurring (15–30 min) decrease in BCA and MVP as well as an increase in BRP (67–813 %) occurred after the initial excitatory effect of 30 µg of PACAP in 3 of 5 rats. These late changes were accompanied by overflow incontinence. Similar changes occurred in one rat after the 10 µg dose. The 30 µg dose of PACAP-38 increased the BCD (19–55 %) (Table 3).
Effects of PACAP-38 in spinal cord intact rats with intact or bilaterally sectioned hypogastric nerves, under isovolumetric conditions
In spinal cord intact rats with HGNs transected bilaterally the intravesical volume required to induce large amplitude reflex bladder contractions (>15 cm H2O) was significantly smaller (mean: 0.12 ± 0.02 ml, range: 0.07–0.18 ml, n=4) than the volume in rats with intact HGNs (mean: 0.37 ± 0.10, range: 0.17–0.75 ml, n=4) (P<0.05, unpaired t test), suggesting that voiding reflexes were modulated by a tonic sympathetic inhibitory control.
Under isovolumetric conditions spinal cord intact rats with intact HGNs (n=4) exhibited reflex bladder contractions (BCF: 0.41 ± 0.07 contractions/min) that were significantly lower in amplitude (BCA: 59.4 ± 3.3 cm H2O) and shorter in duration (BCD: 49 ± 5 sec) than the reflex contractions in rats with the HGNs transected bilaterally (BCA: 103.5 ± 7.5 cm H2O; BCD: 138 ± 27 sec), suggesting that transection of the HGNs in decerebrate unanesthetized rats facilitates bladder activity. PACAP-38 in low doses (0.1–1 µg) did not change bladder activity (n=4). Unfortunately full dose response studies with PACAP could not be conducted in these animals due to variability of bladder activity and the inability to obtain consistent recordings over a long period of time.
Effects of PACAP-38 on EUS EMG activity during CMGs in spinal cord transected and spinal cord intact rats with intact and bilaterally sectioned hypogastric nerves
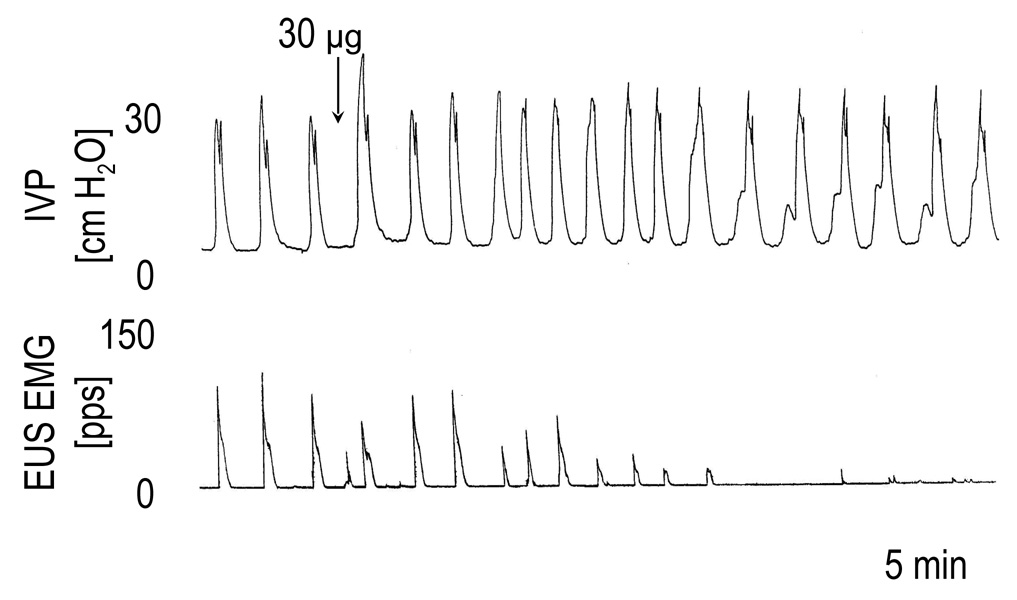
PACAP (30 µg) significantly decreased the EUS EMG activity in spinal cord intact animals with intact HGNs (by 49–100%, P=0.025, n=4), or transected HGNs (42–100 %, P=0.0086, n=5). The PACAP inhibitory effect on the EUS EMG activity did not occur in lower doses and was accompanied by a delayed suppression of bladder contractions in 5 of 9 rats. In the other 4 rats (HGNs intact, n=2 and transected, n=2), the EUS EMG activity was suppressed without a decrease in the amplitude of bladder contractions (Figure 6).
FIG. 6.
The effects of PACAP-38 (30 µg i.t.) on the bladder and EUS EMG activity during continuous filling (0.21 ml/min) cystometrograms in a spinal cord intact rat with intact hypogastric nerves. Note that PACAP-38 increased the micturition pressure immediately after its injection and that the dose reduced the EUS EMG activity without the decrease of bladder contraction amplitude.
In SCT rats PACAP 30 µg also decreased EUS EMG activity (by 64–100 %, P=0.0005, n=4) in animals with intact (n=2) and transected (n=2) HGNs. Lower doses (0.1–10 µg) were inactive.
DISCUSSION
These studies demonstrated that i.t. administration of PACAP-38 modulated EUS EMG activity and reflex bladder contractions in decerebrate unanesthetized rats with the spinal cord intact or chronically transected (SCT) at the mid-thoracic level. In spinal cord intact rats during continuous infusion CMGs, PACAP-38 decreased EUS activity, enhanced the amplitude of bladder contractions and in some experiments produced a late occurring suppression of bladder contractions. Transection of hypogastric nerves enhanced the facilitatory effects on the bladder but did not alter the late occurring suppression. In SCT rats the effect on bladder activity depended on the conditions of the experiment. During isovolumetric recording PACAP-38 produced an initial enhancement in the amplitude of the bladder contractions followed in 60% of the experiments by a decrease in amplitude. Transection of the hypogastric nerves reduced the threshold dose of PACAP-38 for eliciting facilitation and reduced the number of rats that exhibited a late occurring suppression of bladder activity. During CMGs in SCT rats PACAP-38 consistently reduced EUS EMG activity but also reduced the amplitude of bladder contractions. These observations coupled with previous demonstrations of PACAP-immunoreactivity (IR) in bladder afferent neurons and in nerves in the lumbosacral parasympathetic nucleus and dorsal horn of the spinal cord and upregulation of PACAP-IR at these sites after spinal cord injury (Zvarova et al., 2005) raises the possibility that PACAP may function as a neurotransmitter in micturition reflex pathways.
We propose several hypotheses to explain the complex effects of PACAP-38 on the lower urinary tract. First it is clear that PACAP-38 like PACAP-27 (Ishizuka et al. 1995) and VIP in the rat (Igawa et al., 1993) and cat (de Groat et al., 1990) has a facilitatory effect at the level of the lumbosacral spinal cord on the parasympathetic reflex pathway controlling bladder activity. The peptide enhanced reflex bladder contractions in spinal intact as well as in chronic spinal cord transected animals in which the supraspinal micturition reflex pathway was eliminated. PACAP-38 might act at several sites in the spinal cord, because patch clamp studies in neonatal rat spinal slices revealed that PACAP-38 has direct excitatory effects on parasympathetic preganglionic neurons (PPGN) and also can enhance excitatory synaptic input to the PPGN (Miura et al., 2001). PACAP-38 decreased the electrical threshold for triggering action potentials, increased the number of action potentials induced by depolarizing current pulses and suppressed a 4-aminopyridine sensitive outward current in PPGN. PACAP-38 also induced spontaneous firing and increased the frequency of spontaneous excitatory postsynaptic potentials in the presence of tetrodotoxin. Because excitatory synaptic inputs to PPGN are mediated primarily by AMPA and NMDA glutamatergic synapses (Araki and de Groat, 1996) it was proposed that PACAP-38 facilitates glutamatergic excitatory synaptic input to the PPGN in addition to directly enhancing the excitability of the PPGN by blocking K+ channels (Miura et al., 2001). PACAP-38 could act presynaptically to enhance the firing of interneurons, enhance glutamate release from interneuronal terminals or act postsynaptically directly on the PPGN to enhance glutamatergic currents as noted in cortical neurons where PACAP-38 potentiates N-methyl-d-aspartate (NMDA) induced currents (Liu and Madsen, 1997). Thus PACAP might function as an excitatory transmitter at spinal synapses in the micturition reflex pathway.
However in many experiments PACAP-38 also produced a late occurring suppression of bladder activity. This was detected in both spinal cord intact and SCT rats but in SCT rats it was less common after transection of the HGNs. The latter observation suggests that the PACAP-38 inhibition in SCT rats might be mediated by stimulation of inhibitory sympathetic nerve pathways to the bladder that arise in the rostral lumbar and caudal thoracic segments of the spinal cord (Dun et al., 1996). Our previous study revealed that sectioning the HGNs enhanced nonvoiding contractions in SCT rats (Yoshiyama & de Groat, 2002) demonstrating that sympathetic inhibitory pathways are important in regulating bladder activity after spinal cord injury. The delayed onset of the PACAP-38 inhibition is consistent with the longer time required for diffusion of the peptide from the site of injection at the caudal lumbosacral level to the location of sympathetic nuclei in the more rostral spinal segments. The demonstration by other investigators (Lai et al., 1997) that PACAP can stimulate sympathetic preganglionic neurons in in vitro spinal cord slice preparations and increase blood pressure in in vivo experiments in rats when administered i.t. at the thoracic spinal level provides support for a possible role of sympathetic pathways in the PACAP-38 inhibition. On the other hand, late occurring inhibition in spinal cord intact rats was still elicited after HGN transection indicating that another mechanism must also be involved possibly mediated by activation of inhibitory neurons in the spinal cord.
In cats i.t. administration of VIP, another member of the secretin-glucagon-VIP family of peptides with 68 % homology to PACAP, also modulates reflex bladder activity; and the effect of VIP changes after spinal cord injury. In cats with an intact spinal cord VIP inhibited bladder activity; whereas after chronic spinal cord transection small doses of VIP facilitated reflex bladder activity (de Groat et al., 1990). In the cat VIP is prominently expressed in several populations of pelvic visceral afferent neurons (e.g., uterine cervix, colon and bladder afferents) (de Groat, 1987; de Groat et al., 1987; Kawatani and de Groat, 1991) and is present in C-fiber afferent projections (Kawatani et al., 1985; Morgan et al., 1999) to the region of the sacral parasympathetic nucleus in the spinal cord. It has been speculated that in cats with an intact spinal cord VIP may function as a spinal neurotransmitter in the reflex inhibition of bladder activity evoked by mechanical stimulation of the uterine cervix or distal bowel (Kawatani and de Groat, 1991). Furthermore since VIP has primarily a neuronal excitatory effect it was suggested that it might produce inhibition by activating interneuronal inhibitory pathways in the spinal cord. On the other hand after chronic SCT in cats which is accompanied by an expansion of VIP containing afferent projections in the spinal cord (Thor et al., 1986) it has been proposed that VIP may be involved as an excitatory transmitter in the C-fiber afferent evoked spinal micturition reflex pathway (de Groat et al., 1990). PACAP expression is increased in micturition reflex pathways in the rat after SCT and therefore it may play a similar role as an excitatory transmitter in micturition in chronic SCT rats (Zvara et al., 2006).
However, in SCT rats the effects of PACAP-38 were different under different experimental conditions. When recording isovolumetric bladder contractions with the urethra ligated in SCT rats, PACAP-38 (10 µg and 30 µg i.t.) produced large amplitude and long duration bladder contractions. Transection of the HGNs enhanced this excitatory effect of PACAP-38. On the other hand, during continuous infusion CMGs with the urethra open to allow voiding, PACAP at 10–30 µg doses markedly decreased the amplitude of bladder contractions. It seems unlikely that ligating the urethral outlet to produce isovolumetric contractions would alter the effects of PACAP-38 in the spinal cord and reverse the effects of the peptide on bladder activity. Thus it seems reasonable to speculate that the excitatory effect of PACAP-38 on bladder activity during continuous infusion CMGs is masked by a simultaneous inhibitory effect on the EUS that in turn blocks detrusor-sphincter-dyssynergia (DSD) and reduces urethral outlet resistance. This would indirectly lower intravesical pressure during voiding. In a previous study we demonstrated a similar effect in SCT rats following the administration of α-bungarotoxin, a potent neuromuscular blocking agent, that selectively suppresses striated muscle activity without altering bladder activity (Yoshiyama et al., 2000). Alpha-bungarotoxin reduced maximal voiding pressure and increased voiding efficiency by blocking DSD in SCT rats. Because the high dose of PACAP-38 induced overflow incontinence in a majority of SCT animals with only a small increase in intravesical pressure, it is likely that this is due to a combined action to facilitate the spinal reflex to the bladder and inhibit the motor outflow to the EUS.
It is important to note that in the present studies, a transurethral catheter was used for the cystometrogram recordings. Transurethral catheterization can be used in anesthetized or decerebrate unanesthetized animals but is not suitable for use in conscious animals. The transurethral catheter may be a stimulus to urethra and also be obstructive during voiding, which could potentially affect urodynamic measurements. However, the maximal voiding pressures in these studies were comparable to those in our previous studies using transvesical catheterization (Yoshiyama et al., 2000). Furthermore, voiding efficiencies in rats with a transurethral catheter (Yoshiyama et al., 1997) and a transvesical catheter (Yoshiyama et al., 1999) were similar. Thus, it seems likely that the influence on urodynamics of the transurethral catheter at least during control recording was minor in the present experiments.
PACAP-38 suppressed EUS EMG activity in both spinal cord intact and SCT rats indicating that its inhibitory effects are mediated at the level of the spinal cord and are independent of supraspinal control. However, inhibition of EUS activity would be expected to have different effects on voiding efficiency in SCT and spinal cord intact rats. In spinal cord intact rats the EUS exhibits bursting activity that promotes voiding (Kruse et al 1993). Thus, blocking EUS activity should reduce voiding efficiency. This has been noted after the administration of neuromuscular blocking agents (Yoshiyama et al., 2000). On the other hand, block of DSD with PACAP-38 in SCT rats would be expected to improve voiding efficiency and lower maximal voiding pressure as noted previously after administration of α-bungarotoxin (Yoshiyama et al., 2000). This should be evaluated in future experiments.
It has been proposed that interneurons located in the region of the dorsal commissure provide an inhibitory input to EUS motoneurons (Blok et al., 1998; de Groat et al., 2001; Sie et al., 2001). PACAP-38 could suppress EUS EMG activity by activating these interneurons. Immunohistochemical studies have revealed that low levels of PACAP-IR are present in this region of normal rats and that the levels are increased almost seven fold in this area as well as in the dorsal horn and sacral parasympathetic nucleus in SCT rats (Zvarova et al., 2005). A similar increase in VIP-IR was noted in the cat sacral spinal cord after chronic SCT (Thor et al., 1986). Thus PACAP and VIP could have a more important role in micturition after SCT. It has been suggested that PACAP in the spinal cord might also be involved in the initiation of bladder hyperactivity induced by other pathological conditions such as chronic cystitis (Vizzard, 2000; Herrera et al., 2006).
The physiological role of PACAP in the control of bladder function in chronic SCT rats has been studied by evaluating the effect of PACAP6-38, a PAC1 receptor antagonist on bladder activity during continuous infusion CMGs in awake rats (Zvara et al., 2006). I.t. administration of the antagonist reduced nonvoiding contractions during bladder filling and reduced maximal voiding pressure suggesting that activation of PAC1 receptors by endogenous PACAP was contributing to the micturition reflex and bladder hyperreflexia. However PACAP could potentially act on multiple receptors (PAC1, VPAC1 and VPAC2) in the spinal cord (Sakashita et al., 2001). RT-PCR analysis in the lumbosacral spinal cord demonstrated the presence of VPAC2 as well as PAC1 receptors, but not VPAC1 receptors (Braas et al., 2006). Therefore the effect of PACAP6-38 which blocks one population of receptors may give insights into only part of the function of PACAP; whereas administration of a receptor agonist such as PACAP-38 which could potentially activate multiple receptors might provide a more complete picture of the putative role of PACAP in the control of micturition.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants, DK-49430 (W.C.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Araki I, de Groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J. Neurophysiol. 1996;76:215–226. doi: 10.1152/jn.1996.76.1.215. [DOI] [PubMed] [Google Scholar]
- 2.Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP): Discovery and current status of research. Regul. Pept. 1992;37:287–302. [PubMed] [Google Scholar]
- 3.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 4.Blok BFM, van Maarseveen JTPW, Holstege G. Electrical stimulation of the sacral dorsal gray commissure evokes relaxation of the external urethral sphincter in the cat. Neurosci. Lett. 1998;249:68–70. doi: 10.1016/s0304-3940(98)00382-6. [DOI] [PubMed] [Google Scholar]
- 5.Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am. J. Physiol. Integr. Comp. Physiol. 2006;290:R951–R962. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng C-L, Chai C-Y, de Groat WC. Detrusor-sphincter dyssynergia induced by cold stimulation of the urinary bladder of rats. Am. J. Physiol. 1997;272:R1271–R1282. doi: 10.1152/ajpregu.1997.272.4.R1271. [DOI] [PubMed] [Google Scholar]
- 7.de Groat WC. Identification of neuropeptides in afferent pathways to the pelvic viscera of the cat. Experientia. 1987;43:801–813. doi: 10.1007/BF01945358. [DOI] [PubMed] [Google Scholar]
- 8.de Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor MB, Yoshimura N, Roppolo JR. Neural control of the urethra. Scand. J. Urol. Nephrol. (Suppl.) 2001;207:35–43. doi: 10.1080/003655901750174872. [DOI] [PubMed] [Google Scholar]
- 9.de Groat WC, Kawatani M, Hisamitsu T, Cheng C-L, Ma C-P, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst. 1990;30 (Suppl):S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- 10.de Groat WC, Kawatani M, Houston MB, Rutigliano M, Erdman S. Identification of neuropeptides in afferent pathways to the pelvic viscera of the cat. In: Ciriello J, Calaresu F, Renaud L, Polosa C, editors. Organization of the Autonomic Nervous System: Central and Peripheral Mechanism, Neurology and Neurobiology. Vol. 31. NY: A.R. Liss Inc.; 1987. pp. 81–90. [Google Scholar]
- 11.Dun NJ, Miyazaki T, Tang H, Dun EC. Pituitary adenylate cyclase activating polypeptide immunoreactivity in the rat spinal cord and medulla: implication of sensory and autonomic functions. Neuroscience. 1996;73:677–686. doi: 10.1016/0306-4522(96)00057-7. [DOI] [PubMed] [Google Scholar]
- 12.Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998;83:1261–1272. doi: 10.1016/s0306-4522(97)00474-0. [DOI] [PubMed] [Google Scholar]
- 13.Feldberg W, Fleischhauer K. Penetration of bromophenol blue from the perfused cerebral ventricles into the brain tissue. J. Physiol. (Lond.) 1960;150:451–462. doi: 10.1113/jphysiol.1960.sp006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridolf T, Sundler F, Ahren B. Pituitary adenylate cyclase-activating polypeptide (PACAP): occurrence in rodent pancreas and effects on insulin and glucagon secretion in the mouse. Cell & Tissue Res. 1992;269:275–279. doi: 10.1007/BF00319618. [DOI] [PubMed] [Google Scholar]
- 15.Hernández M, Barahona MV, Recio P, Benedito S, Martínez AC, Rivera L, García-Sacristán A, Prieto D, Orensanz LM. Neuronal and smooth muscle receptors involved in the PACAP- and VIP-induced relaxations of the pig urinary bladder neck. Br. J. Pharmacol. 2006a;149:100–109. doi: 10.1038/sj.bjp.0706832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernández M, Barahona MV, Recio P, Bustamante S, Benedito S, Rivera L, García-Sacristán A, Prieto D, Orensanz LM. PACAP 38 is involved in the non-adrenergic non-cholinergic inhibitory neurotransmission in the pig urinary bladder neck. Neurourol. Urodyn. 2006b;25:490–497. doi: 10.1002/nau.20287. [DOI] [PubMed] [Google Scholar]
- 17.Herrera GM, Braas KM, May V, Vizzard MA. PACAP enhances mouse urinary bladder contractility and is upregulated in micturition reflex pathways after cystitis. Ann. NY Acad. Sci. 2006;1070:330–336. doi: 10.1196/annals.1317.040. [DOI] [PubMed] [Google Scholar]
- 18.Igawa Y, Persson K, Andersson K-E, Uvelius B, Mattiasson A. Facilitatory effect of vasoactive intestinal polypeptide on spinal and peripheral micturition reflex pathways in conscious rats with and without detrusor instability. J. Urol. 1993;149:884–889. doi: 10.1016/s0022-5347(17)36252-3. [DOI] [PubMed] [Google Scholar]
- 19.Ishizuka O, Alm P, Larsson B, Mattiasson A, Andersson K-E. Facilitatory effect of pituitary adenylate cyclase activating polypeptide on micturition in normal, conscious rats. Neuroscience. 1995;66:1009–1014. doi: 10.1016/0306-4522(95)00038-k. [DOI] [PubMed] [Google Scholar]
- 20.Kawatani M, Erdman S, de Groat WC. Vasoactive intestinal polypeptide and substance P in afferent pathways to the sacral spinal cord of the cat. J. Comp. Neurol. 1985;241:327–347. doi: 10.1002/cne.902410307. [DOI] [PubMed] [Google Scholar]
- 21.Kawatani M, de Groat WC. A large proportion of afferent neurons innervating the uterine cervix of the cat contain VIP and other neuropeptides. Cell and Tissue Research. 1991;266:294–304. doi: 10.1007/BF00678724. [DOI] [PubMed] [Google Scholar]
- 22.Kruse MN, Belton AL, de Groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. 1993;264:R1157–R1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- 23.Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Res. 1990;532:182–190. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- 24.Laburthe M, Couvineau A, Marie J-C. VPAC receptors for VIP and PACAP. Receptors and Channels. 2002;8:137–153. [PubMed] [Google Scholar]
- 25.Lai CC, Wu SY, Lin HH, Dun NJ. Excitatory action of pituitary adenylate cyclase activating polypeptide on rat sympathetic preganglionic neurons in vivo and in vitro. Brain Res. 1997;748:189–194. doi: 10.1016/s0006-8993(96)01297-8. [DOI] [PubMed] [Google Scholar]
- 26.Liu GJ, Madsen BW. PACAP38 modulates activity of NMDA receptors in cultured chick cortical neurons. J. Neurophysiol. 1997;78:2231–2234. doi: 10.1152/jn.1997.78.4.2231. [DOI] [PubMed] [Google Scholar]
- 27.Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats. J. Pharmacol. Methods. 1986;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- 28.Mallory B, Steers WD, de Groat WC. Electrophysiological study of micturition reflexes in rats. Am. J. Physiol. 1989;257:R410–R421. doi: 10.1152/ajpregu.1989.257.2.R410. [DOI] [PubMed] [Google Scholar]
- 29.Miura A, Kawatani M, de Groat WC. Effects of pituitary adenylate cyclase activating polypeptide on lumbosacral preganglionic neurons in the neonatal rat spinal cord. Brain Res. 2001;895:223–232. doi: 10.1016/s0006-8993(01)02112-6. [DOI] [PubMed] [Google Scholar]
- 30.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang LA, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 31.Morgan CW, Ohara PT, Scott DE. Vasoactive intestinal polypeptide in sacral primary sensory pathways in the cat. J. Comp. Neurol. 1999;407:381–394. doi: 10.1002/(sici)1096-9861(19990510)407:3<381::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Reubi JC. In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann N.Y. Acad. Sci. 2000;921:1–25. doi: 10.1111/j.1749-6632.2000.tb06946.x. [DOI] [PubMed] [Google Scholar]
- 33.Sakashita Y, Kurihara T, Uchida D, Tatsuno I, Yamamoto T. Involvement of PACAP receptor in primary afferent fibre-evoked responses of ventral roots in the neonatal rat spinal cord. Br. J. Pharmacol. 2001;132:1769–1776. doi: 10.1038/sj.bjp.0703980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapru HN, Krieger AJ. Procedure for the decerebration of the rat. Brain Res. Bull. 1978;3:675–679. doi: 10.1016/0361-9230(78)90016-3. [DOI] [PubMed] [Google Scholar]
- 35.Sie JAML, Blok BFM, De Weerd H, Holstege G. Ultrastructural evidence for direct projections from the pontine micturition center to glycine-immunoreactive neurons in the sacral dorsal gray commissure in the cat. J. Comp. Neurol. 2001;429:631–637. doi: 10.1002/1096-9861(20010122)429:4<631::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Sundler F, Ekblad E, Absood A, Hakanson R, Koves K, Arimura A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience. 1992;46:439–454. doi: 10.1016/0306-4522(92)90064-9. [DOI] [PubMed] [Google Scholar]
- 37.Thor K, Kawatani M, de Groat WC. Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal cord injury. In: Goldberger M, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord. Fidia Research Series Vol. III. Padova, Italy: Fidia Press; 1986. pp. 65–81. [Google Scholar]
- 38.Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J. Comp. Neurol. 2000;420:335–348. [PubMed] [Google Scholar]
- 39.Wang Z-Y, Alm P, Håkanson R. Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye. Neuroscience. 1995;69:297–308. doi: 10.1016/0306-4522(95)00258-k. [DOI] [PubMed] [Google Scholar]
- 40.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 41.Yoshiyama M, de Groat WC. Effects of intrathecal administration of pituitary adenylate cyclase activating polypeptide (PACAP) on the lower urinary tract in the rat. Soc. Neurosci. Abstr. 1997;23:1523. [Google Scholar]
- 42.Yoshiyama M, de Groat WC. Effect of bilateral hypogastric nerve transaction on voiding dysfunction in rats with spinal cord injury. Exp. Neurol. 2002;175:191–197. doi: 10.1006/exnr.2002.7887. [DOI] [PubMed] [Google Scholar]
- 43.Yoshiyama M, de Groat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55:956–960. doi: 10.1016/s0090-4295(00)00474-x. [DOI] [PubMed] [Google Scholar]
- 44.Yoshiyama M, Nezu FM, Yokoyama O, de Groat WC, Chancellor MB. Changes in micturition after spinal cord injury in conscious rats. Urology. 1999;54:929–933. doi: 10.1016/s0090-4295(99)00234-4. [DOI] [PubMed] [Google Scholar]
- 45.Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J. Pharmacol. Exp. Ther. 1997;280:894–904. [PubMed] [Google Scholar]
- 46.Zvara P, Braas KM, May V, Vizzard MA. A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI) Ann. NY Acad. Sci. 2006;1070:622–628. doi: 10.1196/annals.1317.092. [DOI] [PubMed] [Google Scholar]
- 47.Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp. Neurol. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]