Abstract
Transfer of iron from the mucosa is a critical step in dietary iron assimilation that is tightly regulated to ensure the appropriate amount of iron is absorbed to meet the body's demands. Too much iron is highly toxic, and failure to properly control intestinal iron export causes iron overload associated with hereditary forms of hemochromatosis. One form of genetic iron overload, ferroportin disease, originates due to defects in ferroportin, the membrane iron exporter. Ferroportin acts in conjunction with the intestinal ferroxidase hephaestin to mediate release of iron from the enterocyte. How iron is then acquired by transferrin and released into circulation remains an unknown step in this process.
Keywords: ferroportin, hephaestin, intestinal, iron transport
ASSIMILATION OF DIETARY IRON begins by its transport across the duodenal brush-border membrane, a process mediated by divalent metal ion transporter-1 (DMT1) and reviewed earlier in this series. On entering the enterocyte, iron is then either used for metabolic purposes, stored in ferritin, or translocated through the enterocyte in preparation for its release. This article focuses on the final step of dietary iron assimilation: the exsorption of iron from the mucosa into circulation. Release of iron from the intestine represents a key intersection between copper and iron metabolism. Basolateral efflux of iron into circulation from the enterocyte is not only mediated by the iron transport protein ferroportin but also requires a copper-dependent ferroxidase, hephaestin. Body iron stores, hypoxia, the rate of erythropoiesis, inflammation, and pregnancy all have dramatic effects on the assimilation of dietary iron. How these factors influence the activity of ferroportin and hephaestin to modify iron absorption is an active area of interest in the field. The fact that inherited forms of hemochromatosis are associated with impaired regulation of intestinal iron export underscores the importance of this process in human health and nutrition.
HEPHAESTIN AND THE ROLE OF COPPER IN IRON EFFLUX
Copper deficiency is known to induce microcytic hypochromic anemia that cannot be resolved by iron administration. Early tracer experiments demonstrated that absorption of dietary iron by copper-deficient animals is suppressed, and the observation that iron accumulates in the gut of copper-deficient pigs led to the model that copper was required for the release of iron from the intestinal epithelia. Molecular support for this idea was ultimately obtained in studies of sex-linked anemia (sla) mice. These animals have a block in mucosal iron transfer, and the defective gene was discovered to encode a membrane protein called hephaestin (25). Hephaestin displays a high degree of homology (50% identity, 68% similarity) to the serum ferroxidase ceruloplasmin. In contrast to this soluble homolog, hephaestin contains an additional 86 COOH-terminal amino acids comprising a predicted single transmembranespanning domain. Otherwise, all of the copper-binding sites and key structural features of ceruloplasmin are conserved in its predicted structure suggesting that hephaestin also has ferroxidase activity.
On the basis of its structural homology with ceruloplasmin, our current model for intestinal iron export predicts that hephaestin is responsible for oxidation of iron to the ferric state before its release and high-affinity binding by serum transferrin. Ceruloplasmin is known to mediate the release of iron from the liver and other tissues, but it does not appear to play a role in iron export from the intestinal mucosa. However, despite intense efforts to ascertain the precise role of hephaestin in intestinal iron absorption, our knowledge about its exact activity is actually quite limited. Although copper-deficient pigs show marked iron accumulation in the intestine, this phenomenon is not as pronounced in rodent models of copper deficiency. Conversely, copper deficiency in swine is associated with diminished liver iron, whereas iron accumulates in hepatocytes of copper-deficient rats (24). The latter observation suggests that intestinal iron absorption may not be as severely compromised in copper-deficient rodents, but unfortunately conflicting data indicate both decreased (21) or increased uptake (24) in copper-deficient rats. On the basis of the latter observations, the authors suggest that hephaestin may have functions in the pathway of iron absorption that do not require its copper-dependent ferroxidase activity. Nonetheless, depletion of copper in cultured cells prevents the maturation of hephaestin to its 160-kDa glycosylated form, because apohephaestin is rapidly ubiquinated and degraded in the proteosome (20). The presence of this quality control mechanism indicates that during copper deficiency, synthesis of the protein would be disrupted, providing a direct link between nutritional copper status and iron absorption. In fact, Reeves and DeMars (21) have reported decreased hephaestin levels in intestinal enterocytes from copper-deficient animals, strongly supporting the idea that reduced ferroxidase function impairs iron assimilation.
FERROPORTIN: AN IRON EXPORT PROTEIN
Three independent studies seeking to further elucidate the molecular basis for the export of iron led to the identification of the same factor: ferroportin-1 was found through positional cloning of a defective gene in anemic zebrafish (6), Ireg-1 was discovered by hybridization of mRNAs induced in hypotransferrinemic (hpx) mice (17), and metal transport protein-1 (MTP1) was cloned by construction of a library enriched for iron-responsive protein-1 (IRP-1) binding (1). Also known by its gene name SLC40A1 (previously called Slc39A1 and SLC11A3), there is some agreement that the name “ferroportin” best describes this transporter.
Ferroportin is a highly conserved 571-amino acid protein with human, mouse, and rat clones being 90–95% homologous at the protein level. Ferroportin mRNA and protein are abundant in the liver, spleen, kidney, heart, and placenta as well as the duodenum, and all of these organs can export iron. The protein has been localized to the basolateral membrane of polarized epithelial cells (17), supporting the idea that it functions in the assimilation of dietary iron by exporting the metal from intestinal enterocytes.
The iron export function of ferroportin has been demonstrated in Xenopus oocytes (6, 17), but the presumed role for ferroxidase activity in this process is unclear. One group demonstrated iron efflux in the absence of ceruloplasmin but in the presence of transferrin (6), whereas another showed ceruloplasmin was necessary for efflux but that transferrin was not (17). Neither group rigorously addressed whether ferroxidase activity per se was required to support iron efflux. However, a glycosylphosphatidylinositol (GPI)-linked form of ceruloplasmin has been found to associate with ferroportin and to facilitate iron efflux from brain astrocytes (12). There is speculation that a similar complex forms in the enterocyte between membrane-bound hephaestin and ferroportin, but strong evidence for this binding interaction is lacking. Regardless of its precise export mechanism and the presumed role for ferroxidase activity, overexpression of ferroportin in tissue culture cells confirms its function in iron release because depletion of cytosolic iron can be shown by decreased levels of the ironstorage protein ferritin (1).
Disruption of ferroportin expression in zebrafish (6) and mice (7) results in embryonic lethality, but for both animals this effect appears to be due to impaired maternal-fetal iron transfer. To establish the role of murine ferroportin function in vivo, Donovan et al. (7) selectively inactivated its postnatal expression in the intestine. Once intestinal expression was suppressed, the mice developed severe iron-deficiency anemia and accumulated duodenal iron consistent with a critical role for the transporter in dietary iron assimilation. On the basis of these data, the authors concluded that ferroportin provides the major, if not the only, iron export pathway in the small intestine.
FERROPORTIN IS A RECEPTOR FOR HEPCIDIN
The most important recent discovery in our understanding of intestinal iron absorption has been the role of the liver-derived peptide hepcidin. Hepcidin is produced under iron-loading and inflammatory conditions to suppress iron absorption, and its synthesis is diminished in response to iron deficiency, pregnancy, or enhanced erythropoiesis to promote iron uptake. This peptide therefore provides a direct link between the liver, bone marrow, and intestine to adjust dietary iron absorption to meet the needs of the body.
Direct functional evidence for regulation of ferroportin by hepcidin was established by collaborative work from the Ganz and Kaplan laboratories (18). These investigators showed that hepcidin regulates ferroportin protein levels by inducing its internalization and lysosomal degradation. Through an elegant series of labeling experiments, ferroportin was shown to bind hepcidin, i.e., ferroportin is a receptor for hepcidin. This finding implicates a homeostatic mechanism that allows systemic regulation of intestinal iron absorption directly in response to the body's iron demands. Donovan et al. (7) have further suggested that hepcidin may affect body iron status by influencing levels of “excretion.” Reduced ferroportin levels due to increased circulating levels of hepcidin during iron overload would result in increased enterocyte iron, an effect observed in zebrafish and mice with impaired ferroportin function. These authors point out that in addition to newly absorbed dietary iron, the amount of plasma iron entering enterocytes is also quite large given the surface area of the villous epithelium. Because enterocytes are continually sloughing off into the gut as they mature, any iron they contained due to reduced ferroportin-mediated efflux would be also be lost from the body. Although the hypothesis that hepcidin regulates body iron excretion remains to be rigorously tested, this exciting new model certainly challenges the established view that a mechanism for regulated iron loss does not exist.
CRYPT PROGRAMMING VERSUS SYSTEMIC SIGNALING
The discovery of hepcidin has also challenged the prevailing model that systemic regulation of iron homeostasis involves the “programming” of crypt enterocytes to ensure the appropriate balance of dietary absorption. Because transferrin saturation correlates with body iron stores, iron uptake by crypt cells via the transferrin receptor would be reduced by iron deficiency. Conversely, when stores are high and transferrin saturation is increased, delivery of iron would be enhanced. Early animal studies indicated that iron levels in crypt enterocytes reflected body iron status. Because responses in iron absorption take several days, the view emerged that programming took place in crypt cells that later mature to villus cells (the absorptive enterocytes). At the molecular level, programming in the crypt cell was presumed to involve the modulation of the gene expression of the key factors involved in iron transport. This model predicts iron deficiency would lower crypt cell iron status such that gene expression of critical transport factors would be upregulated on cellular differentiation and migration to the villus tip. However, Frazer et al. (9) have more recently shown that the “lag period” for changes in iron absorption coincides with the period of time necessary to alter hepcidin expression in models of hemolytic anemia and diet-induced iron deficiency. Once circulating hepcidin is increased, changes in absorption occur quite rapidly. Thus there is no need to invoke programming of gene expression to accommodate the time period required for adjustments in dietary iron absorption.
The sla mouse model provided an opportunity to test the crypt cell programming model because disruption of hephaestin results in retention of iron by duodenal enterocytes. Chen et al. (2) observed that whereas DMT1 mRNA levels were increased in iron-deficient mice, sla mice failed to upregulate DMT1 gene expression despite their anemia. In contrast, hephaestin and ferroportin were upregulated in both iron-deficient and sla mice. Thus gene expression of the iron efflux machinery appears to be regulated in a manner that is independent of enterocyte iron status as predicted by crypt programming. What remains to be better understood, however, is how the gradient of intestinal iron transporter expression from the crypt to the villi arises during differentiation.
The pattern of signaling observed for sla enterocyte regulation characterizes a two-step model for dietary iron absorption: transport of iron across the apical surface of the intestinal epithelia reflecting enterocyte iron status and basolateral efflux dictated by systemic factors such as hepcidin. This two-stage model is further supported by the influence of luminal iron content on DMT1 expression, which is suppressed after an intragastric dose of high iron with little effect on mRNA and protein levels of the basolateral export factors (8). Thus local iron status may be the dominant regulator of apical iron absorption, whereas systemic modulators control basolateral export. This two-step model helps to explain how the body can respond to iron deficiency while limiting uptake to prevent toxic accumulation of iron and should be further examined using other genetic models.
REGULATION OF IRON EXPORT AT THE mRNA LEVEL
Although we continue to learn more about hepcidin and the systemic regulation of intestinal iron efflux, there are striking differences in the profile of ferroportin expression between the intestine and liver (2 major sites of iron release) that still remain to be fully explained. Iron loading reduces intestinal expression, whereas it enhances mRNA levels in liver and macrophages (1, 17). How systemic circulation of hepcidin could alter ferroportin mRNA is not fully explained by its action on ligand-induced internalization and degradation of the transporter, and the molecular basis for tissue-specific regulation of ferroportin expression at the transcriptional and/or posttranscriptional level remains to be fully identified. It is quite possible that other receptors for hepcidin (and/or other signals) exist. Early posttranslational responses to inflammation may downregulate ferroportin protein levels, but transcriptional (or posttranscriptional) effects are maintained well after the peak of hepcidin induction to suppress ferroportin mRNA.
Gene expression of many proteins involved in iron metabolism involves regulation by IRPs through interactions mediated by IRP binding to RNA elements called iron-responsive elements (IREs). IRPs bind to IREs in both 5′- and 3′-untranslated regions, but the regulatory mechanism depends on the location: 5′-IREs dictate translational control such that protein synthesis is downregulated on IRP binding, whereas 3′-IREs enhance message stability, thereby upregulating protein levels when IRPs are bound. Recognition of a 5′-IRE in ferroportin mRNA led to in vitro studies of its interaction with IRPs (17). From these investigations, it is clear that IRE/IRP interactions can posttranscriptionally regulate transporter levels in cells such that under low-iron conditions, which stabilize the RNA binding activity of IRP, ferroportin protein synthesis is reduced. Consistent with regulation through IRP control, it has been found that iron depletion diminishes ferroportin expression, whereas iron loading increases its expression in macrophages (1). However, iron deficiency increases ferroportin levels in the small intestine and the intestinal Caco-2 cell line, whereas iron overload decreases its expression (1, 10, 17). It has been suggested that the 5′-IRE of the ferroportin transcript may also influence mRNA stability (17), but this possibility has yet to be tested experimentally and certainly does not fully account for tissue-specific differences in gene regulation. More work is necessary to better define the role of IRE-IRP interactions in the control of ferroportin levels.
Considering the close relationship between copper and iron metabolism, our laboratory has explored the influence of copper status on intestinal iron export and found that treatment of human intestinal Caco-2 cells with high copper upregulated ferroportin mRNA but in a manner dependent on iron status (10). This study showed that copper-treated Caco-2 cells display increased transepithelial transport of iron, suggesting that enterocyte synthesis of the copper-dependent ferroxidase hephaestin and the iron transporter ferroportin is coordinately regulated by both metals. Because copper-deficient mice fail to upregulate ferroportin expression, as would be expected due to their microcytic anemia (3), these combined data strongly suggest that copper sufficiency is required for normal maintenance of iron export not only to support ferroxidase activity, but also to adjust ferroportin expression to appropriate levels.
FERROPORTIN DISEASE
Several different mutations of the human ferroportin (Fpn) gene have been unequivocally associated with iron overload (Table 1). In contrast to other genetic causes of hemochromatosis, “ferroportin disease” is autosomal dominant. The clinical symptoms of patients with mutations in ferroportin are somewhat variable but have been generally classified into two groups. One group of patients has high ferritin levels with low-to-normal transferrin saturation accompanied by macrophage iron loading. The other group has high transferrin saturation typically associated with other inherited forms of hemochromatosis, along with iron accumulation predominantly in hepatocytes.
Table 1.
Functional consequences of ferroportin mutations
| Mutation | Clinical Features | Cellular Location of Ferroportin Variant |
Hepcidin Interactions | Functional Effects of Mutation |
|---|---|---|---|---|
| Y64N | Parenchymal iron overload | Cell surface (22) | Resistant (22) | Normal iron export activity (22); reduced cellular ferritin (22); increased transferrin receptor (22) |
| A77D | Increased macrophage iron | Reduced cell surface expression (16, 22); intracellular localization (16, 22) |
Resistant (16) | Impaired iron export activity (22); some IRP-1 activation (16); slightly reduced cellular ferritin (16) |
| N144X | Parenchymal iron overload | Cell surface (4, 16) | Resistant (4, 16); reduced hepcidin-induced degradation (4) |
Normal iron export activity (16, 22); reduced cellular ferritin (4, 16, 22); increased transferrin receptor (22); IRP-1 activation (16) |
| D157G | Increased macrophage iron | Cell surface (4, 16); intracellular localization (16) |
Resistant (4, 16) | Reduced (4) or no change (16) in cellular ferritin; some IRP-1 activation (16) |
| Δ162 | Increased macrophage iron | Reduced cell surface expression (4, 22); intracellular localization (4, 16, 22) |
Resistant (4,16) | Impaired iron export (16, 22); reduced (22) or slightly reduced (4, 16) cellular ferritin; some IRP-1 activation (16) |
| Q182H | Increased macrophage iron | Cell surface (4, 16) | Resistant (partial) (4, 16); delayed hepcidin- induced degradation (4) |
Reduced cellular ferritin (4, 16); IRP-1 activation (16) |
| Q248H | Parenchymal iron overload | Cell surface (22) | Sensitive (22) | Normal iron export activity (22); reduced cellular ferritin (22); increased transferrin receptor (22) |
| G323V | Increased macrophage iron | Normal (16) or reduced cell surface expression (4); intracellular localization (4) |
Resistant (4) | Slightly reduced cellular ferritin (4, 16); some IRP-1 activation (16) |
| C326Y | Parenchymal iron overload | Cell surface (22) | Resistant (22) | Normal iron export activity (22); reduced cellular ferritin (22); increased transferrin receptor (22); |
| G490D | Increased macrophage iron | Reduced cell surface expression (4); intracellular localization (4) |
Resistant (4) | Impaired iron export activity (22); reduced (22) or slightly reduced cellular ferritin (4) |
The functional consequences of several human mutations have been analyzed in vitro with results summarized in Table 1. Exogenous expression of ferroportin activity can be directly measured by the amount of radioactive iron efflux after loading or indirectly through assessment of low cellular iron status (decreased ferritin levels, increased transferrin receptor levels, and activation of the RNA-binding activity of IRP1). Most of the variants appear to be “hepcidin-resistant,” but these fall into two classes: those with impaired iron export activity and resistance to hepcidin due to an intracellular distribution and those with cell-surface localization and normal iron-export activity but impaired hepcidin-induced degradation.
Schimanski et al. (22) argued that ferroportin disease promoted by mutations associated with impaired export activity arises due to haploinsufficiency in the transporter's activity. In contrast, De Domenico et al. (4) provided strong evidence that such ferroportin mutants can act in a dominant negative manner. The latter group has shown that ferroportin can form multimers and that complexes formed by wild-type and mutant ferroportin are misdirected to intracellular membranes. Such dominant negative interactions serve to decrease the number of functional transporters at the cell surface. Loss of ferroportin activity at the plasma membrane would reduce both intestinal iron absorption and the recycling of iron from macrophages, leading to macrophage iron loading, high-serum ferritin, with low-to-normal transferrin saturation. Although Schimanski et al. (22) failed to detect oligomerization of ferroportin (and its mutants), the dominant negative model for ferroportin disease mutations is further bolstered by the fact that the only known mutations are missense mutations causing amino acid substitution or deletions: no nonsense mutations have been reported for patients with iron overload. Moreover, Fpnnull/+ mice do not manifest the same pattern of iron loading observed in patients with ferroportin disease, suggesting that haploinsufficiency does not fully account for the pathology observed (7).
Despite controversy over haploinsufficiency versus dominant negative effects, there does appear to be a consensus about possible “gain of function” mutations in ferroportin that confer true “resistance” to hepcidin regulation. In this scenario, impaired hepcidin-induced degradation results in an activated iron exporter at the cell surface that is resistant to downregulation. For the best-characterized example (N144X), hepcidin binds to the mutant transporter but the endocytic signal conferred by these interactions appears to be impaired, thereby preventing lysosomal degradation. Patients harboring such gain of function alleles would have abnormally high intestinal iron absorption, leading to increased transferrin saturation and iron accumulation in hepatocytes. Indeed, several ferroportin mutants display normal iron export activity and of these, Y64N, N144X, and C326Y appear to resist inhibition by hepcidin and are associated with increased liver iron deposition. However, there are exceptions; for example, whereas the Q182H mutant appears to have normal iron export activity but delayed hepcidin-induced downregulation, clinical information suggests that this mutation is associated with macrophage iron accumulation similar to the dominant negative mutations discussed previously. More work is necessary to better understand how the function of ferroportin is perturbed by such gain of function mutations.
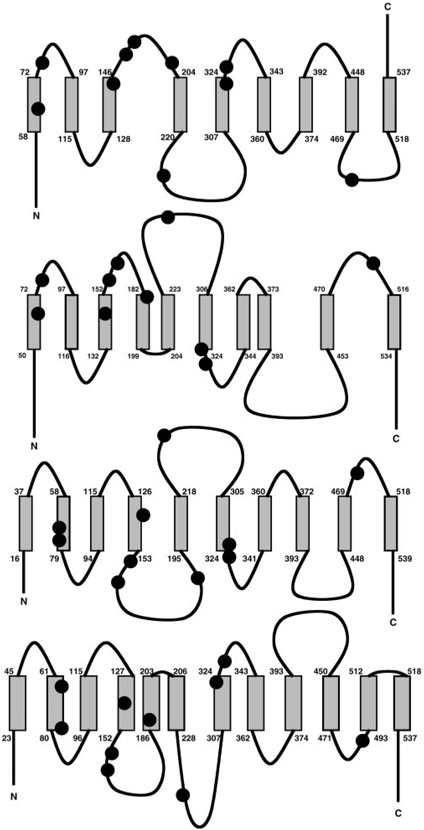
The phenotypes associated with different ferroportin mutations may also provide some clues about the transporter's structure. Four different models have been proposed for this transmembrane protein (Fig. 1). Only one suggests that the COOH terminus of ferroportin is extracellular (5), an idea that is supported by work of Schimanski et al. (22), showing that a COOH-terminal c-myc epitope tag can be identified via FACS analysis using anti c-myc antibodies. In contrast, Lui et al. (16) have found that a COOH-terminal hemagglutin tag cannot be detected by immunofluorescence microscopy unless cells are first permeabilized, indicating an intracellular placement for this epitope. Unfortunately, little information is available regarding ferroportin's native topology to resolve this controversy, and it is quite possible that the presence of a nonnative epitope-tagged structure interferes with proper folding and membrane insertion. In this regard, it is important to note that Schimanski et al. (22) were unable to detect ferroportin oligomers, suggesting that epitope tagging could disrupt proteinprotein interactions.
Fig. 1.
Predicted topologies for ferroportin. Schematic diagrams noting the amino acid segments of predicted membrane helices for the 9 (5)-, 10 (7, 17)-, and 12-transmembrane domain (16) models are shown in order from top to bottom. Known human mutations are represented by the filled circles for the amino acids listed in Table 1.
All other proposed models place the COOH-terminal domain of ferroportin inside of the cell (Fig. 1). It has been noted that ferroportin harbors a putative PDZ domain at its COOH terminus and that this motif is possibly important for basolateral targeting of the transporter (17). It would seem likely that if functional, the PDZ domain would be intracellularly disposed. Two of the intracellular COOH-terminal models suggest that ferroportin has 10 transmembrane spanning helices, however, topological predictions for ferroportin mutations are quite different. In one model, the mutations are largely distributed on the transporter's exofacial surface, whereas the other model predicts these mutations are present on the intracellular side of the membrane. Because both the dominant negative and gain-of-function mutations underlying ferroportin disease appear to interfere with membrane trafficking, it seems more probable that mutations on the intracellular face would interfere with membrane-targeting signals. Finally, the structural topology predicted by Lui et al. (16) suggests ferroportin has 12 membrane-spanning domains. This group used a combination of epitope tagging and cysteine mutagenesis strategies to support their model. Similar to the model proposed by McKie et al. (17), most of the mutations cluster between the second and fourth predicted membrane helix and the second intracellular loop of the protein. For reasons discussed above, this work needs to be corroborated by further analysis of the native (nontagged) transporter, however, it does represent the initial step toward a detailed understanding of ferroportin's secondary structure within the membrane.
DIETARY IRON SENSING AND JUVENILE HEMOCHROMATOSIS
The idea that the hepcidin-ferroportin axis is critical in dietary iron sensing is strongly supported by studies of inherited forms of hemochromatosis. Genetic lesions in HFE, transferrin receptor-2, and ferroportin all result in increased dietary iron absorption. Juvenile hemochromatosis is another inherited disorder of iron loading that is very rare with severe pathology characterized by early onset. Most mutations identified in patients with this disease have been found to disrupt the HJV gene encoding a protein called hemojuvelin. Mouse models of this disease have recently been established (11, 19). Despite high iron, these mice have decreased hepcidin levels resulting in increased intestinal ferroportin expression. Niederkofler et al. (19) have proposed a selective role for hemojuvelin in dietary iron sensing because hepcidin synthesis in these animals was still induced by cytokines. They suggest that two independent pathways for hepcidin regulation exist: one that senses dietary iron through hemojuvelin and another that responds to inflammatory signals independent of this factor. Clearly, hemojuvelin must be an upstream regulator of hepcidin expression, but its mechanism of action remains controversial. There appear to be two forms: a GPI-linked cellassociated hemojuvelin may interact with a transmembrane receptor to induce changes in hepcidin synthesis, whereas a soluble circulating form can serve as an antogonist to disrupt these interactions. Lin et al. (15) have also shown that the cell-associated hemojuvelin regulates hepcidin synthesis independent of the cytokine IL-6, supporting the idea that two independent control pathways exist. However, these authors argue that the soluble hemojuvelin antagonist can reduce hepcidin expression in the presence of IL-6. They propose that baseline hepcidin expression is set by hemojuvelin and that inflammatory responses subsequently depend on this level (19). The relationship between these models is further clouded by the fact that inflammation, but not iron status, modulates hemojuvelin expression (13), whereas the amount of the soluble form that is released may be suppressed by high iron (15). If there are two distinct pathways (dietary iron sensing and inflammation) to control hepcidin, extensive cross talk must occur to integrate these signals.
LOCATION AND FUNCTION OF IRON EXPORT MACHINERY TO LOAD TRANSFERRIN
Both ferroportin and hephaestin are expressed throughout the gut, not just the proximal small intestine where iron absorption is thought to specifically occur. Throughout the gut, villous rather than crypt enterocytes are predominantly stained for ferroportin and hephaestin expression. Surprisingly, whereas ferroportin protein is found at the basolateral surface of enterocytes, hephaestin appears to be predominantly distributed in apical intracellular membranes as well as the basolateral membrane. Because a truncated but partially active form of hephaestin is localized to the apical compartment in sla enterocytes, Kuo et al. (14). have suggested that its absence at the basolateral surface may account for the iron-export defect in these mice. Alternatively, intracellular hephaestin may have additional functions. How iron exported from the enterocyte reaches transferrin is not well understood. Intracellular trafficking of apotransferrin has been proposed in one model (23). This pathway may account for intracellular placement for ferroxidase activity of hephaestin, but it does not account for the appearance of ferroportin at the basolateral membrane. On the other hand, because extracellular ceruloplasmin does not appear to contribute to dietary iron absorption, intracellular ferroxidase activity may be required to load iron onto transferrin. This final step of iron exsorption from the mucosa remains to be fully defined. The possible intracellular function of hephaestin leaves us with fewer clues as to the thermodynamic driving force for ferroportin-mediated efflux of Fe3+ against its electrical gradient. Given that rapid progress being made in our understanding of dietary iron assimilation, however, we are likely to soon gain a comprehensive model to explain the molecular basis for each step in this process and its regulation.
REFERENCES
- 1.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Su T, Attieh ZK, Fox TC, McKie AT, Anderson GJ, Vulpe CD. Systemic regulation of hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood. 2003;102:1893–1899. doi: 10.1182/blood-2003-02-0347. [DOI] [PubMed] [Google Scholar]
- 3.Chung J, Prohaska JR, Wessling-Resnick M. Ferroportin-1 is not upregulated in copper-deficient mice. J Nutr. 2004;134:517–521. doi: 10.1093/jn/134.3.517. [DOI] [PubMed] [Google Scholar]
- 4.De Domenico I, Ward DM, Nemeth E, Vaughn MB, Musci G, Ganz T, Kaplan J. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci USA. 2005;102:8955–8960. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devalia V, Carter K, Walker AP, Perkins SJ, Worwood M, May A, Dooley JS. Autosomal dominant reticuloendothelial iron overload associated with a 3-base pair deletion in the ferroportin 1 gene (SLC11A3) Blood. 2002;100:695–697. doi: 10.1182/blood-2001-11-0132. [DOI] [PubMed] [Google Scholar]
- 6.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 7.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Frazer DM, Wilkins SJ, Becker EM, Murphy TL, Vulpe CD, McKie AT, Anderson GJ. A rapid decrease in the expression of DMT1 and Dcytb but not Ireg1 or hephaestin explains the mucosal block phenomenon of iron absorption. Gut. 2003;52:340–346. doi: 10.1136/gut.52.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frazer DM, Inglis HR, Wilkins SJ, Millard KN, Steele TM, McLaren GD, McKie AT, Vulpe CD, Anderson GJ. Delayed hepcidin response explains the lag period in iron absorption following a stimulus to increase erythropoiesis. Gut. 2004;53:1509–1515. doi: 10.1136/gut.2003.037416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han O, Wessling-Resnick M. Copper repletion enhances apical iron uptake and transepithelial iron transport by Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G527–G533. doi: 10.1152/ajpgi.00414.2001. [DOI] [PubMed] [Google Scholar]
- 11.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115:2187–2191. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong SY, David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem. 2003;278:27144–27148. doi: 10.1074/jbc.M301988200. [DOI] [PubMed] [Google Scholar]
- 13.Krijt J, Vokurka M, Chang KT, Necas E. Expression of Rgmc the murine ortholog of hemojuvelin gene is modulated by development and inflammation but not by iron status or erythropoietin. Blood. 2004;104:4308–4310. doi: 10.1182/blood-2004-06-2422. [DOI] [PubMed] [Google Scholar]
- 14.Kuo YM, Su T, Chen H, Attieh Z, Syed BA, McKie AT, Anderson GJ, Gitschier J, Vulpe CD. Mislocalisation of hephaestin a multicopper ferroxidase involved in basolateral intestinal iron transport in the sex linked anaemia mouse. Gut. 2004;53:201–206. doi: 10.1136/gut.2003.019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005 doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- 16.Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis. 2005;35:33–46. doi: 10.1016/j.bcmd.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 18.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 19.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing and its mutation leads to severe iron overload. J Clin Invest. 2005;115:2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nittis T, Gitlin JD. Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J Biol Chem. 2004;279:25696–25702. doi: 10.1074/jbc.M401151200. [DOI] [PubMed] [Google Scholar]
- 21.Reeves PG, DeMars LC. Copper deficiency reduces iron absorption and biological half-life in male rats. J Nutr. 2004;134:1953–1957. doi: 10.1093/jn/134.8.1953. [DOI] [PubMed] [Google Scholar]
- 22.Schimanski LM, Drakesmith H, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, Bastin JM, Cowley D, Chinthammitr Y, Robson KJ, Townsend AR. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood. 2005;105:4096–4102. doi: 10.1182/blood-2004-11-4502. [DOI] [PubMed] [Google Scholar]
- 23.Simovich MJ, Conrad ME, Umbreit JN, Moore EG, Hainsworth LN, Smith HK. Cellular location of proteins related to iron absorption and transport. Am J Hematol. 2002;69:164–170. doi: 10.1002/ajh.10052. [DOI] [PubMed] [Google Scholar]
- 24.Thomas C, Oates PS. Copper deficiency increases iron absorption in the rat. Am J Physiol Gastrointest Liver Physiol. 2003;285:G789–G795. doi: 10.1152/ajpgi.00509.2002. [DOI] [PubMed] [Google Scholar]
- 25.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]