Abstract
Epidemiological studies have shown a relationship between diets rich in tomato and/or lycopene and a reduction in cancer rates. Several studies reported reductions in proliferation of certain cell lines when treated with lycopene. This study used seven human cell lines to measure the effect of lycopene on cell proliferation across normal human plasma concentrations of lycopene. Seven cell types, cancerous and noncancerous, were treated with lycopene from 0.0001 to 10 μM for 24, 48, and 72 hours and counted electronically. Controls and experimental samples were compared using the Mann-Whitney U-test at a 95% confidence level. All cells grew normally and there was no significant difference between any of the controls. The Hep-G2, liver adenocarcinoma cell line, showed a reduction at the high doses after 24 hours and the IMR-90, noncancerous lung cell line, showed a reduction at the highest dose after 72 hours when compared to the solvent control. The A431, skin carcinoma, DU-145, prostate carcinoma, HS-68, noncancerous skin, A549, lung carcinoma, and HS-578T, breast carcinoma, all showed no reduction in proliferation. This indicated that lycopene at the physiological range does not significantly affect cell proliferation in an in vitro model and requires more careful investigations.
Keywords: lycopene, cell proliferation, cell culture
Introduction
Lycopene is a 40-carbon acyclic carotenoid which contains 11 conjugated double bonds and belongs to a subgroup of carotenes consisting only of hydrogen and carbon atoms (Stahl and Sies, 1996). Lycopene is a red plant pigment found in tomatoes, apricots, guavas, watermelons, papayas, pink grapefruits and rosehips, with tomatoes being the largest contributor to the dietary intake of humans (Chalabi et al., 2004).
Interest in lycopene as a molecule with biological activities began in 1958, at the University of Stockholm, where they demonstrated the curative action of injected lycopene in irradiated mice (Forssberg et al., 1959), and when administered to mice 24 hours prior to being challenged with X-rays, the bacteria, Klebsiella pneumonia, and Ehrlich ascites tumor cells showed a nonspecific resistance (Lingen et al., 1959).
Several epidemiology studies strongly suggest the hypothesis that the consumption of foods containing high concentrations of lycopene reduces the risk for certain types of cancer (Gann et al., 1999; Giovannucci et al., 2002; Jian et al., 2005). This hypothesis has not been upheld by many researchers who suggest that lycopene alone may not be the compound that reduces cancer rates, but rather the group of carotenoids and nutrients as a whole may be responsible for the lower cancer rates seen in groups with higher dietary intake of tomatoes and tomato-based products (Bosetti et al., 2004).
The physiological level of lycopene in human blood and tissues varies with intake and tissue type (Diwadkar-Navsariwala et al., 2003; van Breeman et al., 2002). Lycopene in human blood averages ~0.5 μM of plasma and tissue levels vary from 0.001 μM wet weight in adipose tissue to 0.02 μM wet weight in adrenals and testes (Stahl and Sies, 1996).
Researchers have reported increases in lycopene in the blood of humans after dosing with lycopene or tomato products. Human subjects had a plasma increase of 0.47–0.58 μM after two weeks with a 25 mg supplement/day of lycopene or tomato paste (Richelle et al., 2002). Other researchers reported a serum concentration of 0.34–0.65 μM after dosing with 10 to 120 mg/day of lycopene with a peak serum concentration two days after the dose was given (Diwadkar-Navsariwala et al., 2003).
The mechanism of lycopene is still under investigation and lycopene has been proposed to negatively affect cancer cells or development of cancer by antioxidant activity, inhibition of cell cycle progression/cell proliferation, apoptosis induction, inhibition of several cytokines, and increased gap-junctional communication (Wertz et al., 2004).
The ability of a compound to inhibit the proliferation of cancer cells is very desirable. Most studies on cell proliferation with lycopene treatment show a decrease in cell proliferation with an increasing dose of lycopene (Chalabi et al., 2004; Fornelli et al., 2007; Hwang and Bowen, 2004; Kim et al., 2002; Levy et al., 1995; Limpens et al., 2006; Livny et al., 2002; Park et al., 2005; Pastori et al., 1998; Prakash et al., 2001; Salman et al., 2007; Tang et al., 2005). Very few have reported no effect on cell proliferation from lycopene treatment (Hantz et al., 2005). Of these reports, one researcher has reported no effect in one malignant cell line and increases in two others (Prakash et al., 2001), and two reports have shown a reduction in prostate cell proliferation with lycopene and vitamin E (Limpens et al., 2006; Pastori et al., 1998). Most reports have used lycopene concentrations starting at 1 μM and going as high as 100 μM (Chalabi et al., 2004; Levy et al., 1995; Livny et al., 2002; Park et al., 2005; Prakash et al., 2001; Salman et al., 2007).
The objective of the present study was to use a variety of human cell lines as an in vitro model and measure the effect of lycopene on the proliferation of these cells. This was done with a dose range that covers the physiological range of lycopene that represented the plasma levels of an average human diet with and without lycopene supplementation and a large variety of cultured human cells.
Methods
Experimental Design
Human cell lines that were from both cancerous and noncancerous tissues were used to determine if exposure to lycopene would affect the proliferation of the cells. The dose range used was 100X higher to 10X lower than the normal physiological concentrations of lycopene in various tissues (Diwadkar-Navsariwala et al., 2003; van Breeman et al., 2002).
The following human cell lines from American Tissue Type Collection (ATTC) were used: DU-145, a prostate adenocarcinoma; HS-68, a noncancerous foreskin fibroblast; A549, a lung carcinoma; IMR-90, a noncancerous lung; HS-578T, a breast carcinoma; A431, an epidermoid carcinoma; and Hep G2, a hepatocellular carcinoma.
Cell culture
All cell lines were grown as recommended by ATCC. The cell lines DU-145, HepG2, and IMR-90 were grown in Modified Eagle’s Medium (MEM), nonessential amino acids, and sodium pyruvate (HyClone). HS-578T was grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (HyClone), and bovine insulin (Sigma). A431 and HS-68 were grown in DMEM. A431 cells were grown in HAMS F12K medium (HyClone). All media included Bovine Growth Serum (BGS) (HyClone) and penicillin (100 units/ml) and streptomycin (100 μg/ml) (HyClone). The cells were cultured at 37° C in 5% CO2 and 95% air humidified environment. Cells were grown in various media and serums for 24, 48, and 72 hours to demonstrate normal growth characteristics.
Treatment of cells
Lycopene at >90% purity (MP Biomedicals) was dissolved in acetone to a stock concentration of 10−3 M. The lycopene was serially diluted in the various media to concentrations of 0.0001 to 10 μM with 1% acetone. Twenty-four well plates were seeded with 1.5–3.0 × 106 cells per well, determined with the aid of an electronic cell counter (Coulter). Media with lycopene were added and the cells were incubated for 24, 48, or 72 hours and then counted.
Nonsolvent and solvent controls were also included. No detectable effect was seen with the use of 1% acetone in any of the cell lines. Also, the cells were grown with 0.5% tetrahydrofuran and lycopene to determine if a different solvent would affect the cells’ proliferation and no effect was seen. To determine if the half-life of the lycopene in culture was affecting cell proliferation, lycopene was added again after 24 and 48 hours, with no significant effect seen.
Cell Counts
Cells were removed from the plates by aspirating the media, then rinsed with Hanks Balanced Salt Solution 1X (HyClone), and 1ml of 0.25% trypsin (Hyclone) was added. Counting vials were prepared by adding 18 ml of Isotone II (Medix) solution, 1 ml of trypsin inhibitor (Worthington Biochemical) at 1 mg/ml, and the 1 ml sample of cells. Each vial was counted with the electronic cell counter (Coulter). Six wells per lycopene treatment were used and three trials for each cell line were conducted.
Data analysis
The effects of the lycopene were determined using the Mann-Whitney U-test at a 95% confidence level. First, the two controls were compared, control without and control with solvent and no effect of the solvent was seen. Then the control with the solvent was compared to the lycopene treated cells. Three separate trials were used for each cell line and the Mann-Whitney U-test was used to test for reduction of cell proliferation.
Results
All cell lines were shown to have normal growth characteristics when cultured as recommended by ATCC. During each trial and for all the cell lines both controls were included and counted to demonstrate that the cells grew normally over the 72-hour test period and that there was no effect from the solvent (data not shown). This was tested when the solvent and nonsolvent controls were compared with the Mann Whitney U-test, and there was no significant difference in cell proliferation at 95% confidence level.
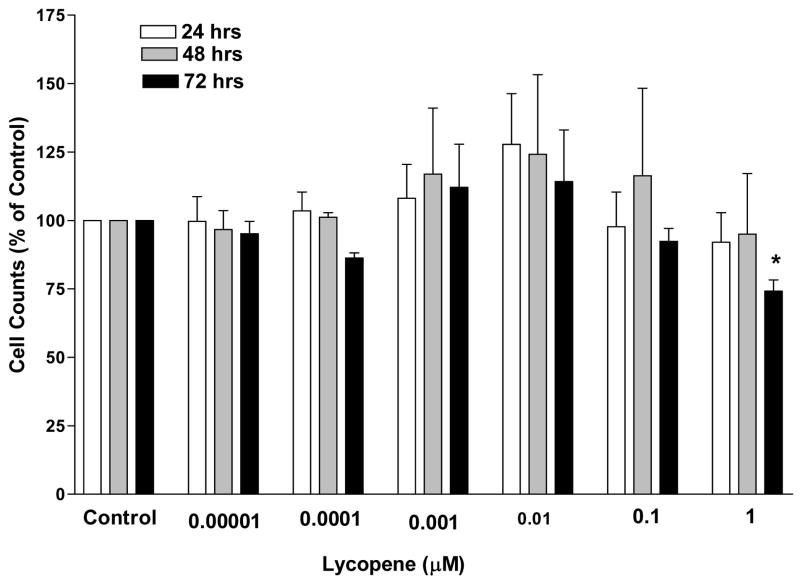
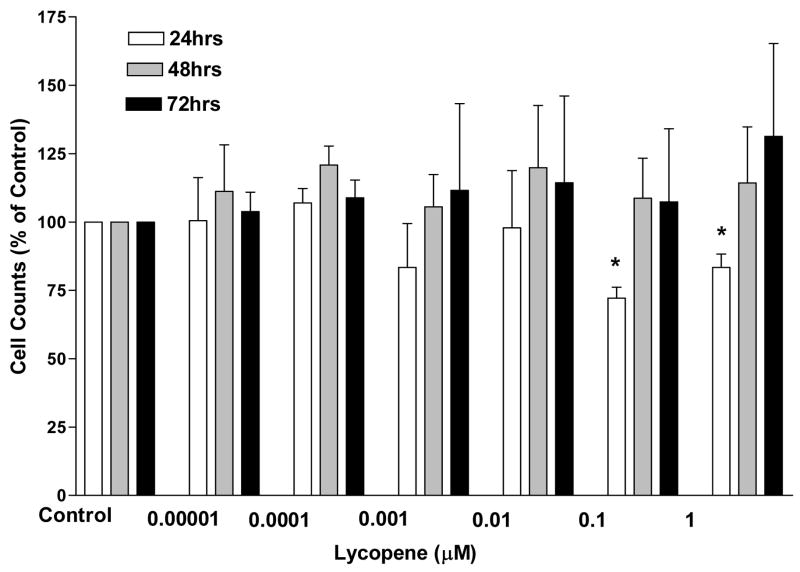
The treated cell samples were each compared to the solvent control and only the IMR-90 cells showed any significant difference in the cell proliferation over the 72 hours at a 95% confidence level. This cell line only showed a reduction at the 10 μM dose (Fig. 1). The only other cell line to show a reduction was the Hep-G2 cells at the 1 and 10 μM doses after 24 hours of incubation (Fig. 2). There were no significant differences at any of the dose levels at any of the time periods for any of the other cell lines (data not shown).
Figure 1. Cell counts of noncarcinoma lung cells, IMR- 90, after 24, 48, and 72 hours of incubation in the presence of log doses of lycopene.
Cell counts are expressed as a percent of the solvent control. Error bars represent the standard error of the mean which was determined from three separate trials each with six replicates of each cell type for every lycopene treatment. Asterisks indicate a significant difference from the solvent control (p<0.05). Significance was determined with the Mann-Whitney U-Test based on three trials.
Figure 2. Cell counts of liver carcinoma cells, Hep-G2, after 24, 48, and 72 hours of incubation in the presence of log doses of lycopene.
Cell counts are expressed as a percent of the solvent control. Error bars represent standard error of the mean which was determined from three separate trials each with six replicates of each cell type for every lycopene treatment. Asterisks indicate significant difference from controls (p<0.05). Significance was determined with the Mann-Whitney U-Test based on three trials.
Discussion
Past research has suggested that lycopene may reduce cancer risks by a variety of mechanisms including the inhibition of cell proliferation (Campbell et al., 2004; Everson and McQueen, 2004; Gann et al., 1999; Giovannucci et al., 2002; Jian et al., 2005; Stahl and Sies, 1996; Wane and Lengacher, 2006; Wertz et al., 2004). The specific mechanism of lycopene is unclear and is made complicated rather than more lucid due to the various in vitro model systems of various investigators (Hantz et al., 2005).
Interestingly, we observed very few changes in cellular proliferation at any concentration of lycopene. Our samples treated with the higher concentration of lycopene were at the lower range of many of the other investigators and we included two cell lines that are not malignant. Other researchers have limited their experiments to malignant cell lines and generally have used lycopene concentrations that may not be obtainable in human blood or tissues. The present research differs from other reports in two major areas. First, we are the only report of which we are aware, that has used acetone as the solvent for lycopene. We reported that acetone is a useable vehicle for cell culture with retinoids (Burgess and Hall, 2001), and in this research we never observed any differences in cell growth rates between our acetone-treated and nonacetone-treated controls, which were included in every trial. In the present research we tested 0.5% THF as a vehicle and had no significant decrease in cell proliferation. Of the other researchers who reported which vehicle and concentration were used in their research, most used tetrahydrofuran at various concentrations including 1–2 mM (Hantz et al., 2005; Levy et al., 1995; Pastori et al., 1998), 0.25g/l (Chalabi et al., 2004; Prakash et al., 2001), or 0.5% (Livny et al., 2002). Several reports did not state what concentration of tetrahydrofuran or vehicle was used (Fornelli et al., 2007; Kim et al., 2002; Salman et al., 2007; Tang et al., 2005), two used DMSO at 0.1% (Hwang and Bowen, 2004; Park et al., 2005), and two used water soluble lycopene (Limpens et al., 2006; Park et al., 2005). Tetrahydrofuran has been reported to be toxic to some cell lines (including prostate cell lines), this limits the concentration of lycopene delivered, and does not stabilize lycopene in the cell medium (Park et al., 2005).
Other researchers have reported that several of the cell lines we used in our research showed no effect on their proliferation from lycopene. HS-578T’s proliferation was not affected at lycopene doses of 7–20 μM (Prakash et al., 2001) and DU-145 was not affected at doses of 1–5 μM (Pastori et al., 1998). DU-145 was not affected until a dose of 20 μM for 72 hours was used (Tang et al., 2005). None of the other cell lines used in the present research has been reported on in studies with lycopene and cell proliferation to the authors’ knowledge. Many researchers have reported using dose ranges that were greater than the physiological range to show decreases in cellular proliferation (Fornelli et al., 2007; Livny et al., 2002; Park et al., 2005; Salman et al., 2007; Tang et al., 2005).
Another area where there was a difference between our research findings and other researchers’ findings was in the application of the statistical tests. We selected the Mann-Whitney U-test because it is a nonparametric alternative to the t test that is applied in situations in which the samples are drawn from the same population but different treatments are used on each set (Johnson, 1984), and cultured cells are drawn from the same population.
Conclusions
The data presented here demonstrate that exposure of lycopene to cells grown in culture will not always affect the cells’ proliferation when the concentration of lycopene is not dramatically higher than the normal physiological range for a human. Our study has added more confusion to the question of whether lycopene alone is an anticarcinogenic compound or whether it requires the consumption of the entire tomato or other fruit to produce the cancer rate reductions previously reported (Bosetti et al., 2004; Gann et al., 1999; Giovannucci et al., 2002; Jian et al., 2005; Stahl and Sies, 1996) There is another issue that has not yet been resolved by researchers. That is whether lycopene or diets with these types of compounds are preventing cancer or treating cancer. The testing of lycopene on established cancer cell lines may be superfluous. It seems that future research is needed to answer these questions and it needs to include studies dealing with normal cells, their transformation into malignant cells, and the association between malignant cells and the surrounding normal cells.
Acknowledgments
This publication was made possible by NIH grant number P20 RR016741 from the INBRE program of the National Center for Research Resources.
Footnotes
Competing Interests
None of the authors have any financial or other type of competing interests.
Authors’ Contributions
LCB: conception and design of the study, drafted the manuscript and is the instructor for the other authors who were, at the time of their involvement, undergraduate students. ER: draft of the manuscript, editing, production of data. TF: draft of the manuscript, editing, production of data. JRS: Statistical analysis, production of data. TPB: draft of the manuscript, editing, production of data, technical assistance. SJS: editing, production of data. KK: editing, technical assistance, production of data. All authors have read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bosetti C, Talamini R, Montella M, Negri E, Conti E, Franceschi S, La Vecchia C. Retinol, Carotenoids and the risk of prostate cancer: A case control study from Italy. International Journal of Cancer. 2004;112:689–692. doi: 10.1002/ijc.20486. [DOI] [PubMed] [Google Scholar]
- Burgess LC, Hall JO. Conditioned media from solid tumor cell lines treated with retinoic acids both decreases and increases proliferation of capillary endothelial cells. Life Sciences. 2001;69:2819–2831. doi: 10.1016/s0024-3205(01)01353-4. [DOI] [PubMed] [Google Scholar]
- Campbell JK, Canene-Adams K, Lindshield BL, Boileau TWM, Clinton SK, Erdman JWJ. Tomato phytochemicals and prostate cancer risk. Journal of Nutrition. 2004;134:3486S–3492S. doi: 10.1093/jn/134.12.3486S. [DOI] [PubMed] [Google Scholar]
- Chalabi N, Le Corre L, Mauizis JC, Bignon YJ, Bernard-Gallon D. The effects of lycopene on the proliferation of human breast cells and BRCA1 and BRCA2 gene expression. European Journal of Cancer. 2004;40:1768–1775. doi: 10.1016/j.ejca.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Diwadkar-Navsariwala V, Novotny JA, Gustin DM, Sosman JA, Rodvold KA, Crowell JA, Stacewicz-Sapuntzakis M, Bowen PE. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. Journal of Lipid Research. 2003;44:1927–1939. doi: 10.1194/jlr.M300130-JLR200. [DOI] [PubMed] [Google Scholar]
- Everson KM, McQueen CE. Lycopene for prevention and treatment of prostate cancer. American Journal of Health-System Pharmacy. 2004;61:1562–1566. doi: 10.1093/ajhp/61.15.1562. [DOI] [PubMed] [Google Scholar]
- Fornelli F, Leone A, Verdesca I, Minervini F, Zacheo G. The influence of lycopene on the proliferation of breast cell line (MCF-7) Toxicology In Vitro. 2007;21:217–223. doi: 10.1016/j.tiv.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Forssberg A, Lingen C, Ernster L, Lindberg O. Modification of the X-irradiation syndrome by lycopene. Experimental Cell Research. 1959;16:7–14. doi: 10.1016/0014-4827(59)90190-9. [DOI] [PubMed] [Google Scholar]
- Gann PH, Ma J, Giovannucci E, Willett WC, Sacks FM, Hennekens CH, Stampfer MJ. Lower prostate cancer risk in men with elevated plasma lycopene levels: Results of a prospective analysis. Cancer Research. 1999;59:1225–1230. [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. Journal of the National Cancer Institution. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- Hantz HL, Young LF, Martin KR. Physiologically attainable concentrations of lycopene induce mitochondrial apoptosis in LNCaP human prostate cancer cells. Experimental Biology and Medicine. 2005;230:171–179. doi: 10.1177/153537020523000303. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Bowen PE. Cell cycle arrest and induction of apoptosis by lycopene in LNCaP human prostate cells. Journal of Medicinal Food. 2004;7:284–289. doi: 10.1089/jmf.2004.7.284. [DOI] [PubMed] [Google Scholar]
- Jian L, Du CJ, Lee AH, Binns CW. Do dietary lycopene and other carotenoids protect against prostate cancer? International Journal of Cancer. 2005;113:1010–4. doi: 10.1002/ijc.20667. [DOI] [PubMed] [Google Scholar]
- Johnson R. Elementary Statistics. 4. Duxbury Press; Boston, MA: 1984. The Mann-Whitney U test; pp. 479–485. [Google Scholar]
- Kim L, Rao V, Rao L. Effects of Lycopene on prostate LNCaP cancer cells in culture. Journal of Medicinal Food. 2002;5:181–187. doi: 10.1089/109662002763003320. [DOI] [PubMed] [Google Scholar]
- Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, Sharoni Y. Lycopene is a more potent inhibitor of human cancer cell proliferation then either alpha-carotene or beta-carotene. Nutrition and Cancer. 1995;24:257–266. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- Limpens J, Schroder F, de Ridder CMA, Bolder CA, Wildhagen MF, Obermuller-Jevic UC, Kramer K, van Weerden W. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. Journal of Nutrition. 2006;136:1287–1293. doi: 10.1093/jn/136.5.1287. [DOI] [PubMed] [Google Scholar]
- Lingen C, Ernster L, Lindberg O. The promoting effect of lycopene on the non-specific resistance of animals. Experimental Cell Research. 1959;16:384–393. doi: 10.1016/0014-4827(59)90267-8. [DOI] [PubMed] [Google Scholar]
- Livny O, Kaplan I, Reifen R, Polak-Charcon S, Madar Z. Lycopene inhibits proliferation and enhances gap-junctional communication on KB-1 human oral tumor cells. Journal of Nutrition. 2002;132:3754–3759. doi: 10.1093/jn/132.12.3754. [DOI] [PubMed] [Google Scholar]
- Park YO, Hwang ES, Moon TW. The effect of lycopene on cell growth and oxidative DNA damage of Hep3B hepatoma cells. Biofactors. 2005;23:129–139. doi: 10.1002/biof.5520230302. [DOI] [PubMed] [Google Scholar]
- Pastori M, Pfander H, Boscoboinik D, Azzi A. Lycopene in association with α-tocopherol inhibits at physiological concentrations proliferation of prostate carcinoma cells. Biochemical and Biophysical Research Communications. 1998;250:582–585. doi: 10.1006/bbrc.1998.9351. [DOI] [PubMed] [Google Scholar]
- Prakash P, Russell RM, Krinsky NI. In vitro inhibition of proliferation of estrogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. Journal of Nutrition. 2001;131:1574–1580. doi: 10.1093/jn/131.5.1574. [DOI] [PubMed] [Google Scholar]
- Richelle M, Bortlik K, Lairdet S, Hager C, Lambelet P, Baur M, Applegate LA, Offord E. A food-based formulation provides lycopene with the same bioavailability to humans as that from tomato paste. Journal of Nutrition. 2002;132:404–408. doi: 10.1093/jn/132.3.404. [DOI] [PubMed] [Google Scholar]
- Salman H, Bergman M, Djaldetti M, Bessler H. Lycopene affects proliferation and apoptosis of four malignant cell lines. Biomedicine & Pharmacotherapy. 2007 doi: 10.1016/j.biopha.2007.02.015. in press. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Lycopene: A biologically important carotenoid for humans. Archives of Biochemistry and Biophysics. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- Tang L, Jin T, Zeng X, Wang JS. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. Journal of Nutrition. 2005;135:287–290. doi: 10.1093/jn/135.2.287. [DOI] [PubMed] [Google Scholar]
- van Breeman RB, Xu X, Viana MA, Chen L, Stacewicz-Sapuntzakis M, Duncan C, Bowen PE, Sharifi R. Liquid chromatography-mass spectrometry of cis-and all-trans-lycopene in human serum and prostate tissue after dietary supplementation with tomato sauce. Journal of Agricultural and Food Chemistry. 2002;50:2214–2219. doi: 10.1021/jf0110351. [DOI] [PubMed] [Google Scholar]
- Wane D, Lengacher CA. Integrative review of lycopene and breast cancer. Oncology Nursing Forum. 2006;33:127–134. doi: 10.1188/06.ONF.127-137. [DOI] [PubMed] [Google Scholar]
- Wertz K, Siler U, Goralczyk R. Lycopene: modes of action to promote prostate health. Archives of Biochemistry and Biophysics. 2004;430:127–134. doi: 10.1016/j.abb.2004.04.023. [DOI] [PubMed] [Google Scholar]