Abstract
Salvinorin A is a psychoactive natural product that has been found to be a potent and selective κ opioid receptor agonist in vitro and in vivo. The activity of salvinorin A is unusual compared to other opioids such as morphine in that it mediates potent κ opioid receptor signaling yet leads to less receptor downregulation than observed with other κ agonists. Our initial chemical modifications of salvinorin A have yielded one analogue, herkinorin (1c), with high affinity at the μOR. We recently reported that 1c does not promote the recruitment of βarrestin-2 to the μOR or receptor internalization. Here we describe three new derivatives of 1c (3c, 3f, and 3i) with similar properties and one, benzamide 7b, that promotes recruitment of βarrestin-2 to the μOR and receptor internalization. Considering the important role μ opioid receptor regulation plays in determining physiological responsiveness to opioid narcotics, μ opioids derived from salvinorin A may offer a unique template for the development of functionally selective μ opioid receptor ligands with the ability to produce analgesia while limiting adverse side effects.
Keywords: salvinorin A, Salvia divinorum, kappa, mu, opioid, agonist, βarrestin
Introduction
Increasing evidence indicates that chemically distinct ligands can elicit different receptor regulation pathways.1 For example, the opioids morphine, methadone, and fentanyl each promote μ opioid receptor (μOR) coupling to G proteins, but they differ in their ability to direct receptor trafficking.2, 3 This may be due to differences in agonist-induced receptor conformations, resulting in different degrees of phosphorylation, arrestin recruitment and vesicular trafficking. Such differences in μOR regulation and trafficking may be physiologically relevant as mice lacking βarrestin2 display enhanced antinociception, decreased tolerance, and greatly diminished side effects (constipation and respiratory depression) following morphine treatment.4–7 Therefore, an opioid agonist conferring non-conventional receptor conformations may yield novel analgesics with reduced potential to produce unwanted side effects.
Currently, there are no selective pharmaceutical or biochemical inhibitors of GPCR desensitization nor are there specific inhibitors of the GRKs or βarrestins. A therapeutic approach in which β-arrestins or GRKs were individually inhibited might produce unwanted alterations of the function of other GPCRs. Furthermore, since arrestins regulate >1000 different GPCRs,8, 9 it will be exceedingly difficult to produce receptor selective effects using this approach. An alternate approach would be to selectively target μOR regulation by designing ligands that confer μOR conformations that allow for signaling yet disrupt receptor regulation.
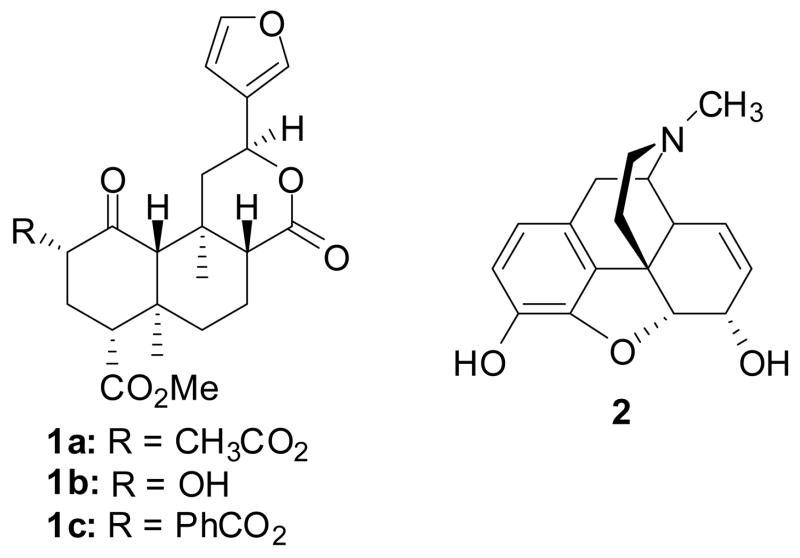
Salvinorin A (1a, Figure 1) is a neoclerodane diterpene isolated from Salvia divinorum, a member of the Lamiaceae family native to Oaxaca, Mexico.10, 11 S. divinorum has been used as a vision-inducing plant by the Mazatec Indians in their divination rituals for centuries.12 Previous studies have shown that 1a is a potent and selective κ opioid receptor agonist in vitro and in vivo.13–20 Interestingly, 1a activates κ opioid receptor signaling with less receptor internalization than observed with other κ agonists.21 These studies suggest that the κOR conformation induced by 1a binding is conducive to G protein mediated signal transduction but resistant to internalization-mediated regulation. Recent biochemical and site-directed mutagenesis studies indicate that 1a has a unique binding epitope at κORs.22–24 These findings support a novel mode by which subtype selectivity for GPCR ligands is induced by a change in the topology of conserved residues within a common binding pocket.23, 24
Figure 1.
Structures of salvinorin A (1a), salvinorin B (1b), herkinorin (1c), and morphine (2).
Our initial chemical modifications of 1a yielded several ligands, some agonists and some antagonists at μ, δ or κ ORs.25–27 In particular, herkinorin (1c) was identified as the first nonnitrogenous μ opioid receptor agonist and does not lead to receptor internalization under any conditions tested, but more interestingly, it does not promote the recruitment of βarrestin-2 to the μOR.28 As part of our ongoing program to develop analgesics with reduced propensity to induce tolerance and dependence, we synthesized several analogues of 1c. These analogues were prepared to further elucidate the role of structure on μOR affinity, activity, and regulatory pathways.
Chemistry
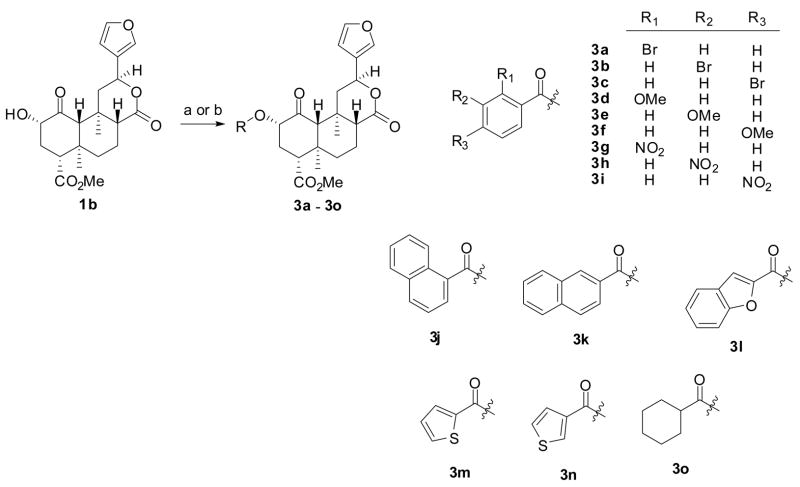
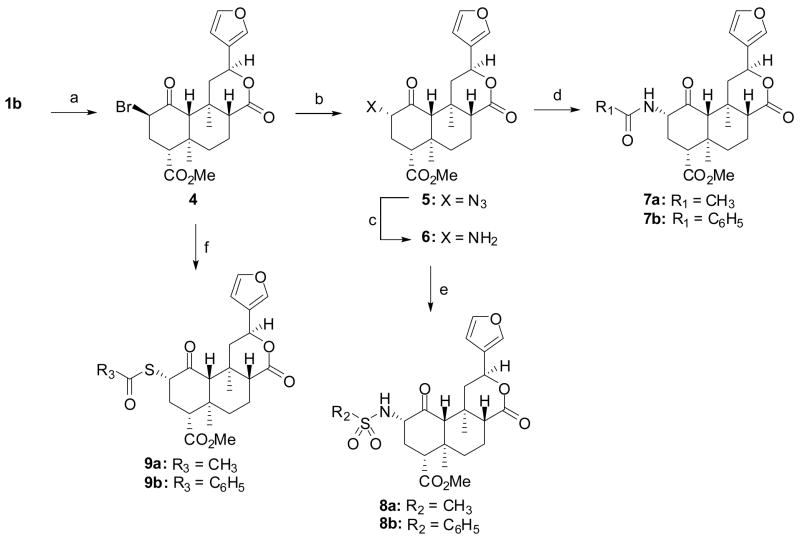
We synthesized compounds 3d – 3o, 7a, 7b, 8a, 8b, 9a, and 9b as described in Scheme 1. Diterpene 1a was isolated from S. divinorum and then converted to salvinorin B (1b) as described previously.29 The reaction of 1b with the appropriate acid halide or anhydride under basic conditions afforded compounds 3d – 3o.30, 31 Alternately, the reaction of 1b with CBr4 and PPh3 afforded a mixture of 432(59%) and its C2 epimer (14%). However, addition of the PPh3 in two portions afforded almost exclusively the β isomer. This method results in higher yields than previously described methods using SOBr2.32 The reaction of 4 with sodium azide in DMF was unsuccessful. However, if the reaction was conducted in a mixture of acetic acid and DMF,33 azide 532 was formed in 86% yield, a higher yield than previously described.32 Interestingly, when the C2 epimer of 4 was subjected to identical conditions, azide 5 was also formed. Reduction of 5 using Zn metal and NH4Cl34 afforded amine 635 in 36% yield. Staudinger reduction (PPh3, H2O)36 of 5 was also attempted but led mainly to decomposition of starting material.35 The treatment of amine 6 with acetic anhydride or benzoyl chloride under basic conditions and in the presence of a catalytic amount of DMAP afforded amides 7a32, 35 and 7b, respectively. The reaction of amine 6 with methanesulfonyl chloride or benzenesulfonyl chloride using similar conditions afforded 8a and 8b. Finally, the reaction of the potassium salt of thioacetic acid or thiobenzoic acid with 4a gave 9a32, 37 and 9b, respectively.
Scheme 1.
aReagents and conditions: (a) Appropriate acid chloride, DMAP, NEt3, CH2Cl2; (b) Appropriate acid, EDCI, HOBT, CH2Cl2
Results
Newly synthesized compounds 3d – 3o, 7b, 8a, 8b, and 9b were then evaluated for affinity at opioid receptors using methodology previously described (Table 1).38 These analogues were prepared to give insight as to the nature of the high affinity and selectivity of 1a and 1c for κ and μ receptors, respectively. Recently, we investigated the effects of the addition of a bromo group to 1c (i.e. 3a – 3c).30 It was found that substitution of the bromo group in the 4-position (3c) retained high affinity at μ receptors. This modification also increased μ/κ selectivity compared to 1c. Given the clear effects of ring substitution, we sought to probe additional modifications of the benzene ring.
Table 1.
Binding affinities of salvinorin A analogues at opioid receptors using [125I]IOXY as radioligand.39, 40
| Ki ± SD, nM | Selectivity | ||||
|---|---|---|---|---|---|
| Compound | μ | δ | κ | μ/κ | δ/κ |
| 1aa | > 1,000b | 5790 ± 980 | 1.9 ± 0.2 | > 526 | 3050 |
| 1b | > 10,000 | > 10,000 | 280 ± 20 | > 35 | > 35 |
| 1ca | 12 ± 1 | 1170 ± 60 | 90 ± 2 | 0.13 | 12 |
| 3ac | 110 ± 1 | > 10,000 | 90 ± 7 | 1.2 | > 100 |
| 3bc | 110 ± 1 | > 10,000 | 70 ± 7 | 1.6 | > 100 |
| 3cc | 10 ± 1 | 1410 ± 80 | 740 ± 40 | 0.01 | 2 |
| 3d | 1640 ± 90 | > 10,000 | 230 ± 20 | 7 | > 43 |
| 3e | 30 ± 2 | 1140 ± 60 | 550 ± 30 | 0.05 | 2 |
| 3f | 70 ± 4 | 1860 ± 140 | 540 ± 40 | 0.12 | 3 |
| 3g | 7550 ± 970 | > 10,000 | 900 ± 50 | 8 | > 11 |
| 3h | > 10,000 | > 10,000 | 800 ± 50 | > 12 | > 12 |
| 3i | 260 ± 210 | > 10,000 | 570 ± 40 | 13 | 0.45 |
| 3j | > 10,000 | > 10,000 | 410 ± 40 | > 24 | > 24 |
| 3k | 180 ± 20 | > 10,000 | 5490 ± 640 | 0.03 | > 2 |
| 3l | 10 ± 1 | 580 ± 30 | 70 ± 2 | 0.14 | 8 |
| 3mc | 10 ± 2 | 1380 ± 130 | 260 ± 20 | 0.04 | 5 |
| 3n | 10 ± 1 | 690 ± 30 | 80 ± 3 | 0.16 | 9 |
| 3o | 1030 ± 80 | > 10,000 | 2010 ± 110 | 0.5 | > 5 |
| 7a | 4180 ± 310 | > 10,000 | 30 ± 2 | 13 | > 330 |
| 7b | 3.1 ± 0.4 | 810 ± 30 | 7430 ± 880 | 0.0004 | 0.11 |
| 8a | > 10,000 | > 10,000 | 260 ± 30 | > 38 | > 38 |
| 8b | > 10,000 | > 10,000 | 1400 ± 110 | > 7 | > 7 |
| 9a | 4370 ± 310 | 3990 ± 290 | 5.7 ± 0.4 | 767 | 700 |
| 9b | 290 ± 70 | 1930 ± 70 | 1410 ± 80 | 0.21 | 1.4 |
The addition of a 2-methoxy group (3d) decreased affinity for μ receptors over 130-fold compared to 1c (Ki = 1640 vs. 12 nM). Introduction of a methoxy group in the 3-position of the benzene ring (3e) also decreased affinity for μORs and κORs compared to 1c (Ki = 30 vs. 12 nM and Ki = 550 vs. 90 nM, respectively). This modification, however, increased affinity 55-fold for μORs compared to 3d (Ki = 30 vs. 1640 nM) and improved selectivity for μORs over κORs compared to 1c (μ/κ = 0.05 vs. μ/κ = 0.13). The presence of 4-methoxy group (3f) leads to an approximately 6-fold decrease in affinity (Ki = 70 vs. 12 nM) and similar selectivity (μ/κ = 0.12 vs. μ/κ = 0.13) for μORs compared to 1c. This observation and our previous finding that 3c has equal affinity when compared to 1c,30 suggest that an electron-withdrawing group in the 4-position is more favorable for μOR affinity.
The introduction of a 2-nitro group (3g) decreased affinity for μORs over 600-fold compared to 1c (Ki = 7550 vs. 12 nM). This modification was better tolerated at κORs where only a 10-fold loss in affinity was observed (Ki = 900 vs. 90 nM). This result, coupled with those observed for 3a, 3d, 3g, would indicate that factors other than electronics are likely involved in the binding of 2-position analogues. Substitution of a 3-nitro group (3h) abolished affinity at μORs (Ki > 10,000) and decreased affinity approximately 10-fold at κORs compared to 1c (Ki = 800 vs. 90 nM). Finally, a 4-nitro group (3i) was also explored. This modification decreased affinity over 20-fold for μORs and over 6-fold for κORs compared to 1c (Ki = 260 vs. 12 nM and Ki = 570 vs. 90 nM, respectively). This result, coupled with those observed for 3c and 3f, would indicate that factors other than the strength of the electron withdrawing group are likely involved in the binding of 4-position analogues.
We then sought to further explore the size requirements for the aromatic substituent. First, we annulated an additional benzene ring onto the 2 and 3 positions (3j).31 This modification resulted in a roughly 1000-fold loss of affinity at μOR compared to 1c (Ki > 10,000 vs. 12 nM). This change, however, was better tolerated at κORs with roughly a 5-fold loss in affinity compared to 1c (Ki = 410 vs. 90 nM). This is interesting given the observation that replacement of the acetoxy group in 1 with an 1-naphthoate abolishes affinity for κORs (Ki > 10,000 nM).31 This difference is likely due to the different radioligands used ([3H]bremazocine vs. [125I]IOXY) or the possibility of misidentification since these compounds were not rigorously evaluated for purity.31 Annulation of the benzene ring into the 3 and 4 positions (3k) reduced affinity at μORs approximately 10-fold compared to 1c (Ki = 180 vs. 12 nM). This modification also decreased affinity for κORs greater than 50-fold (Ki = 5,490 vs. 90 nM). This suggests that a β,γ-annulated system increases selectivity for μORs over κORs. To probe this we prepared 2-benzofuran 3l as an analogue that possesses a β,γ-annulated system. Somewhat surprisingly, 3l had equal affinity at μORs compared to 1c (Ki = 10 vs. 12 nM). However, it retained selectivity for μORs over κORs. Previously, we showed that bioisosteric replacement of the benzene ring with a 2-thiophene (3m) retained affinity at μORs.30 We were curious if the point of attachment might play a role in its affinity. To probe this, we synthesized the corresponding 3-thiophene (3n). Compound 3n had similar affinity to 1c for μORs and κORs (Ki = 10 vs. 12 nM and Ki = 80 vs. 90 nM, respectively) indicating that the point of attachment on the thiophene ring does not play a role in μOR binding. This change, however, increases affinity 3-fold for κORs (Ki = 80 vs. 260 nM). Finally, we sought to further confirm the role of the aromatic moiety in the selectivity of 1c. To address this we prepared cyclohexyl analogue (3o). As expected, 3o had reduced affinity for μORs and κORs compared to 1c (Ki = 1030 vs. 12 nM and Ki = 2010 vs. 90 nM, respectively). This change also decreased selectivity for μORs over κ ORs (μ/κ = 0.5 vs. μ/κ = 0.13).
While our studies were in progress, several groups reported the effects of bioisosteric replacement of the 2-acetoxy group in 1a with an acetamido group (7a).32, 35 Consistent with those reports, we found this change resulted in a loss in affinity at κ receptors (Ki = 30 vs. 1.9 nM). However, 7a was found to have affinity for μORs (Ki = 4,180 nM). Given our previous finding that introduction of a benzene ring increases μ affinity,25 we synthesized benzamide 7b. As expected, introduction of the benzene ring resulted in a decreased affinity at κ receptors and increased affinity at μ receptors. To our delight, 7b has 4-fold higher affinity than 1c (Ki = 3.1 vs. 12 nM) and is more selective for μ receptors over κ receptors (κ/μ = 0.0004 vs. κ/μ = 0.13. To further explore these developments, we synthesized sulfonamides 8a and 8b.
Previously, we showed that the addition of a methanesulfonyl group retained high affinity and activity at κORs.25 The replacement the acetamido group with a methanesulfonylamino group (8a) decreased affinity approximately 9-fold for κORs compared to 7a (Ki = 260 vs. 30 nM). This change also abolished affinity for μORs (Ki > 10,000 nM). The addition of a benzene ring to 8a (8b) decreased affinity approximately 5-fold for κORs (Ki = 1,400 vs. 260 nM). The loss of affinity at κORs may be due to the increased ionizability of a sulfonamide compared to a sulfonyl ester. Recently, it has been shown that a tertiary amide has higher affinity for κORs than a secondary amide.35 This data would seem to confirm this observation, as well as, our previous finding that sulfonyl esters of 1a are not binding in an identical manner to alkyl esters.30
Finally, we probed the replacement of the 2-acetoxy group with a 2-acetylthio group. As seen previously,37 this change resulted in a slight reduction in affinity at κ receptors (Ki = 5.7 vs. 1.9 nM). However, 9a was found to have low affinity for μORs (Ki = 4,370 nM). The addition of a benzene ring to 9a (9b) lead to an increase in affinity at μORs (Ki = 290 vs. 4,370 nM). However, this change lead to a 24-fold decrease in affinity compared to 1c, indicating an ester or amide linkage is preferential for binding at μORs.
To test the hypothesis that μ opioids derived from 1a have functional activity at opioid receptors, several analogues were then evaluated in a [35S]GTP-γ–S assay (Table 2).39, 40 The introduction of a 4-bromo substituent (3c) resulted in an approximately 10-fold decrease in activity compared to 1c (EC50 = 4,890 vs. 500 nM). This modification also reduced the efficacy compared to 1c (Emax = 108 vs. 130) but 3c is just as efficacious as DAMGO (Emax = 108 vs. 100). The presence of a 3-methoxy group (3e) resulted in an approximately 3-fold loss in activity at μORs compared to 1c (EC50 = 1,670 vs. 500 nM). A similar effect was seen at κORs. Interestingly, 3e is not as efficacious as 1c (Emax = 72 vs. 130) and appears to be a partial agonist when compared to DAMGO (Emax = 72 vs. 100). A 4-methoxy group (3f) had similar activity compared to 1c (EC50 = 830 vs. 500 nM). However, 3f is not as efficacious at μORs as 1c (Emax = 94 vs. 130) but is approximately as efficacious as DAMGO (Emax = 94 vs. 100). A 4-nitro group (3i) decreased activity at μORs approximately 3-fold compared to 1c (EC50 = 1370 vs. 500 nM). This change however resulted in a large decrease in efficacy compared to 1c and DAMGO (Emax = 46 vs. 130 and Emax = 46 vs. 100).
Table 2.
Results from [35S]GTP-γ-S Functional Assay Carried Out in CHO Cells Containing DNA for Human μ and κ receptors.39, 40
| Compound | μEC50 ± SD, nM | μEmaxa ± SD | κEC50 ± SD, nM | κEmaxa ± SD |
|---|---|---|---|---|
| 1a | NTb | NTb | 40 ± 10 | 120 ± 2 |
| 1c | 500 ± 140 | 130 ± 4 | 1320 ± 150 | 140 ± 2 |
| 3c | 4890 ± 980 | 108 ± 8* | NTb | NTb |
| 3e | 1670 ± 250 | 72 ± 3* | 3590 ± 550 | 97 ± 2* |
| 3f | 830 ± 100 | 94 ± 3* | 2610 ± 470 | 106 ± 5* |
| 3i | 1370 ± 230 | 46 ± 2* | NTb | NTb |
| 3l | 1680 ± 250 | 104 ± 5* | 1120 ± 170 | 109 ± 5* |
| 3m | 1150 ± 250 | 95 ± 3* | NTb | NTb |
| 3n | 690 ± 60 | 108 ± 3* | 840 ± 210 | 95 ± 8* |
| 7a | NTb | NTb | 120 ± 20 | 108 ± 3* |
| 7b | 360 ± 60 | 134 ± 5 | NTb | NTb |
| (−)-U50,488 | 4840 ± 890 | 35 ± 2 | 30 ± 6 | 100 ± 4 |
| DAMGO | 40 ± 4 | 100 ± 4 | NTb | NTb |
Emax is % which compound stimulates binding compared to DAMGO (10 μM) at μ, and (−)-U50,488 (500 nM) at κ receptors respectively;
Not tested.
P<0.05 when compared to the Emax of 1c at μ and κ receptors (Student’s t test).
Substitution of the benzene ring in 1c with a 2-benzofuran (3l) resulted in an approximately 3-fold loss in activity and decreased efficacy at μORs compared to 1c (EC50 = 1680 vs. 500 nM and Emax = 104 vs. 130). However, 3l is still a full agonist when compared to DAMGO (Emax = 104 vs. 100). Benzofuran 3l had similar activity at κORs compared to 1c (EC50 = 1120 vs. 1320 nM). Strikingly, 3l was less efficacious as an agonist at κORs compared to 1a (Emax = 109 vs. 140) but more efficacious than U50,488H (Emax = 109 vs. 100). Bioisosteric replacement of the benzene ring in 1c with a 2-thiophene (3m) reduced activity and efficacy at μORs compared to 1c (EC50 = 1150 vs. 500 nM and Emax = 95 vs. 130). Substitution of a 3-thiophene (3n) had little effect on activity at μORs (EC50 = 690 nM vs. 500 nM) and decreased efficacy (Emax = 108 vs. 130). Compound 3n, however, is roughly as efficacious as DAMGO (Emax = 108 vs. 100) at μORs.
Replacement of the 2-acetoxy group in 1a with a 2-acetamido group (7a) resulted in a 3-fold loss in activity at κORs compared to 1a (EC50 = 120 vs. 40 nM). This change however had little effect on efficacy (Emax = 108 vs. 120). Replacement of the 2-benzoyloxy group in 1c with a 2-benzoylamino group (7b) resulted in a slight increase in activity and no change in efficacy (EC50 = 360 vs. 500 nM and Emax = 134 vs. 130).
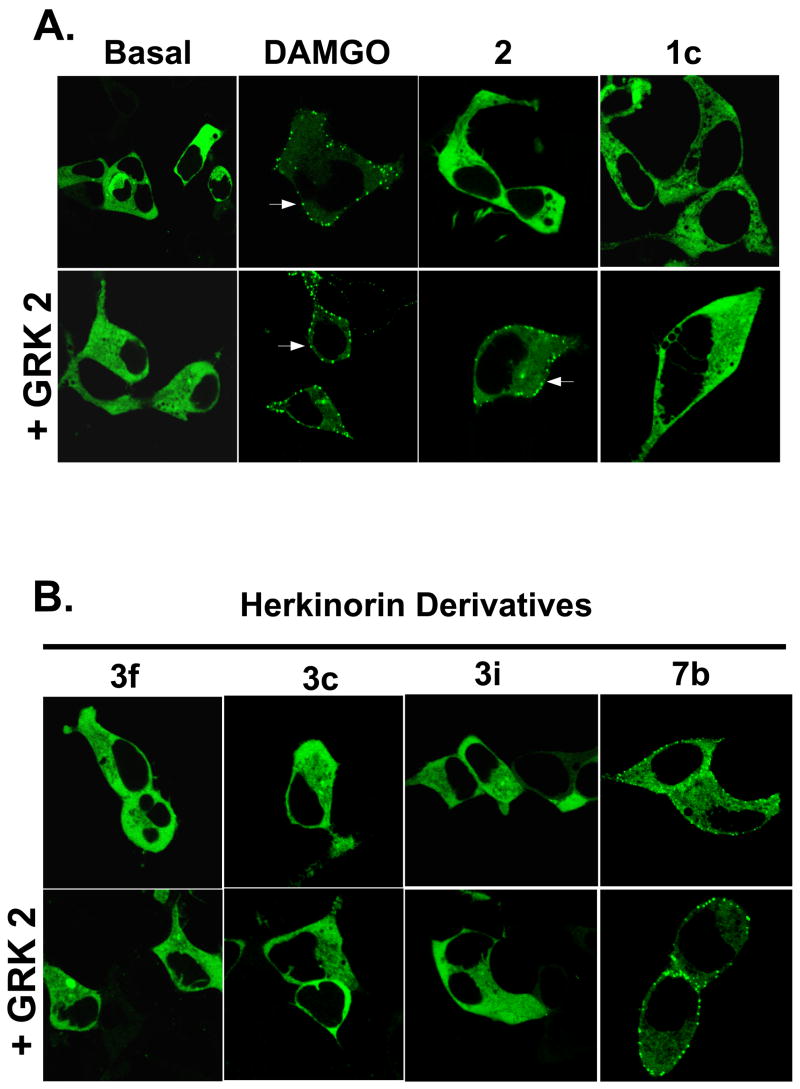
To better understand the role of drug structure on μOR regulation pathways, we examined the ability of 3c, 3f, 3i, and 7b to induce β-arrestin2-GFP translocation HEK-293 cells (Figure 2). The effects of DAMGO, morphine and 1c are shown for comparison.3, 28 DAMGO induces robust translocation of βarr2-GFP to the plasma membrane. Morphine, however, can only induce translocation when GRK2 is over-expressed. Compounds 3c, 3f, and 3i, like 1c, are unable to induce robust βarr2-GFP translocation to the plasma membrane even in the presence of GRK2 over-expression. Amide 7b induces robust βarr2-GFP translocation under both conditions.
Figure 2.
Agonist-induced β-arrestin2-GFP translocation. HEK-293 cells transfected with MOR1 and βarr2-GFP and with or without GRK2 over-expression were treated with the indicated drugs. Representative cells of at least 3 independent experiments are shown in which several cells were imaged. A. DAMGO induces robust translocation of βarr2-GFP (puncta; arrows) to the plasma membrane. Morphine, however, can only induce translocation when GRK2 is over-expressed. Ester 1c is unable to induce robust βarr2-GFP translocation to the plasma membrane even in the presence of GRK2 over-expression. B. Amide 7b is the only herkinorin derivative that induces βarr2-GFP translocation in the absence or presence of GRK2 over-expression.
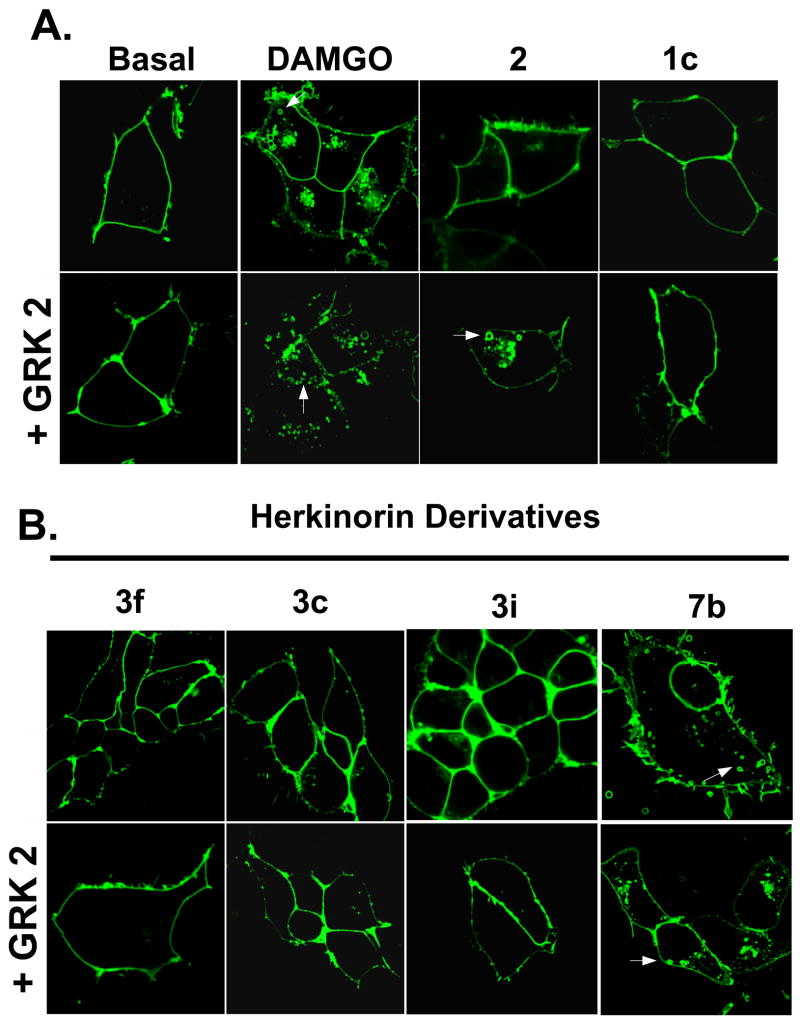
To further support the conclusion that μ opioids derived from 1a have altered receptor regulation, we examined the ability of 3c, 3f, 3i, and 7b to induce μOR-YFP internalization in HEK-293 cells (Figure 3). The effects of DAMGO, morphine and 1c are shown for comparison.3, 28 DAMGO induces robust internalization of μOR-YFP. Morphine, however, can only induce μOR-YFP internalization when GRK2 is over-expressed. Unlike DAMGO and morphine and similar to 1c, 3c, 3f, and 3i are unable to induce robust μOR-YFP internalization even in the presence of GRK2 over-expression. However, 7b induces robust μOR-YFP internalization in HEK-293 cells under both conditions.
Figure 3.
Agonist-induced MOR1-YFP internalization. HEK-293 cells stably transfected with MOR1-YFP were treated with the indicated drugs with or without GRK2 over-expression. Representative cells of at least 3 independent experiments are shown in which several cells were imaged. A. DAMGO induces robust internalization of MOR1-YFP (vesicles; arrows). Morphine, however, can only induce robust MOR1-YFP internalization when GRK2 is over-expressed. Ester 1c is unable to induce robust MOR1-YFP internalization even in the presence of GRK2 over-expression. B. Amide 7b is the only herkinorin derivative that can induce MOR1-YFP internalization, even in the in the presence of GRK2 over-expression.
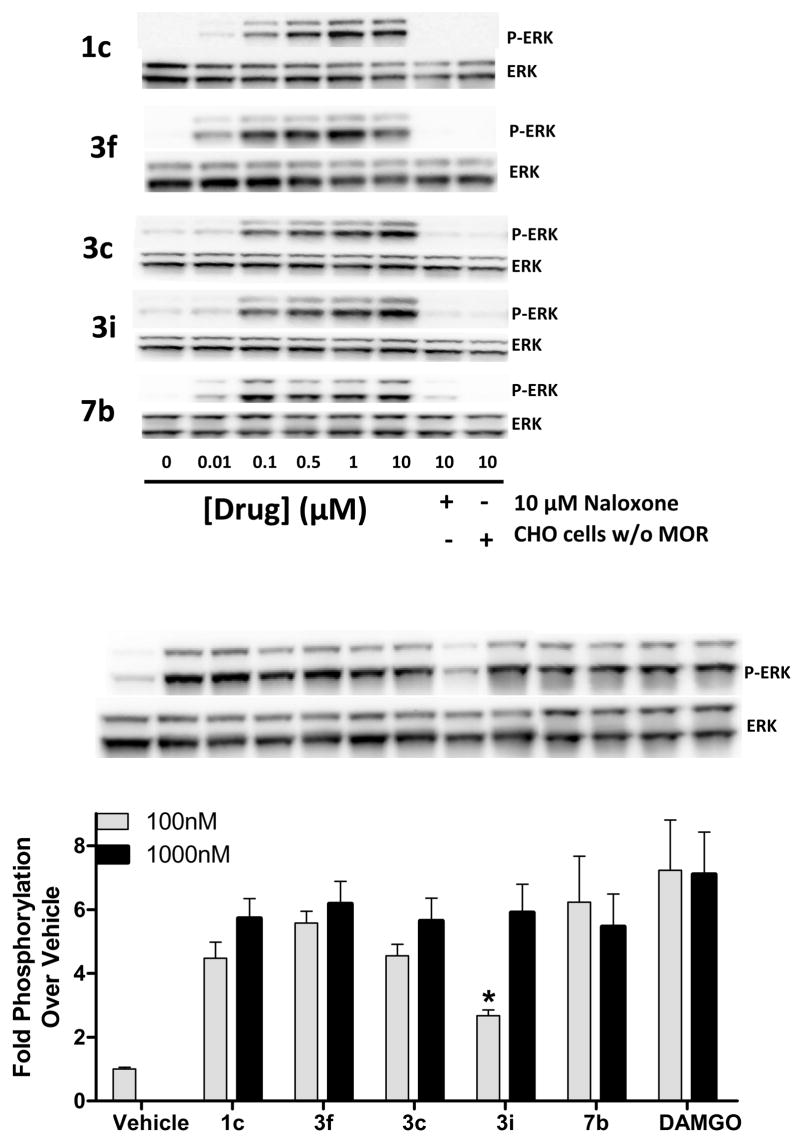
To further assess agonist activity in parallel with the current studies, we used the phosphorylation of the downstream MAP kinases (ERK1/ERK2) as an indicator of receptor activation. Compounds 3c, 3f, 3i, and 7b were examined for their ability to activate ERK in hμOR-CHO cells. In Figure 3, 3c, 3f, 3i, and 7b are similar to DAMGO in that they are able to induce a μOR1-mediated, dose-dependent increase in ERK phosphorylation that is blocked by naloxone.28
Taken together, these data indicate that 3c, 3f, and 3i are able to induce receptor conformations that are able to activate both G protein coupling and MAP Kinase activation pathways, yet have unique properties compared to the morphine or DAMGO bound μOR rendering the receptor resistant to βarrestin interactions or internalization. Amide 7b appears to induce receptor conformations that are different than other derivatives of 1c and produces effects similar to other opioids such as DAMGO.
Discussion
Our results indicate that the structure-activity relationships for affinity and activity at μ opioid receptors are not identical to those for receptor regulation. Addition of substituents to the aromatic ring of 1c results in agonists and partial agonists at μORs and similar receptor regulation to 1c. These changes do not affect the unique receptor regulation properties of 1c. Analogues 3c, 3f, 3i are unable to induce robust βarr2-GFP translocation and μOR-YFP internalization even in the presence of GRK2 over-expression in HEK-293 cells. Replacement of the ester linkage in 1c with an amide linkage (7b) increases affinity at μORs compared to 1c. Amide 7b has been identified as the most potent neoclerodane μ agonist described to date. However, this change promotes βarrestin translocation and receptor internalization in HEK-293 cells. The discovery of two compounds with nearly identical chemical structure and similar binding affinity and efficacies which elicit differential signaling at the cellular level would suggest that not only receptor conformation but also ligand structure contribute to signaling events. Future studies of the effect of chemical alterations of 1c on the activation of cellular pathways may serve as a basis for the development of compounds which can selectively activate or block βarrestin-receptor interactions may determine specific physiological responses.
The differences in affinity and receptor regulation between 1c and 7b are interesting. One potential explanation is that these two molecules, while very similar in structure are not binding in an identical manner at the μOR. This type of phenomenon has been seen previously with other opioids.41 Another explanation is that the benzene rings in 1c and 7b may have different orientations relative to the A ring of the salvinorin core. X-ray crystallographic studies26, 30 indicate that the benzene ring in 1c is out of the plane of the A ring of the salvinorin core. Preliminary molecular modeling indicates that the benzene ring in 7b is in the plane of the A ring. This orientation of 7b may be responsible for the increased affinity and activity at μORs compared to 1c. However, the out of the plane orientation of the benzene ring in 1c and esters 3c, 3f, 3i may be required for the lack of the βarrestin translocation and receptor internalization. Conformationally constrained analogues will need to be prepared to further delineate the role of the benzene ring on affinity, activity, and receptor regulation pathways.
An alternate explanation for the differences seen in affinity and receptor regulation is that ester 1c hydrolyzes too rapidly in serum to cause internalization and other chronic effects. Amide 7b would be expected to be more stable in serum, as recently shown for 7a.42 Additional stability studies of 1c and 7b will be necessary to further investigate the role of metabolism in the differences seen in receptor regulation pathways. However, 1b the likely metabolism product of ester 1c has no affinity for μORs (Ki > 10,000 nM)43 and after 30 minutes, 1c still produces a 3.5-fold increase in ERK phosphorylation demonstrating a persisting agonistic activity.28 Moreover, cells treated with DAMGO will internalize the μOR in approximately 10–15 minutes, therefore, the compound, which is still active at 30 minutes in the ERK activation assay, should be sufficiently potent to induce internalization. Furthermore, chronic treatment of 1c produces desensitization in cells suggesting that it is active long enough to induce some yet undescribed mode of receptor desensitization.44
The molecular basis for the unique signaling properties of 1c is not clear at this time. A likely explanation is that they are the result of a unique binding mode at the μOR relative to other opioids. Most nonpeptide opioid ligands, which contain a basic nitrogen atom, interact with aspartate 147 in TM III 45. Given the structure of 1c, this interaction is unlikely. This explanation is further supported by recent studies indicating that 1a utilizes unique residues in binding to κORs.22–24 Ester 1c and related analogues may have a similar mode of binding at μORs. The exact nature of the interaction of 1c with the μOR will have to be confirmed through site directed mutagenesis and/or affinity labeling experiments.
With regards to chemical structure, 1a and 1c have an interesting structural motif for GPCR ligands. The neoclerodane nucleus is not considered to be a privileged structure which is defined as a selected substructure that is able to provide high-affinity ligands for more than one type of receptor.46, 47 However, natural products, such as 1a, can be viewed as a population of privileged structures selected by evolutionary pressures to interact with a wide variety of proteins and other biological targets for specific purposes.48 Finding additional molecules that have unique receptor regulation pathways for GPCRs may require examining additional natural products or natural product-like libraries.49
The life cycle of a G-protein couple receptor (GPCR) is to reside at the cell surface and upon activation, become phosphorylated, desensitized, internalized, and then either degraded or recycled. While internalized, the GPCR may also take part in activating signaling cascades.50, 51 Usual drug discovery efforts for GPCRs are to develop agonists, antagonists, or inverse agonists for the GPCR of interest. In our case, this is the μ opioid receptor. Our results illustrate a novel drug discovery strategy that seeks to develop a series of compounds that retain signaling properties at a GPCR but avoid typical regulation pathways. This has a clear impact on the development of novel opioids with reduced side-effects and GPCR drug discovery, because this finding illustrates the ability of selecting or designing novel agents that differentially activate regulation pathways of a single receptor. This has the potential to optimize therapeutic action in vivo by alleviating unwanted side-effects.
Experimental Section
General Methods
Unless otherwise indicated, all reagents were purchased from commercial suppliers and are used without further purification. All melting points were determined on a Thomas–Hoover capillary melting apparatus and are uncorrected. The 1H NMR spectra were recorded at 300 MHz on a Bruker Avance-300 spectrometer using CDCl3 as solvent, δ values in ppm (TMS as internal standard), and J (Hz) assignments of 1H resonance coupling. Thin-layer chromatography (TLC) was performed on 0.25 mm plates Analtech GHLF silica gel plates using n-hexanes/EtOAc, 1:1 as the solvent system. Spots on TLC visualized with vanillin/H2SO4 in ethanol. Column chromatography was performed with Silica Gel (32–63 μ particle size) from Bodman Industries (Atlanta, GA). Analytical HPLC was carried out on an Agilent 1100 Series Capillary HPLC system with diode array detection at 254.8 nm on an Agilent Eclipse XDB-C18 column (4.6 × 150 mm, 5 μm) with isocratic elution in 70% CH3CN/30% H2O at a flow rate of 5.0 mL/min.
General Procedure A
A solution of 1b (1 equiv), appropriate acid chloride (1–3 equiv), NEt3 (3 equiv) and a catalytic amount of DMAP in CH2Cl2 was stirred at room temperature. Absolute MeOH was added and the solvent was removed under reduced pressure. CH2Cl2 was added to the residue and the solution was washed with 10% HCl (3 × 20 mL) and saturated NaCl (3 × 20 mL) and dried (Na2SO4). Removal of the solvent under reduced pressure afforded a crude solvent which was purified by column chromatography (eluent: n-hexanes/EtOAc) to yield the desired compound.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(4-bromobenzoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho-[2,1-c]pyran-7-carboxylic acid methyl ester (3c)
3c was synthesized from 1b using general procedure A and 4-bromobenzoyl chloride to afford 0.083 g (57%) as a white solid, mp 190–192°C; 1H NMR (CDCl3): d 1.17 (s, 3H), 1.46 (s, 3H), 1.65 (m, 3H), 1.83 (dd, J = 3.3, 9.9 Hz, 1H), 2.10 (dd, J = 2.7, 11.4 Hz, 1H), 2.17 (s, 1H), 2.20 (m, 1H), 2.50 (m, 3H), 2.83 (dd, J = 11.1, 11.7 Hz, 1H), 3.75 (s, 3H), 5.38 (dd, J = 9.9, 10.2 Hz, 1H), 5.52 (dd, J = 5.1, 11.7 Hz, 1H), 6.38 (dd, J = 0.9, 1.8 Hz, 1H), 7.41 (m, 2H), 7.61 (m, 2H), 7.94 (m, 2H).
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(2-Methoxybenzoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3d)
3d was synthesized from 1b using general procedure A and 2-anisoyl chloride to afford 0.010 g (14%) as a white solid, mp 105–107 °C; 1H NMR (300 MHz, CDCl3): δ 1.16 (s, 3H), 1.47 (s, 3H), 1.65 (m, 3H), 1.84 (m, 1H), 2.14 (m, 2H), 2.27 (s, 1H), 2.43 (m, 2H), 2.55 (dd, J = 5.1, 13.2 Hz, 1H), 2.83 (dd, J = 8.4, 8.7 Hz, 1H), 3.74 (s, 3H), 3.90 (s, 3H), 5.38 (dd, J = 9.9, 9.9 Hz, 1H), 5.52 (dd, J = 5.4, 11.7 Hz, 1H), 6.38 (s, 1H), 7.00 (dd, J = 7.5, 8.1 Hz, 2H), 7.40 (m, 2H), 7.51 (ddd, J = 1.8, 7.5, 8.1 Hz, 1H), 7.95 (d, J = 7.5 Hz, 1H); HRMS (m/z): [M+] calcd. for C29H32O9, 525.2125; found, 525.2117; HPLC tR = 4.43 min; Purity = 97.76%.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(3-Methoxybenzoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3e)
3e was synthesized from 1b using general procedure A and 3-anisoyl chloride to afford 0.017 g (26%) as a white solid, mp 200–202°C; 1H NMR (300 MHz, CDCl3): δ 1.17 (s, 3H), 1.46 (s, 3H), 1.65 (m, 3H), 1.82 (dd, J = 2.4, 9.9 Hz, 1H), 2.14 (m, 2H), 2.27 (s, 1H), 2.46 (m, 2H), 2.54 (dd, J = 5.4, 13.8 Hz, 1H), 2.84 (dd, J = 6.3, 10.5 Hz, 1H), 3.75 (s, 3H), 3.86 (s, 3H), 5.39 (dd, J = 9.6, 10.5 Hz, 1H), 5.51 (dd, J = 5.1, 11.7 Hz, 1H), 6.39 (d, J = 0.9 Hz, 1H), 7.13 (ddd, J = 0.9, 0.9, 7.1 Hz, 1H), 7.40 (m, 3H), 7.58 (dd, J = 1.5, 2.4 Hz, 1H), 7.69 (dt, J = 0.9, 0.9, 7.5 Hz, 1H); HRMS (m/z): [M+] calcd. for C29H32O9, 525.2125; found, 525.2140; HPLC tR = 5.14 min; Purity = 98.16%.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(4-Methoxybenzoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3f)
3f was synthesized from 1b using general procedure A and 4-anisoyl chloride to afford 0.083 g (60%) as a white solid, mp 185–187°C; 1H NMR (300 MHz, CDCl3): δ 1.17 (s, 3H), 1.46 (s, 3H), 1.65 (m, 3H), 1.83 (dd, J = 2.7, 11.7 Hz, 1H), 2.15 (m, 2H), 2.25 (s, 1H), 2.45 (m, 2H), 2.55 (dd, J = 5.1, 13.2 Hz, 1H), 2.83 (dd, J = 7.8, 8.7 Hz, 1H), 3.74 (s, 3H), 3.87 (s, 3H), 5.37 (dd, J = 9.6, 10.2 Hz, 1H), 5.52 (dd, J = 5.1, 11.7 Hz, 1H), 6.38 (dd, J = 0.9, 1.8 Hz, 1H), 6.93 (dt, J = 2.1, 3.0, 8.7 Hz, 2H), 7.39 (dd, J = 1.8, 1.8 Hz, 1H), 7.41 (dd, J = 0.9, 1.5 Hz, 1H), 8.04 (dt, J = 2.1, 3.0, 9.0 Hz, 2H); Anal. (C29H32O9): C, H.
2S,4aR,6aR,7R,9S,10aS,10bR)-9-(2-Nitrobenzoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3g)
3g was synthesized from 1b using general procedure A and 2-nitrobenzoyl chloride to afford 0.103 g (75%) as a white solid, mp 144–146°C; 1H NMR (300 MHz, CDCl3): δ 1.15 (s, 3H), 1.48 (s, 3H), 1.64 (m, 3H), 1.83 (dd, J = 2.7, 11.7 Hz, 1H), 2.15 (m, 2H), 2.27 (s, 1H), 2.40 (m, 2H), 2.55 (dd, J = 5.4, 12.3 Hz, 1H), 2.83 (dd, J = 3.6, 13.2 Hz, 1H), 3.75 (s, 3H), 5.42 (dd, J = 7.5, 12.6 Hz, 1H), 5.54 (dd, J = 5.1, 11.4 Hz, 1H), 6.41 (dd, J = 0.9, 1.8 Hz, 1H), 7.42 (dd, J = 1.5, 1.8 Hz, 1H), 7.45 (dd, J = 0.9, 1.5 Hz, 1H), 7.69 (td, J = 1.8, 7.8 Hz, 1H), 7.74 (td, J = 1.5, 7.5 Hz, 1H), 7.92 (dd, J = 1.8, 7.8 Hz, 1H), 8.00 (dd, J = 1.8, 7.5 Hz, 1H); Anal. (C28H29NO10•0.25H2O): C, H, N.
2S,4aR,6aR,7R,9S,10aS,10bR)-9-(3-Nitrobenzoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3h)
3h was synthesized from 1b using general procedure A and 3-nitrobenzoyl chloride to afford 0.110 g (80%) as a white solid, mp 148–150°C; 1H NMR (300 MHz, CDCl3): δ 1.19 (s, 3H), 1.46 (s, 3H), 1.64 (m, 3H), 1.85 (dd, J = 2.7, 9.9 Hz, 1H), 2.15 (m, 2H), 2.28 (s, 1H), 2.52 (m, 3H), 2.85 (dd, J = 5.1, 11.7 Hz, 1H), 3.76 (s, 3H), 5.43 (dd, J = 8.4, 11.7 Hz, 1H), 5.53 (dd, J = 5.1, 11.7 Hz, 1H), 6.39 (dd, J = 0.9, 1.8 Hz, 1H), 7.41 (m, 2H), 7.69 (t, J = 8.1, 8.1 Hz, 1H), 8.41 (dt, J = 1.5, 1.5, 7.5 Hz, 1H), 8.46 (ddd, J = 0.9, 2.4, 8.1 Hz, 1H), 8.91 (t, J = 2.1, 2.1 Hz, 1H); Anal. (C28H29NO10•0.5H2O) C, H, N.
2S,4aR,6aR,7R,9S,10aS,10bR)-9-(4-Nitrobenzoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3i)
3i was synthesized from 1b using general procedure A and 4-nitrobenzoyl chloride to afford 0.093 g (67%) as a white solid, mp 195–200°C; 1H NMR (300 MHz, CDCl3): δ 1.18 (s, 3H), 1.46 (s, 3H), 1.66 (m, 3H), 1.84 (dd, J = 3.0, 9.9 Hz, 1H), 2.15 (m, 2H), 2.27 (s, 1H), 2.51 (m, 3H), 2.85 (dd, J = 6.9, 15.9 Hz, 1H), 3.76 (s, 3H), 5.42 (dd, J = 9.3, 10.8 Hz, 1H), 5.53 (dd, J = 5.1, 11.7 Hz, 1H), 6.39 (dd, J = 0.9, 1.8 Hz, 1H), 7.40 (dd, J = 1.8, 1.8 Hz, 1H), 7.42 (dd, J = 0.9, 1.8 Hz, 1H), 8.25 (dt, J = 1.8, 2.1, 9.3 Hz, 2H), 8.31 (dt, J = 1.8, 2.1, 9.0 Hz, 2H); Anal. (C28H29NO10) C, H, N.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(1-Naphthoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3j)
3j was synthesized 1b using general procedure A and 1-naphthoyl chloride to afford 0.044 g (63%) of 103 as a white solid, mp 155–160°C. 1H NMR (CDCl3): d 1.19 (s, 3H); 1.49 (s, 3H); 1.66 (m, 3H); 1.84 (dd, J = 2.7, 9.9 Hz, 1H); 2.15 (m, 2H); 2.30 (s, 1H); 2.55 (m, 3H); 2.87 (dd, J = 8.1, 8.4 Hz, 1H); 3.75 (s, 3H); 5.53 (m, 2H); 6.40 (dd, J = 0.9, 1.8 Hz, 1H); 7.40 (dd, J = 1.8, 1.8 Hz, 1H); 7.43 (d, J = 0.9 Hz, 1H); 7.56 (m, 3H); 7.89 (dd, J = 1.2, 7.8 Hz, 1H); 8.05 (d, J = 8.1 Hz, 1H); 8.32 (dd, J = 1.5, 7.5 Hz, 1H); 8.86 (d, J = 8.7 Hz, 1H).
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(2-Naphthoyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3k)
3k was synthesized from 1b using general procedure A and 2-naphthoyl chloride to afford 0.020 g (29%) as a white solid, mp 155–160°C; 1H NMR (300 MHz, CDCl3): δ 1.19 (s, 3H), 1.48 (s, 3H), 1.67 (m, 3H), 1.85 (dd, J = 2.7, 9.9 Hz, 1H), 2.15 (m, 2H), 2.29 (s, 1H), 2.56 (m, 3H), 2.86 (dd, J = 6.0, 10.8 Hz, 1H), 3.76 (s, 3H), 5.44 (m, 1H), 5.55 (dd, J = 5.4, 12.0 Hz, 1H), 6.39 (dd, J = 0.9, 1.8 Hz, 1H), 7.40 (dd, J = 1.8, 3.0 Hz, 1H), 7.42 (dd, J = 0.9, 1.8 Hz, 1H), 7.59 (m, 2H), 7.90 (m, 2H), 7.97 (d, J = 8.1 Hz, 1H), 8.08 (dd, J = 1.8, 10.2 Hz, 1H), 8.67 (m, 1H); HRMS (m/z): [M]+ calcd for C32H32O8Cs, 677.1152; found, 677.1150. HPLC tR = 7.38 min; Purity = 98.22%.
Benzofuran-2-carboxylic acid (2S,4aR,6aR,7R,9S,10aS,10bR)-7-carbomethoxy-2-(3-furanyl)dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-9-yl ester (3l)
A solution of 1b (0.10 g, 0.26 mmol), benzofuran 2-carboxylic acid (0.08 mg, 0.51 mmol), HOBT (0.07 g, 0.51 mmol), and EDCI (0.120 g, 0.64 mmol) in CH2Cl2 (20 mL) was stirred at room temperature for 4 d. The mixture was washed with 2N HCl (3 × 15 mL), saturated NaHCO3 (3 × 15 mL) and H2O (3 × 15 mL) and dried (Na2SO4). Removal of the solvent under reduced pressure afforded a crude product that was purified by column chromatography (eluent: n-hexanes/EtOAc, 1:1) to afford 0.05 g of 1b and 0.016 g (22%) of 3l as a white solid, mp 226–227 °C; 1H NMR (300 MHz, CDCl3): δ 1.19 (s, 3H), 1.48 (s, 3H), 1.64 (m, 3H), 1.84 (dd, J = 2.8, 10.5 Hz, 1H), 2.19 (m, 2H), 2.28 (s, 1H), 2.60 (m, 3H), 2.86 (1H, dd, J = 5.25, 10.5 Hz, 1H), 3.77 (s, 3H), 5.48 (dd, J = 9.3, 10.8 Hz, 1H), 5.57 (dd, J = 5.1, 11.7 Hz, 1H), 6.40 (dd, J = 0.9, 1.2 Hz, 1H), 7.36 (s, 1H), 7.43 (m, 2H), 7.52 (m, 1H), 7.66 (m, 2H), 7.73 (dd, J = 0.6, 7.8 Hz, 1H); Anal. (C30H30O9): C, H.
Thiophene-3-carboxylic acid (2S,4aR,6aR,7R,9S,10aS,10bR)-7-carbomethoxy-2-(3-furanyl)dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-9-yl ester (3m)
3m was synthesized from 1b using general procedure A and 3-thiophenoyl chloride to afford 0.056 g (44 %) as a white solid, mp 211–212°C; 1H NMR (300 MHz, CDCl3): δ 1.18 (s, 3H), 1.47 (s, 3H), 1.69 (m, 3H), 1.82 (dd, J = 2.7, 10.0 Hz, 1H), 2.18 (m, 3H), 2.27 (s, 1H), 2.42 (m, 2H), 2.52 (dd, J = 5.1, 13.2 Hz, 1H), 2.84 (dd, J =7.5, 12.6 Hz, 1H), 3.76 (s, 3H), 5.39 (m, 1H), 5.56 (dd, J = 5.1, 11.7 Hz, 1H), 7.36 (dd, J = 3.0, 5.1 Hz, 1H), 7.42 (m, 2H), 7.57 (dd, J = 1.0, 4.6 Hz, 1H), 8.22 (dd, J = 0.6, 2.7 Hz, 1H); Analysis (C26H2808S): C, H.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(1-Cyclohexanecarbonyloxy)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3o)
3o was synthesized from 1b using general procedure A and cyclohexane carbonyl chloride to afford 0.091 g (71%) as a white solid, mp 104–107°C; 1H NMR (300 MHz, CDCl3): δ 1.12 (s, 3H), 1.28 (m, 4H), 1.51 (m, 2H), 1.60 (m, 4H), 1.79 (m, 3H), 1.94 (m, 1H), 2.02 (m, 1H), 2.08 (m, 1H), 2.16 (m, 1H), 2.19 (s, 1H), 2.29 (dd, J = 8.7, 9.8 Hz, 2H), 2.42 (tt, J = 3.6, 11.3 Hz, 1H), 2.51 (dd, J = 5.1, 13.5 Hz, 1H), 2.76 (dd, J = 7.5, 9.3 Hz, 1H), 3.73 (s, 3H), 5.14 (dd, J = 9.8, 10.4 Hz, 1H), 5.52 (dd, J = 5.3, 11.6 Hz, 1H), 6.38 (dd, J = 0.8, 1.7 Hz, 1H), 7.32 (dd, J = 1.4, 1.4 Hz, 1H), 7.42 (m, 1H); Anal. (C28H36O8•0.5H2O): C, H.
(2S,4aR,6aR,7R,9R,10aS,10bR)-9-(Bromo)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (4a) and (2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Bromo)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (4b)
A mixture of salvinorin B (1b)29 (0.15 g, 0.38 mmol), triphenylphosphine (0.21 g, 0.80 mmol), and carbon tetrabromide (0.15 g, 0.45 mmol) in CH2Cl2 (30 mL) was stirred at room temperature overnight. TLC indicated that starting material was still present after 16 h, thus additional triphenylphosphine (0.11 g, 0.42 mmol) and carbon tetrabromide (0.07 g, 0.21 mmol) were added and the mixture was stirred for an additional 3 h. The solvent was removed under reduced pressure affording a crude residue. The residue was purified by column chromatography (eluent: 30% EtOAc/n-hexanes) to afford 0.10 g (59%) of 4a as a white solid, mp 170–173 °C (Lit.32 156–158 °C); 1H NMR (300 MHz, CDCl3): δ 1.15 (s, 3H), 1.48 (s, 3H), 1.60 (m, 3H), 1.81 (dd, J = 2.7, 9.9 Hz, 1H), 1.95 (dd, J = 13.2, 26.1 Hz, 1H), 2.1 (m, 2H), 2.27 (s, 1H), 2.47 (dd, J = 4.8, 13.2 Hz, 1H), 2.66 (m, 1H), 2.80 (dd, J = 3.3, 13.2 Hz, 1H), 3.70 (s, 3H), 3.89 (d, J = 2.4 Hz, 2H), 4.45 (m, 1H), 5.55 (dd, J = 4.8, 11.7 Hz, 1H), 6.38 (dd, J = 0.9, 1.5 Hz, 1H), 7.4 (m, 2H).
A more polar spot was isolated to afford 0.02 g (14%) of 4b as an oil; 1H NMR (300 MHz, CDCl3): δ 1.14 (s, 3H), 1.48 (s, 3H), 1.52 – 1.73 (m, 4H), 1.80 (dd, J = 3.0, 9.6 Hz, 1H), 2.08 (dd, J = 3.0, 11.4 Hz, 1H), 2.18 (m, 1H), 2.24 (s, 1H), 2.57 (dd, J = 5.1, 13.2 Hz, 1H), 2.63 – 2.70 (m, 2H), 2.76 (dd, J = 3.3, 12.9 Hz, 1H), 3.73 (s, 3H), 4.60 (dd, J = 7.8, 12.3 Hz, 1H), 5.56 (dd, J = 5.1, 11.7 Hz, 1H), 6.38 (dd, J = 0.9, 0.9 Hz, 1H), 7.41 (m, 1H).
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Azido)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (5)
A solution of 4a (0.10 g, 0.22 mmol), sodium azide (0.05 g, 0.77 mmol) and glacial acetic acid in DMF (3 mL) was stirred at room temperature for 4 h. H2O (30 mL) was added and the mixture was extracted with EtOAc (20 mL). The EtOAc solution was washed with H2O (2 × 20 mL) and saturated NaCl (20 mL) and dried (Na2SO4). Removal of the solvent under reduced pressure afforded a crude solid. The crude solid was purified by column chromatography (eluent: 30% EtOAc/n-hexanes) to afford 0.08 g (86%) of 5 as a white solid, mp 200–203 °C (Lit.32 179–181 °C) (EtOAc/n-hexanes). 1H NMR spectra was in agreement with that previously reported.32
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Amino)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (6)
A mixture of 5 (0.21 g, 0.50 mmol), Zn dust (0.33 g, 5.0 mmol) and NH4Cl (0.27 g, 5.0 mmol) in a mixture of CH2Cl2/MeOH (1:4, 10 mL) was stirred at room temperature for 3 h. The mixture was filtered and the filtrate was concentrated to dryness under reduced pressure. 2N NaOH (30 mL) was added to the residue and the mixture was extracted with CH2Cl2 (2 × 20 mL). The combined CH2Cl2 portion was washed with H2O (30 mL) and dried (Na2SO4). Removal of the solvent under reduced pressure afforded 0.07 g (36%) of 6 as an orange solid, mp 237–240 °C (EtOAc/n-hexanes). The 1H NMR spectra was in agreement with that previously reported.35
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Acetylamino)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (7a)
7a was prepared from 6 using a method similar to that previously described35 to afford 0.04 g (58%) as a white solid, mp 222–224 °C (Lit.32 137–138 °C)(EtOAc/n-hexanes). The 1H NMR spectra was in agreement with that previously reported.35 Anal. (C23H29NO7): C, H, N.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Benzoylamino)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (7b)
A solution of 6 (0.10 g, 0.26 mmol), benzoyl chloride (0.11 g, 0.78 mmol) and DMAP (0.08 g, 0.78 mmol) in CH2Cl2 (20 mL) was stirred at room temperature for 2 h. Absolute MeOH (15 mL) was added and the solvent was removed under reduced pressure. CH2Cl2 (25 mL) was added to the residue and the solution was washed with 10% HCl (2 × 20 mL), H2O (3 × 20 mL), and saturated NaCl (3 × 20 mL) and dried (Na2SO4). Removal of the solvent under reduced pressure afforded 0.09 g (67%) of 7b as a white crystalline solid, mp 155–157 °C (EtOAc/n-hexanes); 1H NMR (300 MHz, CDCl3): δ 1.44 (s, 3H), 1.50 (s, 3H), 1.63 (m, 3H), 1.82 (dd, J = 2.1, 10.5 Hz, 1H), 2.0 (m, 1H), 2.12 (dd, J = 2.7, 8.4 Hz, 1H), 2.17 (m, 1H), 2.32 (s, 1H), 2.48 (dd, J = 5.4, 13.2 Hz, 1H), 2.79 (dd, J = 3.3, 6.9 Hz, 1H), 2.87 (dd, J = 2.7, 13.5 Hz, 1H), 3.71 (s, 3H), 4.69 (m, 1H), 5.55 (dd, J = 5.1, 11.4 Hz, 1H), 6.37 (dd, J = 0.9, 1.8 Hz, 1H), 7.1 (d, J = 6.0 Hz, 1H), 7.39 (t, J = 1.8 Hz, 1H), 7.41 (dd, J = 0.9, 1.8 Hz, 1H), 7.46 (m, 1H), 7.53 (tt, J = 1.5, 2.7, 7.2 Hz, 1H), 7.80 (t, J = 2.4 Hz, 1H), 7.82 (t, J = 1.2 Hz, 1H); Anal. (C28H31NO7•0.5H2O): C, H, N.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Methanesulfonylamino)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (8a)
A solution of 6 (0.10 g, 0.26 mmol), methanesulfonyl chloride (0.08 mL, 1.03 mmol), NEt3 (0.04 mL, 0.28 mmol) and a catalytic amount of DMAP in CH2Cl2 (50 mL) was stirred at room temperature for 2 h. The mixture was washed with 2N HCl (30 mL), 2N NaOH (30 mL), and H2O (30 mL) and dried (Na2SO4). Removal of the solvent under reduced pressure afforded a crude solid. The crude solid was purified by column chromatography (eluent: 2% MeOH/CH2Cl2) to afford 0.7 g (56%) of 8a as a white crystalline solid, mp 262–265 °C (EtOAc/n-hexanes); 1H NMR (300 MHz, CDCl3): δ 1.09 (s, 3H), 1.46 (s, 3H), 1.60 (m, 3H), 1.79 (dd, J = 2.7, 9.6 Hz, 1H), 2.07 (m, 2H), 2.18 (m, 1H), 2.21 (s, 1H), 2.50 (m, 2H), 2.75 (dd, J = 3.6, 13.2 Hz, 1H), 2.99 (s, 3H), 3.72 (s, 3H), 4.15 (m, 1H), 5.34 (d, J = 5.4 Hz, 1H), 5.55 (dd, J = 5.1, 11.4 Hz, 1H), 6.38 (dd, J = 0.9, 1.2 Hz, 1H), 7.41 (dd, J = 1.5, 1.8 Hz, 1H), 7.43 (dd, J = 0.9, 1.5 Hz, 1H); Analysis (C22H29NO8S): C, H, N.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Benzenesulfonylamino)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (8b)
A solution of 6 (0.08 g, 0.21 mmol), benzenesulfonyl chloride (0.07 g, 0.42 mmol), triethylamine (0.06 g, 0.63 mmol), and a catalytic amount of DMAP in CH2Cl2 (40 mL) was stirred at room temperature for 18 h. Absolute MeOH was then added and the solution was washed with 10% HCl (3 × 25 mL) and saturated NaCl (2 × 25 mL), dried (Na2SO4), filtered, and concentrated under reduced pressure to yield a crude solid. The solid was purified by flash column chromatography (eluent: n-hexanes/EtOAc, 1:1). Removal of the solvent under reduced pressure gave 0.11 g (97%) of 8b as a white solid, mp 271–273 °C (EtOAc/n-hexanes): 1H NMR (300 MHz, acetone-d6): d 0.98 (s, 3H), 1.29 (s, 3H), 1.52 (m, 2H), 1.65 (m, 1H), 1.70 (ddd, J = 3.0, 3.0, 12.6 Hz, 1H), 1.82 (ddd, J = 1.8, 5.1, 13.5 Hz, 1H), 1.95 (ddd, J = 6.3, 6.3, 10.2 Hz, 1H), 2.09 (d, J = 13.2 Hz, 1H), 2.22 (dd, J = 2.7, 11.7 Hz, 1H), 2.29 (ddd, J = 3.3, 6.9, 13.5 Hz, 1H), 2.62 (s, 1H), 2.96 (dd, J = 3.5, 13.4 Hz, 1H), 3.66 (s, 3H), 4.19 (m, 1H), 5.47 (dd, J = 5.4, 12.0 Hz, 1H), 6.53 (dd, J = 0.9, 1.5 Hz, 1H), 6.69 (d, J = 8.4 Hz, 1H), 7.38 (m, 1H), 7.40 (d, J = 6.9 Hz, 1H), 7.44 (dd, J = 2.1, 3.0 Hz, 1H), 7.60 (m, 2H), 7.80 (m, 2H); 13C NMR (acetone-d6): d 15.7, 16.8, 19.4, 35.5, 36.4, 39.1, 43.4, 44.3, 51.5, 52.2, 54.8, 61.2, 64.7, 72.4, 110.0, 127.5, 128.2, 130.1, 133.5, 141.2, 142.4, 145.1, 171.7, 173.0, 205.3; Analysis (C27H31NO8S•H2O): C, H, N.
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(Benzoylthio)-2-(3-furanyl)-dodecahydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (9b)
A solution of 4 (0.10 g, 0.22 mmol), the potassium salt of thiobenzoic acid (0.194 g, 1.10 mmol) were stirred in acetonitrile at room temperature for 3 hours. Solvent was removed under reduced pressure and it was then redissolved in DCM (30 mL). The DCM solution was washed with H2O (3 × 30 mL) and saturated NaCl (2 × 30 mL) and dried (Na2SO4). Removal of solvent under reduced pressure affored 0.74 g (66%) of 9b as a white solid, mp 212–215 °C. 1H NMR (300 MHz, CDCl3): δ 1.16 (s, 3H), 1.47 (s, 3H), 1.63 (m, 3H), 1.80 (m, 1H), 2.12 (dd, 1H, J = 1.8, 11.1 Hz), 2.17 (m, 1H), 2.36 (m, 1H), 2.39 (s, 1H), 2.49 (m, 1H), 2.57 (dd, 1H, J = 5.1 13.5 Hz), 2.90 (dd, 1H, J = 3.3, 12.9 Hz), 3.72 (s, 3H), 4.52 (dd, J = 6.9, 13.2 Hz, 1H), 5.54 (dd, J = 5.1, 11.4 Hz, 1H), 6.39 (d, J = 0.9 Hz, 1H), 7.39 (dd, J = 1.5, 1.8 Hz, 1H), 7.42 (m, 1H), 7.46 (m, 2H), 7.60 (m, 1H), 7.96 (m, 2H); HRMS (m/z): [M+] calcd. for C28H30O7S, 511.1791; found, 511.1781. HPLC tR = 6.34 min; Purity = 98.94%.
In vitro Pharmacology
Cell culture, [35S]GTP-γ-S binding assay, and [125I]IOXY binding assays proceeded as described elsewhere.43, 52, 53 Recombinant CHO cells (hMOR-CHO, hDOR-CHO, and hKOR-CHO) were produced by stable transfection with the respective human opioid receptor cDNA and provided by Dr. Larry Toll (SRI International, CA).
β-Arrestin2 Translocation
HEK-293 cells stably expressing the mu opioid receptor (~1000 fmol/mg membrane protein) were transiently transfected with 2μg of β-arrestin2 tagged on the C-terminus with Green Fluorescent Protein (βarr2-GFP) and 1.5 μg G-protein receptor kinase 2 (GRK2). Experiments were also done in HEK-293 cells transiently transfected with MOR1. After incubation at 37 °C for 24 to 36 hours, cells were serum-starved for 30 minutes. Basal βarr2-GFP images were obtained, followed by drug treatment for 10min. Drugs included DAMGO (1 μM), morphine (10 μM), and 1c and its derivatives (10 μM). Cells were monitored each minute throughout the 10 minute drug treatment. Representative cells at 5 minutes are shown. Images were taken using an Olympus Fluoview 300 confocal microscope and Olympus Fluoview imaging software version 4.3.
MOR-YFP Internalization
HEK-293 cells stably expressing MOR1 tagged with Yellow Fluorescent Protein at the C-terminus (MOR1-YFP) were transiently transfected with GRK2. After incubation at 37°C for 24 to 36 hours, cells were serum-starved for 30 minutes. Basal MOR1-YFP images were obtained, followed by drug treatment for 2 hours. Drugs included DAMGO (1 μM), morphine (10 μM), and 1c and its derivatives (10 μM). Cells were monitored every 15 minutes throughout the 2 hour drug treatment. In some experiments, cells were left to incubate at 37°C during the hour treatment time and this did not result in different internalization profiles. Representative cells at 60 minutes are shown. Images were taken using the Olympus 300 confocal microscope and Olympus Fluoview imaging software version 4.3.
ERK Activation
CHO cells stably expressing the human MOR1 (~800 fmol/mg membrane protein) were serum-starved for 30 minutes at 37°C. Cells were treated with 1c or derivative for 10 minutes. Where indicated, naloxone was included during serum-starvation and drug treatment. After washing with PBS on ice, cells were collected in lysis buffer (20 mM Tris HCl pH8.0, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% NP-40, 0.25% dioxycholate, 1 mM sodium orthovanedate, 1 mM PMSF, 1 mM NaF, and protease inhibitor cocktail (Roche)) and centrifuged at 20,000 × g for 30 minutes. Supernatants were quantified using Bio-Rad Dc Protein Assay and diluted to equal concentrations with 4 ×XT Sample Buffer (Bio-Rad) (62.5 mM Tris-HCl, pH 6.8, 25% glycerol, 2% SDS, 0.01% Bromophenol Blue) with 5% β-mercaptoethanol and boiled at 95°C for 3 minutes. Samples were subjected to SDS-PAGE and transferred to PVDF membranes. Blots were first probed with an antibody specific to total ERK1/2 (Cell Signaling; 1:1000). Blots were stripped and re-blotted for Phospho-ERK (Tyr204) (Santa Cruz; 1:2000). Bands were detected using secondary antibodies (Amersham) (anti-rabbit IgG 1:2000 and anti-mouse IgG 1:5000, respectively) conjugated to horseradish peroxidase and Supersignal West Pico Chemiluminescent Substrate (Pierce). Densitometric analysis was performed on Kodak 1D Imaging Software. Phospho-ERK bands were normalized to corresponding total ERK bands. Statistical analysis was performed using GraphPad Prism software.
Statistics
Statistical analyses were performed using Prism software (GraphPad Software), and the specific tests used are presented in the figure legends.
Figure 4.
Herkinorin and its four derivatives induce dose-dependent, MOR1-mediated ERK Activation. CHO cells stably expressing the human MOR1 were treated with the indicated drugs for 10 minutes. Top: Representative concentration-response data of 1c, 3c, 3f, 3i, and 7b are shown. Experiments were performed at least three times in triplicate. Bottom: Densitometric analysis of two experiments done in triplicate compare efficacy of 1c, 3c, 3f, 3i, and 7b. Bar graph shows means and S.E.M. for the densitometric analysis (Students t-test p<0.0001 vs vehicle for all treatments; p<0.05 vs 3i (100 nM) for all other treatments) Representative immunoblots of a single experiment are shown.
Scheme 2.
aReagents and conditions: (a) CBr4, PPh3, CH2Cl2; (b) NaN3, DMF, AcOH; (c) Zn, NH4Cl, MeOH, CH2Cl2; (d) Appropriate acid chloride or anhydride, DMAP, NEt3, CH2Cl2; (e) Appropriate sulfonyl chloride, DMAP, CH2Cl2; (f) RCOSK, CH3CN
Acknowledgments
This work described was supported by Grant Numbers K01DA14600 (to LMB), R01DA18860 (to LMB), R01DA018151 (to TEP), and R01DA018151S1 (to TEP) from the National Institute on Drug Abuse. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. The authors also thank support in part from the Intramural Research Program of the NIH, NIDA and acknowledge the expert technical assistance of Mario Ayestas, IRP, NIDA.
Footnotes
Supporting Information Available. HPLC analysis of compounds 3d, 3e, 3k, and 9b. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional Selectivity, Classical and Concepts of Quantitative Pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 2.Zastrow Mv, Svingos A, Haberstock-Debic H, Evans C. Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol. 2003;13:348–353. doi: 10.1016/s0959-4388(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 3.Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- 4.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin F. T Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 5.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 6.Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raehal KM, Walker JKL, Bohn LM. Morphine Side Effects in β-Arrestin 2 Knockout Mice. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 8.Kohout TA, Lefkowitz RJ. Regulation of G Protein-Coupled Receptor Kinases and Arrestins During Receptor Desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Z. The role of opioid receptor internalization and beta-arrestins in the development of opioid tolerance. Anesth Analg. 2005;101:728–734. doi: 10.1213/01.ANE.0000160588.32007.AD. [DOI] [PubMed] [Google Scholar]
- 10.Ortega A, Blount JF, Manchand PS. Salvinorin, a New Trans-Neoclerodane Diterpene from Salvia-Divinorum (Labiatae) J Chem Soc Perkin Trans. 1982;1:2505–2508. [Google Scholar]
- 11.Valdes LJ, III, Butler WM, Hatfield GM, Paul AG, Koreeda M. Divinorin A, a Psychotropic Terpenoid, and Divinorin B from the Hallucinogenic Mexican Mint Salvia divinorum. J Org Chem. 1984;49:4716–4720. [Google Scholar]
- 12.Valdes LJ, III, Diaz JL, Paul AG. Ethnopharmacology of Ska-Maria-Pastora (Salvia, Divinorum, Epling and Jativa-M) J Ethnopharmacol. 1983;7:287–312. doi: 10.1016/0378-8741(83)90004-1. [DOI] [PubMed] [Google Scholar]
- 13.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: A Potent Naturally Occurring Nonnitrogenous Kappa Opioid Selective Agonist. Proc Natl Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butelman ER, Harris TJ, Kreek MJ. The plant-derived hallucinogen, salvinorin A, produces kappa-opioid agonist-like discriminative effects in rhesus monkeys. Psychopharmacology. 2004;172:220–224. doi: 10.1007/s00213-003-1638-0. [DOI] [PubMed] [Google Scholar]
- 15.Butelman ER, Mandau M, Tidgewell K, Prisinzano TE, Yuferov V, Kreek MJ. Effects of Salvinorin A, a κ-Opioid Hallucinogen, on a Neuroendocrine Biomarker Assay in Nonhuman Primates with High κ-Receptor Homology to Humans. J Pharmacol Exp Ther. 2007;320:300–306. doi: 10.1124/jpet.106.112417. [DOI] [PubMed] [Google Scholar]
- 16.Carlezon WA, Jr, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-Like Effects of the κ-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 17.McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol Biochem Behav. 2006;83:109–113. doi: 10.1016/j.pbb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Fantegrossi WE, Kugle KM, Valdes LJ, 3rd, Koreeda M, Woods JH. Kappa-opioid receptor-mediated effects of the plant-derived hallucinogen, salvinorin A, on inverted screen performance in the mouse. Behav Pharmacol. 2005;16:627–633. doi: 10.1097/00008877-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 19.John TF, French LG, Erlichman JS. The antinociceptive effect of Salvinorin A in mice. Eur J Pharmacol. 2006;545:129–133. doi: 10.1016/j.ejphar.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 20.Ansonoff MA, Zhang J, Czyzyk T, Rothman RB, Stewart J, Xu H, Zjwiony J, Siebert DJ, Yang F, Roth BL, Pintar JE. Antinociceptive and Hypothermic Effects of Salvinorin A Are Abolished in a Novel Strain of κ-Opioid Receptor-1 Knockout Mice. J Pharmacol Exp Ther. 2006;318:641–648. doi: 10.1124/jpet.106.101998. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of Pharmacological Activities of Three Distinct κ Ligands (Salvinorin A, TRK-820 and 3FLB) on κ Opioid Receptors in Vitro and Their Antipruritic and Antinociceptive Activities in Vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 22.Kane BE, Nieto MJ, McCurdy CR, Ferguson DM. A unique binding epitope for salvinorin A, a non-nitrogenous kappa opioid receptor agonist. FEBS J. 2006;273:1966–1974. doi: 10.1111/j.1742-4658.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- 23.Vortherms TA, Mosier PD, Westkaemper RB, Roth BL. Differential Helical Orientations among Related G Protein-coupled Receptors Provide a Novel Mechanism for Selectivity: Studies with Salvinorin A and the κ-Opioid Receptor. J Biol Chem. 2007;282:3146–3156. doi: 10.1074/jbc.M609264200. [DOI] [PubMed] [Google Scholar]
- 24.Yan F, Mosier PD, Westkaemper RB, Stewart J, Zjawiony JK, Vortherms TA, Sheffler DJ, Roth BL. Identification of the molecular mechanisms by which the diterpenoid salvinorin A binds to κ-opioid receptors. Biochemistry. 2005;44:8643–51. doi: 10.1021/bi050490d. [DOI] [PubMed] [Google Scholar]
- 25.Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. Neoclerodane Diterpenes as a Novel Scaffold for mu Opioid Receptor Ligands. J Med Chem. 2005;48:4765–4771. doi: 10.1021/jm048963m. [DOI] [PubMed] [Google Scholar]
- 26.Harding WW, Tidgewell K, Schmidt M, Shah K, Dersch CM, Snyder J, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE, Salvinicins AB. New Neoclerodane Diterpenes from Salvia divinorum. Org Lett. 2005;7:3017–3020. doi: 10.1021/ol0510522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding WW, Schmidt M, Tidgewell K, Kannan P, Holden KG, Gilmour B, Navarro H, Rothman RB, Prisinzano TE. Synthetic Studies of Neoclerodane Diterpenes from Salvia divinorum: Semisynthesis of Salvinicins A and B and Other Chemical Transformations of Salvinorin A. J Nat Prod. 2006;69:107–112. doi: 10.1021/np050398i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An Opioid Agonist that Does Not Induce μ-Opioid Receptor--Arrestin Interactions or Receptor Internalization. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tidgewell K, Harding WW, Schmidt M, Holden KG, Murry DJ, Prisinzano TE. A facile method for the preparation of deuterium labeled salvinorin A: synthesis of [2,2,2-2H3]-salvinorin A. Bioorg Med Chem Lett. 2004;14:5099–5102. doi: 10.1016/j.bmcl.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 30.Tidgewell K, Harding WW, Lozama A, Cobb H, Shah K, Kannan P, Dersch CM, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. Synthesis of Salvinorin A Analogues as Opioid Receptor Probes. J Nat Prod. 2006;69:914–918. doi: 10.1021/np060094b. [DOI] [PubMed] [Google Scholar]
- 31.Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. Salvinorin A, an Active Component of the Hallucinogenic Sage Salvia divinorum Is a Highly Efficacious κ-Opioid Receptor Agonist: Structural and Functional Considerations. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- 32.Stewart DJ, Fahmy H, Roth BL, Yan F, Zjawiony JK. Bioisosteric modification of salvinorin A, a potent and selective kappa-opioid receptor agonist. Arzneimittelforschung. 2006;56:269–275. doi: 10.1055/s-0031-1296720. [DOI] [PubMed] [Google Scholar]
- 33.Lamers YMAW, Rusu G, Wijnberg JBPA, de Groot A. Synthesis of chiral methyl-branched linear pheromones starting from (+)-aromadendrene. Part 7. Tetrahedron. 2003;59:9361–9369. [Google Scholar]
- 34.Rai AN, Basu A. Sphingolipid synthesis via olefin cross metathesis: preparation of a differentially protected building block and application to the synthesis of D-erythro-ceramide. Org Lett. 2004;6:2861–2863. doi: 10.1021/ol049183a. [DOI] [PubMed] [Google Scholar]
- 35.Beguin C, Richards MR, Li J-G, Wang Y, Xu W, Liu-Chen L-Y, Carlezon JWA, Cohen BM. Synthesis and in vitro evaluation of salvinorin A analogues: Effect of configuration at C(2) and substitution at C(18) Bioorg Med Chem Lett. 2006;16:4679–4685. doi: 10.1016/j.bmcl.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 36.Vaultier M, Knouzi N, Carrie R. Reduction d’azides en amines primaires par une methode generale utilisant la reaction de staudinger. Tetrahedron Lett. 1983;24:763–764. [Google Scholar]
- 37.Bikbulatov RV, Yan F, Roth BL, Zjawiony JK. Convenient synthesis and in vitro pharmacological activity of 2-thioanalogs of salvinorins A and B. Bioorg Med Chem Lett. 2007;17:2229–2232. doi: 10.1016/j.bmcl.2007.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, Hashimoto A, Rice KC, Jacobson AE, Thomas JB, Carroll FI, Lai J, Rothman RB. Opioid peptide receptor studies. 14. Stereochemistry determines agonist efficacy and intrinsic efficacy in the [35S]GTP-γ-S functional binding assay. Synapse. 2001;39:64–69. doi: 10.1002/1098-2396(20010101)39:1<64::AID-SYN9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 39.Ni Q, Xu H, Partilla JS, de Costa BR, Rice KC, Rothman RB. Selective labeling of κ2 opioid receptors in rat brain by [125I]IOXY: interaction of opioid peptides and other drugs with multiple κ2a binding sites. Peptides. 1993;14:1279–1293. doi: 10.1016/0196-9781(93)90188-m. [DOI] [PubMed] [Google Scholar]
- 40.de Costa BR, Iadarola MJ, Rothman RB, Berman KF, George C, Newman AH, Mahboubi A, Jacobson AE, Rice KC. Probes for narcotic receptor mediated phenomena. 18. Epimeric 6α- and 6β-iodo-3,14-dihydroxy-17-(cyclopropylmethyl)-4,5α-epoxymorphinans as potential ligands for opioid receptor single photon emission computed tomography (SPECT): synthesis, evaluation, and radiochemistry of [125I]-6β-iodo-3,14-dihydroxy-17-(cyclopropylmethyl)-4,5α-epoxymorphinan ([125I]IOXY) J Med Chem. 1992;35:2826–2835. doi: 10.1021/jm00093a016. [DOI] [PubMed] [Google Scholar]
- 41.Portoghese PS. A new concept on the mode of interaction of narcotic analgesics with receptors. J Med Chem. 1965;8:609–616. doi: 10.1021/jm00329a013. [DOI] [PubMed] [Google Scholar]
- 42.Beguin C, Potter DN, DiNieri JA, Munro TA, Richards MR, Paine TA, Berry L, Zhao Z, Roth BL, Xu W, Liu-Chen L-Y, Carlezon WA, Jr , Cohen BM. N-methylacetamide analogue of salvinorin A: a highly potent and selective kappa opioid receptor agonist with oral efficacy. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.107.129023. jpet.107.129023. [DOI] [PubMed] [Google Scholar]
- 43.Simpson DS, Katavic PL, Lozama A, Harding WW, Parrish D, Deschamps JR, Dersch CM, Partilla JS, Rothman RB, Navarro H, Prisinzano TE. Synthetic Studies of Neoclerodane Diterpenes from Salvia divinorum: Preparation and Opioid Receptor Activity of Salvinicin Analogues. J Med Chem. 2007;50:3596–3603. doi: 10.1021/jm070393d. [DOI] [PubMed] [Google Scholar]
- 44.Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) muopioid agonists on cellular markers related to opioid tolerance and dependence. Synapse. 2007;61:166–175. doi: 10.1002/syn.20356. [DOI] [PubMed] [Google Scholar]
- 45.Eguchi M. Recent advances in selective opioid receptor agonists and antagonists. Med Res Rev. 2004;24:182–212. doi: 10.1002/med.10059. [DOI] [PubMed] [Google Scholar]
- 46.Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RS, et al. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J Med Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- 47.Bondensgaard K, Ankersen M, Thogersen H, Hansen BS, Wulff BS, Bywater RP. Recognition of privileged structures by G-protein coupled receptors. J Med Chem. 2004;47:888–899. doi: 10.1021/jm0309452. [DOI] [PubMed] [Google Scholar]
- 48.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 49.Ortholand J-Y, Ganesan A. Natural products and combinatorial chemistry: back to the future. Curr Opin Chem Biol. 2004;8:271–280. doi: 10.1016/j.cbpa.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 51.Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothman RB, Murphy DL, Xu H, Godin JA, Dersch CM, Partilla JS, Tidgewell K, Schmidt M, Prisinzano TE. Salvinorin A: Allosteric Interactions at the μ-Opioid Receptor. J Pharmacol Exp Ther. 2007;320:801–810. doi: 10.1124/jpet.106.113167. [DOI] [PubMed] [Google Scholar]
- 53.Hiebel AC, Lee YS, Bilsky E, Giuvelis D, Deschamps JR, Parrish DA, Aceto MD, May EL, Harris LS, Coop A, Dersch CM, Partilla JS, Rothman RB, Cheng K, Jacobson AE, Rice KC. Probes for narcotic receptor mediated phenomena. 34. Synthesis and structure-activity relationships of a potent mu-agonist delta-antagonist and an exceedingly potent antinociceptive in the enantiomeric C9-substituted 5-(3-hydroxyphenyl)-N-phenylethylmorphan series. J Med Chem. 2007;50:3765–3776. doi: 10.1021/jm061325e. [DOI] [PubMed] [Google Scholar]