Abstract
Potent cell activation by endotoxin requires sequential protein-endotoxin and protein-protein interactions involving lipopolysaccharide-binding protein, CD14, MD-2, and Toll-like receptor 4 (TLR4). MD-2 plays an essential role by bridging endotoxin (E) recognition initiated by lipopolysaccharide-binding protein and CD14 to TLR4 activation by presenting endotoxin as a monomeric E·MD-2 complex that directly and potently activates TLR4. Secreted MD-2 (sMD-2) exists as a mixture of monomers and multimers. Published data suggest that only MD-2 monomer can interact with endotoxin and TLR4 and support cell activation, but the apparent instability of MD-2 has thwarted efforts to more fully separate and characterize the individual species of sMD-2. We have taken advantage of the much greater stability of sMD-2 in insect culture medium to fully separate sMD-2 monomer from dimer by gel sieving chromatography. At low nanomolar concentrations, the sMD-2 monomer, but not dimer, reacted with a monomeric complex of E·sCD14 to form monomeric E·MD-2 and activate HEK293/TLR4 cells. The monomer, but not dimer, also reacted with the ectodomain of TLR4 with an affinity comparable with the picomolar affinity of E·MD-2. These findings demonstrate directly that the monomeric form of sMD-2 is the active species both for reaction with E·CD14 and TLR4, as needed for potent endotoxin-induced TLR4 activation.
Invasion by Gram-negative bacteria (GNB)3 is specifically detected and responded to by mammals through mobilization of the innate immune system. In many strains and species of GNB, this process depends on host recognition of and response to the unique complex glycolipid, endotoxin (lipooligosaccharide (LOS) or lipopolysaccharide (LPS)), that comprises much of the outer leaflet of the outer membrane of GNB (1, 2). Minute amounts of endotoxin (E), presented either as an integral part of the outer membrane of GNB or as large aggregates of extracted and purified endotoxin, can stimulate pro-inflammatory responses (2–5). This sensitivity depends upon an ordered series of endotoxin-protein and protein-protein interactions that include the host proteins LPS-binding protein (LBP), membrane-bound or soluble (s)CD14, secreted or TLR4-associated MD-2, and TLR4 (6–8). Engagement of endotoxin-rich membranes or isolated endotoxin aggregates by LBP facilitates the extraction of endotoxin monomers by CD14 to form monomeric E·CD14 complexes that are the most efficient substrate for transfer of endotoxin to MD-2 (4, 6, 9). The monomeric E·MD-2 complex is necessary and sufficient to induce TLR4-dependent cell activation by endotoxin (4, 6, 10). MD-2 associates noncovalently with the N-terminal ectodomain of TLR4 and plays a pivotal role not only in TLR4 activation but also in trafficking of TLR4 to the cell surface (7, 11–13). MD-2 also likely interacts transiently with CD14 facilitating transfer of endotoxin monomer from CD14 to MD-2 and, at high molar excess of CD14, reverse transfer of endotoxin from MD-2 to CD14 (14). Simultaneous engagement by MD-2 of both endotoxin and TLR4 is required for activation of TLR4 by endotoxin (4, 15).
MD-2 belongs to the ML (MD-2-like lipid recognition) domain family of proteins (16). A structural hallmark of these proteins is the presence of a deep hydrophobic pocket into which specific ligands (e.g. specific glycolipids) bind. The recently reported x-ray structure of MD-2 has confirmed the presence in MD-2 of a β-barrel immunoglobin-fold structure (17). In contrast to the very stable and potently bioactive properties of the monomeric E·MD-2 complex (4, 18), recombinant MD-2 expressed and secreted from mammalian cell cultures, such as human embryonic kidney (HEK) 293 cells, is recovered mainly as inactive multimers with little monomer detected (19–22). The recovery of active secreted MD-2 (sMD-2) from mammalian cell cultures is further compromised by the instability of sMD-2 at 37 °C in serum-free culture medium (9, 23, 24). In contrast to MD-2 secreted from mammalian cell cultures, nearly 50% of MD-2, produced by insect cells after infection with baculovirus containing MD-2 cDNA, is monomeric and remains active during storage at 4 °C for more than 1 year and at 37 °C for more than 6 h (9).4 That monomeric MD-2 is the active form of MD-2 necessary for TLR4-dependent activation has been previously proposed (19, 25, 26). However, this conclusion has been largely based on studies in which the physical state of sMD-2, either alone or in complex with LPS or the TLR4 ectodomain (TLR4ECD), has been deduced from the electrophoretic properties of sMD-2 during SDS-PAGE under nonreducing conditions. Efforts to compare more directly the functional properties of monomeric and multimeric forms of sMD-2 been hampered, to date, by the instability of MD-2.
In this study, we have taken advantage of the stability of MD-2 produced and secreted by insect cells to separate monomeric and dimeric forms of sMD-2 using size exclusion chromatography under nondenaturing and nonreducing conditions. This separation has made it possible to investigate directly the functional properties of the monomer and dimer forms of sMD-2. The studies presented here demonstrate directly that only monomeric MD-2 has the ability to act as an acceptor for transfer of endotoxin from E·sCD14 as shown by generation of E·MD-2 and TLR4 activation in the presence of E·sCD14 and to interact with high affinity with the TLR4 ectodomain. Together, these properties explain the importance of the monomeric form of sMD-2 in activation of cells expressing TLR4 by endotoxin.
EXPERIMENTAL PROCEDURES
Materials—[3H]LOS or [14C]LOS (25,000cpm/pmol; 3000 cpm/pmol, respectively) was isolated from an acetate auxotroph of Neisseria meningitidis serogroup B metabolically labeled and isolated as described (3). LBP and sCD14 were gifts from Xoma (Berkley, CA) and Amgen Corp. (Thousand Oaks, CA), respectively. Human serum albumin (HSA) was obtained as an endotoxin-free, 25% stock solution (Baxter Health Care, Glendale, CA). Chromatography matrices (Sephacryl HR S200 and S300) were purchased from GE Healthcare. Express Five™ medium was purchased from Invitrogen and supplemented with 2 mm glutamine per the manufacturer's instructions.
Production of Recombinant Proteins: Insect Cell-derived Recombinant Polyhistidine-tagged MD-2—cDNA encoding human MD-2 was inserted into pBAC11 (Novagen) using XhoI- and NotI-sensitive restriction sites as described (4). Sf9 cells were used for transfection and amplification of baculovirus, whereas High Five™ cells in serum-free medium (Express Five™) were used for protein production (4). Conditioned medium containing secreted human sMD-2-His6 was prepared in collaboration with Biovest International/National Cell Culture Center (Minneapolis, MN) and stored at 4 °C. The conditioned medium containing human sMD-2-His6 was used either directly or after concentration 10–12-fold using Amicon Ultra Centrifugal devices (Millipore) (molecular weight cut-off, 10,000) followed by sterile filtration.
HEK293 Cell-derived Recombinant Polyhistidine-tagged MD-2 and FLAG-TLR4 Ectodomain—Expression vectors containing DNA for production of FLAG-TLR4ECD (amino acids 24–634; pFLAG-CMV-TLR4) and MD-2-FLAG-His6 (pEF-BOS-MD-2-FLAG-His6) have been previously described and characterized (9, 19). Briefly, HEK293T cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum to ∼80% confluency in T75 flasks and transfected with 12 μg of DNA using PolyFect reagent (Qiagen). After 12 h, the medium was removed and replaced with fresh serum-free medium (293 SFM; Invitrogen). Media containing expressed proteins were collected 24–48 h later and concentrated 10–20-fold by ultrafiltration with Millipore Centricon-Plus 70 before use. Conditioned medium containing secreted TLR4ECD maintained activity to react with [3H]LOS·MD-2 for at least 6 months when stored at 4 °C.
Gel Filtration Chromatography of Recombinant MD-2-His6 Secreted from High Five™ Insect Cells—For preparative runs, 2 ml of 10–12× concentrated conditioned insect medium containing recombinant human sMD-2-His6 was applied to 1.6× ∼70-cm column of Sephacryl S200 pre-equilibrated either in Dulbecco's phosphate-buffered saline (PBS) ± 0.1% HSA or in Express Five™ insect medium supplemented with 2 mm glutamine and eluted in the same medium at a flow-rate 0.5 ml/min with the collection of 1-ml fractions. Small differences in elution of sMD-2-His6 dimer (see Figs. 2 and 3) were a result of small differences in column length. The presence of MD-2-His6 in individual fractions was determined by immunoblot as described below. Peak fractions corresponding to sMD-2 monomer or dimer were pooled, sterile-filtered, and stored at 4 °C. Pools of sMD-2 monomer and dimer were used within 2 days of collection for functional assays (see below). Sephacryl S200 was calibrated with the following proteins using Bio-Rad standards for gel filtration: thyroglobulin (650,000, Vo), IgG (158,000), HSA (66,000), ovalbumin (44,500), myoglobin (17,500), and vitamin B12 (1200, Vi).
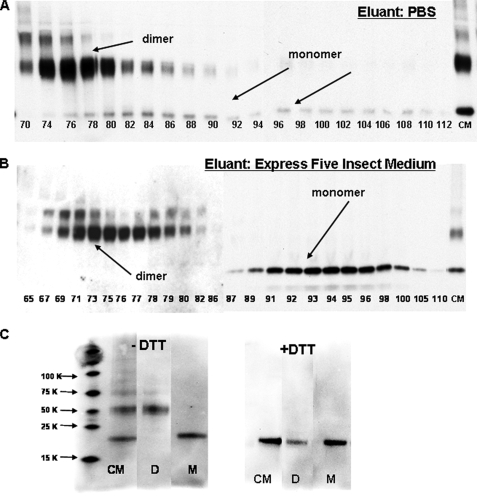
FIGURE 2.
Monomeric MD-2 can be isolated in good yield on Sephacryl S200 using insect medium, but not PBS, as eluant. A and B, concentrated (10×) conditioned insect medium was applied to Sephacryl S200 (1.6 ×∼70 cm) equilibrated and eluted in PBS (A) or Express Five™ medium (B). Aliquots (25 μl) of individual fractions (1 ml) were resolved by SDS-PAGE under nonreducing conditions and after transfer to nitrocellulose probed for MD-2-His by reactivity with an anti-(His)4 antibody. C, fractions from Sephacryl S200 eluted in Express Five™ medium (B) corresponding to dimer and monomer forms of MD-2 as determined by the immunoblot were pooled. Pooled fractions of dimer (lanes D; fractions 73–77) and monomer (lanes M; fractions 92–96) and concentrated conditioned medium (lanes CM) were treated with sample buffer ± dithiothreitol, electrophoresed, transferred to nitrocellulose, and then probed for MD-2 using an anti-(His)4 antibody. The blots shown are representative of fractions derived from two different chromatographic separations for each eluant. Note that testing of fractions shown required two separate blots; however, the gels were electrophoresed, transferred, and probed with anti-(His)4 antibody simultaneously.
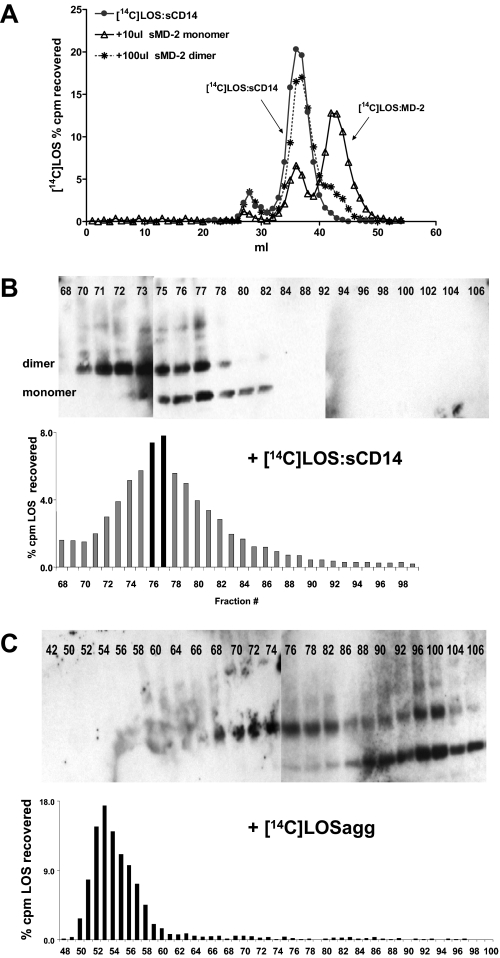
FIGURE 3.
Only the monomeric form of MD-2 reacts with LOS·sCD14, but not LOS aggregates, to generate LOS. MD-2. A, pooled monomer (10 μl) or dimer (100 μl) was incubated with [14C]LOS·sCD14 (2 nm, 10 ng of LOS) for 30 min at 37 °C in PBS with 0.1% HSA. The reaction mixture was separated by chromatography on Sephacryl S200 in PBS. The elution profile of untreated [14C]LOS·sCD14 is shown for reference. The profiles shown are representative of at least two experiments. B and C, conditioned medium containing MD-2-His (2 ml, 10× concentrated) was incubated for 30 min at 37 °C with either (2 μm, 10 μg of LOS) [14C]LOS·sCD14 or [14C]LOS aggregates and the reaction mixture was chromatographed through Sephacryl S200 in Express Five™ medium. The fractions were analyzed for [14C]LOS content by liquid scintillation spectroscopy and the presence of MD-2 by immunoblot using anti-(His)4 antibody. Note fractions 42–72 and 76–102 were analyzed by separate blots. However, the gels were electrophoresed, transferred, and probed with anti-(His)4 antibody simultaneously. The blots shown are representative of fractions derived from two different chromatographic separations from two separate experiments.
Immunoblotting—To detect polyhistidine labeled MD-2, an anti-polyhistidine antibody (Tetra-His antibody; Qiagen) was used. The samples were heated at 100 °C for 10 min in Laemmli sample buffer ± 200 mm dithiothreitol + 6 m urea and electrophoresed through a 4–20% gradient acrylamide gel (Pierce) using Tris/HEPES/SDS buffer and transferred to nitrocellulose. The nitrocellulose was washed with 20 mm Tris, 0.5 m NaCl, pH 7.5, containing 0.05% Tween 20 and 0.2% Triton X-100 (TBSTT), blocked to reduce nonspecific background with 3% bovine serum albumin in 20 mm Tris, 0.5 m NaCl, pH 7.5 for 1 h at 25 °C, and incubated with the anti-His4 antibody in the same buffer overnight. After washing with TBSTT, the blot was incubated with donkey anti-mouse IgG conjugated to horseradish peroxidase (Jackson Immunologicals) for 1 h at 25 °C in 10 mm Tris, 150 mm NaCl, pH 7.5, 0.1% Tween 20 containing 3% goat serum and washed with TBSTT exhaustively. The blots were developed using the Pierce SuperSignal substrate system. The levels of sMD-2-His6 in experimental samples were quantified by densitometric analysis of immunoblots, using known amounts of E·MD-2 as standards. To facilitate quantitative comparisons of sMD-2-His6, the samples were pretreated with dithiothreitol so that all sMD-2 was monomeric.
Preparation of [3H]LOS·Protein Complexes—[3H]LOS·sCD14 and [3H]LOS·MD-2 complexes were prepared as previously described (4, 6, 27). Briefly, [3H]LOS aggregates (Mr > 20 × 106) were obtained after hot phenol/water extraction of [3H]LOS, followed by ethanol precipitation, resuspension in distilled water, sonication, and sedimentation by ultracentrifugation of [3H]LOS aggregates (27). Monomeric [3H]LOS.CD14 complexes (Mr, ∼60,000) were prepared by treatment of [3H]LOS aggregates for 30 min at 37 °C with substoichiometric LBP (molar ratio, 200:1 LOS:LBP) and 1.5× molar excess of sCD14 followed by gel exclusion chromatography (Sephacryl S200, 1.6 × 70-cm column) in PBS, pH 7.4, 0.03% HSA to isolate monomeric [3H]LOS·sCD14 complex. [3H]LOS·MD-2 (Mr ∼25,000) was generated by treatment of [3H]LOS·sCD14 (30 min at 37 °C) with High Five™ insect cell medium containing sMD-2-His6 or the indicated amount of isolated monomeric or dimeric sMD-2-His6. Products of the reaction were resolved by S200 chromatography in PBS as previously described (4, 6) unless otherwise indicated. Fractions containing complex were combined, sterile-filtered, and stored at 4 °C.
Reaction of Secreted TLR4ECD with [3H]LOS·MD-2 ± Various sMD-2 Preparations—[3H]LOS·MD-2 (1 nm) was incubated with concentrated (10–20×) conditioned medium containing TLR4ECD ± samples containing sMD-2 diluted to a final volume of 0.5 or 1 ml in PBS, pH 7.4, for 30 min at 37 °. The reaction products were analyzed by Sephacryl HR S300 (1.6 × 70 cm) chromatography in PBS, pH 7.4. Fractions (1.0 ml) were collected at a flow rate 0.5 ml/min at room temperature using AKTA Purifier or Explorer 100 fast protein liquid chromatography (GE Healthcare). Radioactivity in collected fractions was analyzed by liquid scintillation spectroscopy (Beckman LS liquid scintillation counter). Recoveries of [3H]LOS were ≥70% in all cases. All of the solutions used were pyrogen-free and sterile-filtered. After chromatography, selected fractions were sterile-filtered (0.22 μm) and kept at 4 °C. Size evaluation of products resolved by Sephacryl S300 was based on calibration as described (9) using proteins with apparent Mr: blue dextran (2 × 106, Vo), thyroglobulin (650,000), ferritin (440,000), catalase (232,000), IgG (158,000), HSA (66,000), ovalbumin (44,500), myoglobin (17,500), and vitamin B12 (1200, Vi).
HEK293 Cell Activation Assay—HEK293/TLR4 cells were cultured as has been previously described (28). For cell activation assays, the cells were grown to confluency and then washed twice with warm PBS, pH 7.4, and incubated overnight at 37 °C, 5% CO2, and 95% humidity in 96-well plates containing Dulbecco's modified Eagle's medium, 0.1% HSA with [14C]LOS·sCD14 (2 nm or 60 pm as indicated) or LOS aggregates (20 nm) ± the indicated source of sMD-2. Activation of HEK293/TLR4 cells was assessed by measuring accumulation of extracellular IL-8 by ELISA (BD Clontech, Inc., Palo Alto, CA).
RESULTS
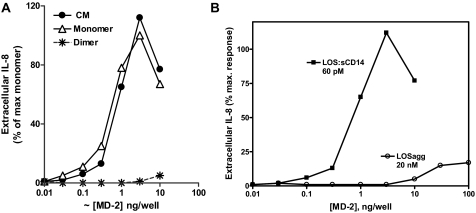
Greater Yield and Stability of Bioactive Recombinant Human sMD-2 in Conditioned Insect Cell versus Mammalian Cell Medium—We have previously shown that harvested conditioned medium of insect cells infected with baculovirus encoding human MD-2-His6 contains significant amounts of bioactive sMD-2 that can readily react with monomeric E·sCD14 complex to generate monomeric E·MD-2 (4, 9). E·MD-2, but not E·sCD14 or purified endotoxin aggregates, can bind to TLR4ECD with picomolar affinity and activate cells expressing TLR4 without MD-2 (e.g. HEK/TLR4 cells) at picomolar concentrations (4, 9, 14). Addition of as little as 1 μl of this conditioned medium (representing ∼1–2 ng of sMD-2; ∼500 pm final concentration of sMD-2) together with 200 pm LOS·sCD14 (1 ng LOS/ml) produced maximum activation of HEK/TLR4 cells (Fig. 1A), whereas parental HEK293 cells lacking TLR4 were not affected (4) (data not shown). Cell activation required co-incubation of the conditioned medium containing sMD-2 with LOS·sCD14 (i.e. generation of LOS·MD-2); neither LOS·sCD14 (Fig. 1A) nor the conditioned medium alone (4) induced activation of the HEK/TLR4 cells.
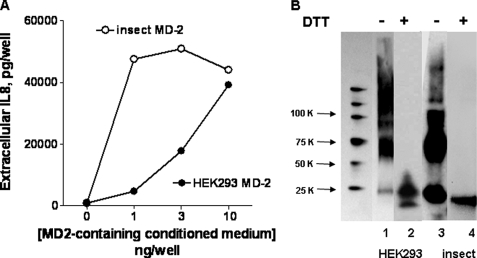
FIGURE 1.
Insect-derived conditioned medium containing secreted MD-2-His contains more active MD-2 and more MD-2 in a monomeric state than HEK-derived MD-2-His. A, functional MD-2 was assayed by measuring activation of HEK/TLR4 cells induced by incubating cells with [14C]LOS·sCD14 (2 nm) and increasing amounts of conditioned medium (insect, ○; HEK-293, •) for 24 h. Cell activation was monitored by extracellular accumulation of IL-8, which was measured by ELISA. The results shown represent the means ± S.E. of three experiments, each in triplicate. B, composition of secreted MD-2 determined by SDS-PAGE ± dithiothreitol (DTT)/immunoblots (25 μl/lane) harvested at 48 h from HEK293T cells transfected with expression vector (pEF-BOS) encoding MD-2-FLAG-His6 and cultured in serum-free medium with 0.4% HSA (lanes 1 and 2) or from insect cells infected with baculovirus containing MD-2-His (lanes 3 and 4). MD-2 was detected using anti-(His)4 antibody. Lane 1 represents molecular weight markers (Perfect Protein; Novagen). The blot shown is representative of more than three experiments.
In contrast to the conditioned insect cell medium containing sMD-2, harvested conditioned medium from HEK293 cells expressing and secreting MD-2 was much less potent in activating HEK/TLR4 cells when incubated together with LOS·sCD14 (Fig. 1A) or in generating monomeric LOS·MD-2 (9, 24). Comparison of the two conditioned media by SDS-PAGE/immunoblot under nonreducing conditions demonstrated a significant difference in the amount and physical state of sMD-2 in the two media under these conditions. As seen in Fig. 1B, the predominant forms of sMD-2 in conditioned medium from infected High Five™ insect cells detected by an anti-His antibody immunoblot were the dimeric and monomeric forms of sMD-2 (consistent with the data of Viriyakosol et al. (22)), whereas HEK293-derived sMD-2 appeared as a collection of oligomers with very little monomer present. Under reducing conditions, all of the secreted recombinant MD-2 migrated as monomers (Mr, ∼20,000; Fig. 1B). These findings indicate that the harvested conditioned medium from infected insect High Five™ cells contain much more active sMD-2 than that present in conditioned medium of transiently transfected HEK293 cells. The yields of bioactive sMD-2 shown were the optimum we could obtain under our culture conditions and were not increased by either higher titers of infection, plasmid DNA, or time of incubation before harvesting.
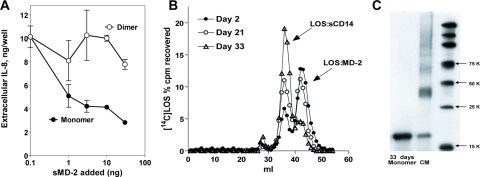
Separation of sMD-2 Monomer and Dimer by Sephacryl S200 Chromatography in Insect Culture Medium—The findings above strongly suggested that conditioned medium from infected High Five™ insect cells provided the more favorable starting material for separation and functional characterization of sMD-2 monomer and multimers. An important limitation of virtually all of the previous studies on the physical state of sMD-2, including our own (Fig. 1B), has been the reliance on SDS-PAGE (i.e. presence of SDS) to resolve sMD-2 monomer and multimers. To better define the physical state of bioactive sMD-2 in the conditioned medium (i.e. under nondenaturing as well as nonreducing conditions), conditioned insect medium containing sMD-2-His6 was concentrated 10–12-fold and subjected to Sephacryl S200 size exclusion chromatography using PBS as eluant. Individual collected fractions were analyzed for MD-2-His6 content by SDS-PAGE under nonreducing conditions followed by immunoblot using an anti-polyhistidine antibody. Fig. 2A shows that, under these chromatographic conditions, fractions containing sMD-2 species eluting with an apparent Mr ∼40,000 (fractions 74–78), corresponding to the expected elution of dimeric sMD-2, contained almost exclusively dimeric MD-2 as judged by SDS-PAGE/immunoblot. Earlier fractions along the upslope of the sMD-2 dimer peak were enriched on immunoblots with an additional, more slowly migrating, sMD-2 species that by behavior on Sephacryl S200 and SDS-PAGE appeared to be a trimer of sMD-2. Comparison of immunoblots of the recovered fractions with the concentrated conditioned medium applied to the column indicated recovery of sMD-2 dimer (and trimer) of >50%, whereas recovery of monomeric sMD-2 was substantially less (Fig. 2A), suggesting a selective loss of sMD-2 monomer. The small amount of recovered immunoreactive material consistent with the size of monomeric sMD-2-His6 eluted over a surprisingly broad range of fractions, including those (after fraction 95) in which molecules much smaller than the monomer of sMD-2 (i.e. Mr, <20,000) would be expected to elute. The poor recovery and broad and late elution of monomeric sMD-2-His6 suggested aberrant behavior of the sMD-2-His6 monomer in the gel filtration matrix in PBS. Supplementing PBS with 0.1% HSA in the column and elution buffer did not significantly increase recovery or change the elution pattern of the sMD-2 monomer (data not shown).
Because bioactive MD-2-His6 is stable for more than 1 year in the conditioned insect cell medium, we repeated size exclusion chromatography using the insect cell medium (Express Five™; Invitrogen) as the equilibration and elution buffer. In contrast to chromatography in PBS, chromatography in Express Five™ medium resulted in markedly improved recovery of the sMD-2 monomer (Fig. 2B). However, even under these conditions, sMD-2 monomer eluted significantly later than that expected for a protein of molecular mass of ∼20,000 daltons. The aberrant elution of monomeric sMD-2 during Sephacryl S200 chromatography was fortuitous in that it resulted in virtually complete separation of sMD-2 dimer and monomer (Fig. 2B). As a result, fractions containing almost exclusively the monomer or dimer forms of sMD-2 could be pooled (Fig. 2 legend) and used for functional assays (see below). The apparent homogeneity of these sMD-2 pools was confirmed by nonreducing SDS-PAGE/immunoblot analysis (Fig. 2C). Under reducing conditions, all of the immunoreactive material migrated as Mr ∼ 20,000, i.e. as monomeric MD-2 (Fig. 2C).
Efficient Production of Monomeric E·MD-2 Requires Monomeric sMD-2 and Presentation of Endotoxin as E·sCD14—Key functional properties of sMD-2 include: 1) reactivity with E·sCD14 to form monomeric E·MD-2; 2) binding to the TLR4 ectodomain; and 3) activation of TLR4 by bound E·MD-2. Thus, we first compared the ability of sMD-2 monomers and dimers to react with [14C]LOS·sCD14 (Mr ∼ 60,000) to generate [14C]LOS·MD-2 (Mr, ∼ 25,000) as assessed by size exclusion chromatography. Fig. 3A shows that incubation of [14C]LOS·sCD14 with sMD-2 monomers caused substantial conversion of [14C]LOS·sCD14 to monomeric [14C]LOS·MD-2. In contrast, the addition of even 10-fold more sMD-2 dimer produced very little monomeric [14C]LOS·MD-2, indicating strong reactivity of sMD-2 monomer but little or no reactivity of sMD-2 dimer with LOS·sCD14. This conclusion was further supported by monitoring the consumption of sMD-2 monomer versus dimer during incubation of [14C]LOS·sCD14 with the sMD-2-containing conditioned insect cell culture medium (Fig. 3B). Formation of monomeric [14C]LOS·MD-2 (Fig. 3B) resulted in 1) the appearance, by immunoblot, of sMD-2 monomers in fractions containing monomeric [14C]LOS·MD-2 (peak is fraction 77); 2) the disappearance of free sMD-2 monomer (note absence of sMD-2 by immunoblot after fraction 84); and 3) no apparent loss of free sMD-2 dimer. These findings directly demonstrate the selective reactivity of monomeric LOS·sCD14 with monomeric sMD-2.
Earlier studies have demonstrated that sMD-2 can also react with purified endotoxin (12, 25). We therefore repeated the same experiment using [14C]LOS aggregates instead of monomeric LOS·sCD14. Fig. 3C shows that, under these experimental conditions, there was neither conversion of LOS aggregates to monomeric LOS·MD-2 nor association of sMD-2 monomer, dimer, or higher order multimers with the LOS aggregates that eluted much earlier in the void volume during Sephacryl S200 chromatography. Taken together, these findings demonstrate that efficient sMD-2-endotoxin interactions require presentation of sMD-2 as a monomer and presentation of endotoxin as a monomeric E·sCD14 complex.
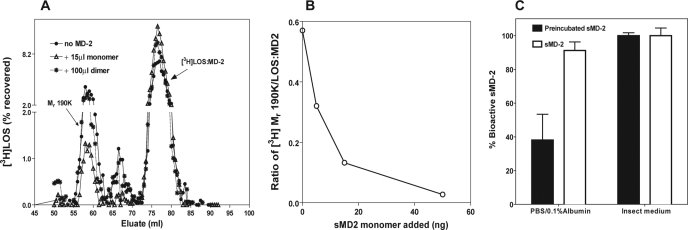
Efficient Interaction of sMD-2 with TLR4 Ectodomain Requires Monomeric sMD-2—Activation of TLR4 by endotoxin requires simultaneous binding of MD-2 to both endotoxin and the TLR4 ectodomain (TLR4ECD). This can occur either by direct binding of monomeric E·MD-2 to TLR4 or by transfer of endotoxin from monomeric E·CD14 to a preformed MD-2/TLR4 heterodimer (4, 9, 12, 14, 25). Previous studies have demonstrated direct interactions of sMD-2 with the TLR4ECD but estimated an affinity that was nearly 50-fold lower than E:MD-2-TLR4ECD binding (12 nm) (26) versus 200–300 pm) (9). To determine whether sMD-2 binds to the TLR4ECD with an affinity comparable with that of E·MD-2, we took advantage of an assay we have recently developed that demonstrates specific binding of [3H]LOS·MD-2 (25,000 cpm/pmol) to TLR4ECD at picomolar concentrations of [3H]LOS·MD-2 (9). Interaction of [3H]LOS·MD-2 with TLR4ECD yields a higher order complex ([3H]LOS·MD-2/TLR4ECD)2 of Mr ∼190,000 that can be quantitatively assayed by gel filtration chromatography. There is little or no transfer of [3H]LOS from [3H]LOS·MD-2 to sMD-2 or to MD-2/TLR4 (9). Therefore, we could measure the ability of monomeric and dimeric sMD-2 to bind to TLR4ECD by testing their ability to inhibit binding of [3H]LOS·MD-2 to TLR4ECD, i.e. as assessed by inhibition of the formation of the 3H-labeled Mr ∼190,000 complex. As shown in Fig. 4 (A and B), the monomeric form of sMD-2 caused dose-dependent inhibition of formation of ([3H]LOS·MD-2/TLR4ECD)2, with half-maximal inhibition produced by ∼1–2 nm sMD-2 monomer (Fig. 4B). Purified sMD-2 monomer also produced dose-dependent inhibition of cell (HEK/TLR4) activation by LOS·MD-2 as measured by the accumulation of extracellular IL-8 (Fig. 4C). In contrast, the dimeric form of sMD-2, even at 10–30× higher concentration, produced little or no inhibition of formation of ([3H]LOS·MD-2/TLR4ECD)2 (Fig. 4A) or of HEK/TLR4 cell activation by LOS·MD-2 (Fig. 4C). Thus, the monomer form of sMD-2, but not the dimer, binds with high affinity to the TLR4 ectodomain.
FIGURE 4.
Monomeric MD-2 competes with [3H]LOS·MD-2 for reaction with the ectodomain of TLR4. A, concentrated (10×) HEK293 conditioned medium containing the ectodomain of TLR4 (100μl) was incubated with [3H]LOS·MD-2 (1 nm)(•) alone or with pooled MD-2 monomer (15μl, ▵) or MD-2 dimer (100μl, *) for 30 min at 37 °C, and the reaction mixture was applied and eluted on Sephacryl S200 in PBS. The fractions were analyzed for [3H]LOS content and formation of the Mr 190 K product by liquid scintillation spectroscopy. B, concentrated (10×) HEK293 conditioned medium containing the ectodomain of TLR4 (100 μl) was incubated with [3H]LOS·MD-2 (1 nm) ± varying amounts of MD-2 for 30 min at 37 °C, and the amount of radiolabeled 190K product formed was determined by liquid scintillation spectroscopy of the chromatographed reaction mixture on Sephacryl S200. The data are expressed as ratios of radiolabeled 190 K complex formed to LOS·MD-2 added in the presence of increasing amounts of MD-2 monomer added. C, dose-dependent inhibition by sMD-2 monomer but not by sMD-2 dimer of HEK/TLR4 cell activation by 60 pm LOS·MD-2. Cell activation was determined by measuring extracellular accumulation of IL-8 by ELISA.
Potent Activation of Cells Expressing TLR4 without MD-2 Requires Presence of Monomeric Form of sMD-2 and Presentation of Endotoxin as Monomeric E·CD14—We have previously shown that insect cell culture medium containing sMD-2 promotes activation by E·sCD14 of cells expressing TLR4 without MD-2 (4, 6). The selective reactivity of the monomeric form of sMD-2 with monomeric LOS·sCD14 shown in Fig. 3 suggests that the activity of the conditioned insect cell medium is due specifically to the presence of monomeric sMD-2. To test this hypothesis, we compared the effect of increasing amounts of unfractionated conditioned insect cell medium containing sMD-2-His6 or of isolated dimeric or monomeric sMD-2-His6 with LOS·sCD14 on activation of HEK/TLR4 cells, as measured by the accumulation of extracellular IL-8. Fig. 5A shows closely similar dose-dependent effects of the conditioned medium containing sMD-2 and of isolated monomeric sMD-2 in promoting activation of HEK/TLR4 cells when co-incubated with LOS·sCD14. In contrast, dimeric MD-2 displayed negligible activity (Fig. 5A). These data indicate that activation of cells expressing TLR4 without MD-2 by LOS·sCD14 requires the presence of monomeric sMD-2.
FIGURE 5.
Activation of HEK/TLR4 cells by incubation of the monomeric form of MD-2 with LOS·sCD14. The HEK/TLR4 cells were incubated overnight with increasing amounts of conditioned medium containing MD-2 (•), isolated dimer (*), or monomer (▵) sMD-2 in the presence of [14C]LOS·sCD14 (2 nm)(A) or with increasing amounts of monomer sMD-2 in the presence of [14C]LOS·sCD14 (60 pm) or [14C]LOS aggregates (20 nm)(B). Cell activation was measured by determining accumulation of extracellular IL-8. Extracellular IL-8 was measured by ELISA. The results shown represent the means ± S.E. of three experiments, each in triplicate.
Potent activation of HEK/TLR4 cells by endotoxin required not only presentation of sMD-2 as a monomer but also presentation of endotoxin as a monomeric complex with CD14. Thus, only limited cell activation was observed when endotoxin was added as LOS aggregates, even when >100-fold greater amounts of endotoxin and of sMD-2 were added (Fig. 5B). These differences in the potency of cell activation parallel the differences observed in formation of monomeric LOS·MD-2 when LOS·sCD14 versus LOS aggregates were incubated with sMD-2-containing culture medium (Fig. 3, compare B and C).
Time-dependent Decay of Bioactive sMD-2 at 37 °C and Protection by Insect Culture Medium—Loss of bioactive sMD-2 has been demonstrated during incubation at 37 °C in serum-free medium (9, 23, 24). In an effort to better understand the apparent protective effects of the insect culture medium on the recovery and preservation of bioactive sMD-2, we compared the stability of insect cell-derived bioactive sMD-2 at 37 °C after dilution in either insect culture (Express Five™) medium or PBS with or without 0.1% albumin. Using inhibition of [3H]LOS·MD-2 binding to TLR4ECD as a functional assay, we observed a significant difference in stability of bioactive sMD-2 at 37 °C after dilution in PBS with (Fig. 6A) or without (data not shown) 0.1% albumin versus dilution in Express Five™ medium (Fig. 6A). As previously seen in serum-free mammalian cell culture medium (9, 23, 24), there was a substantial loss of bioactive sMD-2 during 24 h of incubation in PBS ± 0.1% albumin but no loss of sMD-2 activity during similar incubation in the Express Five™ medium (Fig. 6A). These findings show clearly that the Express Five™ medium provides a favorable environment for preservation of bioactive sMD-2.
FIGURE 6.
Time-dependent decay of bioactive sMD-2 monomer at 37 °C (A) and at 4 °C (B and C). A, insect cell conditioned medium containing sMD-2 was diluted 20-fold in PBS with 0.1% albumin or in Express Five™ medium. Bioactive sMD-2 was measured by assay of inhibition of [3H]LOS·MD-2 binding to TLR4ECD (as in Fig. 4) before and after 24 h of preincubation of sMD-2 at 37 °C. The results represent the means ± S.E. of three separate determinations. B, a sample of pooled sMD-2 monomer (10 μl) stored at 4 °C was taken at days 2, 21, and 33 after isolation in Express Five™ medium and incubated with [14C]LOS·sCD14 (2 nm) for 30 min at 37 °C in PBS with 0.1% HSA. The fractions were analyzed for [14C]LOS content by liquid scintillation spectroscopy. The reaction mixture was separated by chromatography on Sephacryl S200 in PBS. The profiles shown are representative of at least two experiments and show reduced activity of isolated sMD-2 monomer (i.e. formation of [14C]LOS·MD-2) during prolonged storage of 4 °C. C, samples (40 μl) of pooled sMD-2 monomer taken 33 days after isolation and storage at 4 °C and of sMD-2-rich conditioned insect cell medium (CM) were treated with SDS-PAGE sample buffer under nonreducing conditions, electrophoresed, transferred to nitrocellulose, and then probed for MD-2 using an anti-(His)4 antibody. The markers are Perfect Protein markers (Novagen).
Decay of Functional Activity of Isolated sMD-2 Monomer during Storage at 4 °C—Both sMD-2 in the unfractionated conditioned insect culture medium and purified E·MD-2 are stable at 4 °C for at least one year with no apparent loss in potency toward HEK/TLR4 cells or reactivity of the sMD-2-containing conditioned medium with TLR4ECD or with E·sCD14 to form E·MD-2 (data not shown). However, the pooled purified sMD-2 monomer gradually lost activity during storage for several weeks at 4 °C, as manifest by a reduced ability to react with [3H]LOS·sCD14 to form [3H]LOS·MD-2 (Fig. 6B). Remarkably, this functional decay was not accompanied by conversion of sMD-2 monomer to multimeric form, at least as judged by SDS-PAGE/immunoblot under nonreducing conditions (Fig. 6C). Loss of sMD-2 activity could be prevented by storage of sMD-2 monomer at –80 °C (data not shown).
DISCUSSION
We have demonstrated directly, for the first time, that the ability of MD-2 secreted without TLR4 (sMD-2) to contribute to host cell responsiveness to endotoxin depends on the presentation of sMD-2 as a monomer. This was accomplished by the use of conditioned medium from insect cells expressing and secreting recombinant human MD-2 and use of the insect cell medium as the column and elution buffer for separation of sMD-2 monomer and multimers by size exclusion chromatography (Fig. 2). The selective reactivity of the recovered sMD-2 monomer was demonstrated by several different functional parameters relevant to the participation of sMD-2 in host cell responses to endotoxin. These include: reactivity with monomeric E·CD14 to form monomeric E:MD-2 (Fig. 3), binding to the ectodomain of TLR4 (Fig. 4), activation of HEK/TLR4 cells when added together with LOS·sCD14 (Fig. 5), or inhibition of activation of these cells by LOS·MD-2 (Fig. 4C). Marked differences in reactivity of sMD-2 monomer and dimer with LOS·sCD14 were observed both in the unfractionated conditioned medium (Fig. 3B) and after isolation (Fig. 3A), indicating that these differences reflect intrinsic differences in the functional properties of sMD-2 monomer and dimer. Our findings confirm earlier conclusions by other investigators (19, 21, 22, 26) who relied mainly on the behavior of sMD-2 in SDS-PAGE under nonreducing conditions to discern MD-2 monomer and multimers and to demonstrate that the product of interactions of sMD-2 with endotoxin and with TLR4ECD includes, selectively, monomeric MD-2. Comparison of the behavior of sMD-2 in SDS-PAGE under nonreducing conditions with its behavior during size exclusion chromatography under nondenaturing/non reducing conditions (Fig. 2B) revealed a generally good correlation of these two analytical parameters. Thus, at least in conditioned insect cell culture medium and freshly derived protein fractions, the relative levels of monomeric sMD-2 can be assessed by SDS-PAGE/immunoblot under nonreducing conditions (19). However, to demonstrate that monomeric sMD-2 was the selective reactant with E·CD14 and TLR4ECD required the separation of sMD-2 monomer and multimers that we describe in this study.
Our findings suggest that the use of baculovirus-infected High Five™ insect cells to express and secrete potently bioactive sMD-2 monomer was advantageous by increasing the fraction of sMD-2 that was secreted and remained as a bioactive monomer during long periods of storage (Fig. 1). The difference in relative abundance of sMD-2 monomer in conditioned insect cell medium versus conditioned HEK293T cell medium was not due to differences in overall sMD-2 concentration in the two media. Overall sMD-2 content in the two different conditioned media was comparable (Fig. 1B), and concentration of the conditioned insect cell medium did not increase sMD-2 oligomerization (data not shown). Many factors could contribute to the increased secretion and/or stability of insect cell-derived sMD-2 monomer, including differences in glycosylation (29, 30), the lower temperature of culture of insect (27 °C) versus mammalian (37 °C) cells, and components of the culture medium itself. We have no direct evidence that differences in the metabolic properties of insect versus mammalian cells or the different culture temperatures are important, although time-dependent loss of sMD-2 functional activity at 37 °C in serum-free medium has been shown (9, 23, 24). We also observed time-dependent decay of bioactive sMD-2 derived from insect cells but only when sMD-2 was diluted in PBS ± 0.1% albumin and not when diluted in insect culture medium (Fig. 6A). Together with the markedly enhanced recovery of sMD-2 monomer by use of the Express Five™ culture medium in size exclusion chromatography (Fig. 2B), these findings suggest a potentially important role of this culture medium in preserving/maintaining sMD-2 as a bioactive monomer. MD-2 belongs to the ML domain family of proteins, which includes mite allergen proteins (e.g. Der p2, p1, f 2), GM2-activator protein, Npc2, and their orthologs (16). The recent publication of the crystal structure of recombinant MD-2 (17) confirms earlier structural models of MD-2 that were based on the solved structures of other ML domain proteins (31–37). A hallmark of MD-2, as well as other ML domain proteins, is the presence of a deep, hydrophobic cavity that can expand to accommodate specific (glyco)lipid ligands. Studies with MD-2 have been hampered by its instability and propensity to form an array of multimers. MD-2 multimers typically contain intermolecular disulfide(s), as manifest by the complete conversion of multimers to monomers of MD-2 when reducing agents are included in the SDS-PAGE buffer (19–22) (Figs. 1B and 2C). The remarkable stability of E·MD-2 as a monomeric complex and its water solubility suggest that occupation of the hydrophobic cavity of MD-2 by the acyl chains of endotoxin can significantly increase the stability and solubility of monomeric MD-2. Other hydrophobic compounds, including certain free fatty acids, can occupy the hydrophobic cavity of MD-2 (17) and, at high enough concentrations, may have similar stabilizing effects on sMD-2 monomer. The Express Five™ medium contains several detergents, surfactants, and other lipids that could be responsible for the greater recovery of sMD-2 monomer following size exclusion chromatography (Fig. 2). The delayed elution of the sMD-2 monomer, in comparison with LOS·MD-2 (compare Figs. 2B and 3B), manifest in both PBS ± 0.1% albumin and Express Five™ medium, could reflect weak interactions of sMD-2 monomer with the gel matrix that retard elution or a less spherical shape of MD-2 under these experimental conditions.
Our findings also confirm and extend earlier observations indicating that endotoxin must be presented as a monomeric complex with CD14 to react efficiently with MD-2 (4, 14). Neither MD-2 monomer nor dimer showed measurable interaction with endotoxin aggregates under the same reaction conditions in which there was quantitative conversion of sMD-2 monomer to E·MD-2 by incubation with E·CD14 (Figs. 3, B and C). These findings are consistent with the very large differences in apparent affinity that have been previously reported for reaction of MD-2 with immobilized LPS (presumably representing LPS in aggregated form; Kd = ∼65 nm) (25) versus that of MD-2 with E·sCD14 (Kd = ∼100–200 pm) (4). The low levels of HEK/TLR4 cell activation observed during incubation of HEK/TLR4 cells with endotoxin aggregates in the presence of sMD-2 (Fig. 5B) could be explained by a yield of monomeric LOS·MD-2 that was ≤0.1% of that resulting from reaction of LOS·sCD14 with sMD-2, below even the very sensitive limits of detection provided by size exclusion analysis of [3H]LOS of very high specific radioactivity.
Lastly, our studies have not revealed an independent functional role for sMD-2 dimer. The apparent lack of reactivity of sMD-2 dimer with either E·CD14, endotoxin aggregates, or TLR4ECD is inconsistent with a role of sMD-2 dimer as a “decoy” (19) in which interactions could lead to formation of nonproductive E·MD-2 (dimer) or TLR4-MD-2 (dimer) complexes that blunt host cell responsiveness to endotoxin. It remains possible that there are other, as yet unidentified substrates for sMD-2 dimer or other, higher order, sMD-2 multimers. It should be noted, however, that the extent to which sMD-2 exists in biological fluids in monomeric versus multimeric form or in association with other host extracellular proteins is not known. The ability to monitor total sMD-2 by ELISA (38) and sMD-2 monomer by reaction with [3H]LOS·sCD14 and/or TLR4ECD may now make it possible to address these important questions.
Acknowledgments
We thank Xoma Corp. (Berkeley, CA) for recombinant LBP and Amgen Corp. (Thousand Oaks, CA) for sCD14. We are also grateful to DeSheng Zhang for preparation, isolation, and characterization of radiolabeled LOS and to Polonca Prohinar for preparation of concentrated TLR4 ectodomain preparations.
This work was supported, in whole or in part, by National Institutes of Health, United States Public Health Service Grants PO144642 and AI59372 (to J. P. W.). This work was also supported by a Veterans Affairs Merit Review grant (to T. L. G.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GNB, Gram-negative bacteria; E, endotoxin; HEK, human embryonic kidney; HSA, human serum albumin; LBP, lipopolysaccharide-binding protein; LOS, lipooligosaccharide; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; TBSTT, Tris-buffered saline with Tween 20 and Triton X-100; TLR, Toll-like receptor; TLR4ECD, TLR4 ectodomain; sMD-2, secreted MD-2; IL, interleukin; ELISA, enzyme-linked immunosorbent assay.
A. Teghanemt, R. Widstrom, T. L. Gioannini, and J. P. Weiss, unpublished observations.
References
- 1.Beutler, B., Jiang, Z., Georgel, P., Crozat, K., Croker, B., Rutschmann, S., Du, X., and Hoebe, K. (2006) Annu. Rev. Immunol. 24 353–389 [DOI] [PubMed] [Google Scholar]
- 2.Beutler, B., and Rietschel, E. T. (2003) Nat. Rev. Immunol. 3 169–176 [DOI] [PubMed] [Google Scholar]
- 3.Giardina, P. C., Gioannini, T., Buscher, B. A., Zaleski, A., Zheng, D. S., Stoll, L., Teghanemt, A., Apicella, M. A., and Weiss, J. (2001) J. Biol. Chem. 276 5883–5891 [DOI] [PubMed] [Google Scholar]
- 4.Gioannini, T. L., Teghanemt, A., Zhang, D., Coussens, N. P., Dockstader, W., Ramaswamy, S., and Weiss, J. P. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 4186–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post, D. M., Zhang, D., Eastvold, J. S., Teghanemt, A., Gibson, B. W., and Weiss, J. P. (2005) J. Biol. Chem. 280 38383–38394 [DOI] [PubMed] [Google Scholar]
- 6.Gioannini, T. L., Teghanemt, A., Zhang, D., Levis, E. N., and Weiss, J. P. (2005) J. Endotoxin. Res. 11 117–123 [DOI] [PubMed] [Google Scholar]
- 7.Miyake, K. (2003) Int. Immunopharmacol. 3 119–128 [DOI] [PubMed] [Google Scholar]
- 8.Ulevitch, R. J., and Tobias, P. S. (1999) Curr. Opin. Immunol. 11 19–22 [DOI] [PubMed] [Google Scholar]
- 9.Prohinar, P., Re, F., Widstrom, R., Zhang, D., Teghanemt, A., Weiss, J. P., and Gioannini, T. L. (2007) J. Biol. Chem. 282 1010–1017 [DOI] [PubMed] [Google Scholar]
- 10.Jia, H. P., Kline, J. N., Penisten, A., Apicella, M. A., Gioannini, T. L., Weiss, J., and McCray, P. B., Jr. (2004) Am. J. Physiol. 287 L428–L437 [DOI] [PubMed] [Google Scholar]
- 11.Akashi, S., Ogata, H., Kirikae, F., Kirikae, T., Kawasaki, K., Nishijima, M., Shimazu, R., Nagai, Y., Fukudome, K., Kimoto, M., and Miyake, K. (2000) Biochem. Biophys. Res. Commun. 268 172–177 [DOI] [PubMed] [Google Scholar]
- 12.Akashi, S., Saitoh, S., Wakabayashi, Y., Kikuchi, T., Takamura, N., Nagai, Y., Kusumoto, Y., Fukase, K., Kusumoto, S., Adachi, Y., Kosugi, A., and Miyake, K. (2003) J. Exp. Med. 198 1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohnishi, T., Muroi, M., and Tanamoto, K. (2003) Clin. Diagn. Lab. Immunol. 10 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teghanemt, A., Prohinar, P., Gioannini, T. L., and Weiss, J. P. (2007) J. Biol. Chem. 282 36250–36256 [DOI] [PubMed] [Google Scholar]
- 15.Re, F., and Strominger, J. L. (2003) J. Immunol. 171 5272–5276 [DOI] [PubMed] [Google Scholar]
- 16.Inohara, N., and Nunez, G. (2002) Trends Biochem. Sci. 27 219–221 [DOI] [PubMed] [Google Scholar]
- 17.Ohto, U., Fukase, K., Miyake, K., and Satow, Y. (2007) Science 316 1632–1634 [DOI] [PubMed] [Google Scholar]
- 18.Teghanemt, A., Zhang, D., Levis, E. N., Weiss, J. P., and Gioannini, T. L. (2005) J. Immunol. 175 4669–4676 [DOI] [PubMed] [Google Scholar]
- 19.Re, F., and Strominger, J. L. (2002) J. Biol. Chem. 277 23427–23432 [DOI] [PubMed] [Google Scholar]
- 20.Visintin, A., Mazzoni, A., Spitzer, J. A., and Segal, D. M. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 12156–12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen, G. E., Kennedy, M. N., Visintin, A., Mazzoni, A., Leifer, C. A., Davies, D. R., and Segal, D. M. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 3919–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viriyakosol, S., Tobias, P. S., and Kirkland, T. N. (2006) J. Biol. Chem. 281 11955–11964 [DOI] [PubMed] [Google Scholar]
- 23.Kennedy, M. N., Mullen, G. E., Leifer, C. A., Lee, C., Mazzoni, A., Dileepan, K. N., and Segal, D. M. (2004) J. Biol. Chem. 279 34698–34704 [DOI] [PubMed] [Google Scholar]
- 24.Teghanemt, A., Re, F., Prohinar, P., Widstrom, R. L., Gioannini, T. L., and Weiss, J. P. (2008) J. Biol. Chem. 283 1257–1266 [DOI] [PubMed] [Google Scholar]
- 25.Viriyakosol, S., Tobias, P. S., Kitchens, R. L., and Kirkland, T. N. (2001) J. Biol. Chem. 276 38044–38051 [DOI] [PubMed] [Google Scholar]
- 26.Visintin, A., Halmen, K. A., Latz, E., Monks, B. G., and Golenbock, D. T. (2005) J. Immunol. 175 6465–6472 [DOI] [PubMed] [Google Scholar]
- 27.Gioannini, T. L., Zhang, D., Teghanemt, A., and Weiss, J. P. (2002) J. Biol. Chem. 277 47818–47825 [DOI] [PubMed] [Google Scholar]
- 28.Yang, H., Young, D. W., Gusovsky, F., and Chow, J. C. (2000) J. Biol. Chem. 275 20861–20866 [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi, T., Muroi, M., and Tanamoto, K. (2001) J. Immunol. 167 3354–3359 [DOI] [PubMed] [Google Scholar]
- 30.Shi, X., and Jarvis, D. L. (2007) Curr. Drug Targets 8 1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright, C. S., Li, S. C., and Rastinejad, F. (2000) J. Mol. Biol. 304 411–422 [DOI] [PubMed] [Google Scholar]
- 32.Wright, C. S., Mi, L. Z., Lee, S., and Rastinejad, F. (2005) Biochemistry 44 13510–13521 [DOI] [PubMed] [Google Scholar]
- 33.Wright, C. S., Zhao, Q., and Rastinejad, F. (2003) J. Mol. Biol. 331 951–964 [DOI] [PubMed] [Google Scholar]
- 34.Derewenda, U., Li, J., Derewenda, Z., Dauter, Z., Mueller, G. A., Rule, G. S., and Benjamin, D. C. (2002) J. Mol. Biol. 318 189–197 [DOI] [PubMed] [Google Scholar]
- 35.Friedland, N., Liou, H. L., Lobel, P., and Stock, A. M. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2512–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangloff, M., and Gay, N. J. (2004) Trends Biochem. Sci 29 294–300 [DOI] [PubMed] [Google Scholar]
- 37.Gruber, A., Mancek, M., Wagner, H., Kirschning, C. J., and Jerala, R. (2004) J. Biol. Chem. 279 28475–28482 [DOI] [PubMed] [Google Scholar]
- 38.Viriyakosol, S., McCray, P. B., Ashbaugh, M. E., Chu, J., Jia, H. P., Weiss, J., and Kirkland, T. N. (2006) Hybridoma 25 349–357 [DOI] [PubMed] [Google Scholar]