Abstract
Protein kinase A (PKA) or cAMP-dependent protein kinase (cAPK) mediates the synergistic effects of cAMP- and glucocorticoid (GC)-induced apoptosis in lymphoid cells. Using two human acute lymphoblastic leukemia cell (CEM) clones with respective GC-sensitive and GC-resistant phenotypes, we discovered that the PKA regulatory subunit isoform RIIβ is preferentially expressed in the GC-sensitive clone C7–14 cells, whereas other intracellular cAMP receptors, including the exchange proteins directly activated by cAMP (Epac), are expressed at similar levels in both GC-sensitive and GC-resistant clones. High RIIβ expression level in C7–14 cells is associated with elevated total PKA cellular activity and cAMP sensitivity, which consequently lead to an increased basal PKA activity. cAMP analogs that selectively activate type II PKA recapitulate the effects of forskolin of promoting apoptosis and antagonizing AKT/PKB activity in both GC-sensitive and GC-resistant clones, whereas type I PKA-selective agonists do not. Furthermore, down-regulation of RIIβ leads to increased AKT/PKB activation and enhanced GC resistance in C7–14 cells. These results demonstrate that PKA RIIβ is responsible for increased GC sensitivity, critical for cAMP-mediated synergistic cell killing in CEM cells, and may represent a novel therapeutic target for GC-resistant lymphoid malignancy.
Glucocorticoids (GCs)3 induce apoptosis and/or cell growth arrest in lymphoid cells and have been used widely as a mainstay therapy for lymphoid malignancies, especially for acute lymphoblastic leukemia (ALL) (1). Some ALL patients develop resistance during therapy (secondary resistance) or without prior use of GC (primary resistance), and either form coincides with a poor prognosis (2–5). Although extensive studies have revealed that defects in GC-GR signaling pathways, such as impaired GR expression, GR mutation/isoform variants, and target gene expression, can contribute to GC resistance (6), the molecular basis of most GC resistance is still poorly understood (7). GC resistance of several leukemia cell lines can be overcome by increasing cellular cAMP levels (8). Results from our group show that the proapoptotic effect of cAMP is mediated by the classic intracellular cAMP receptor, protein kinase A (PKA)/cAMP-protein kinase (cAPK), and not by the newly discovered exchange protein directly activated by cAMP (Epac) (9).
PKA is a serine-threonine kinase that mediates cAMP regulation for a variety of cellular processes (10). The PKA holoenzyme contains two catalytic (C) subunit and two regulatory (R) subunits. There are two major isoforms of PKA, designated as PKA(I) and PKA(II), due exclusively to differences in the R subunits, RI and RII, that interact with an identical C subunit (11). Four different R subunit genes, RIα (12), RIβ (13), RIIα (14), and RIIβ (15), have been identified. Three C subunit genes, Cα, Cβ, and Cγ, have also been discovered. However, preferential expression of any of these C subunits with either RI or RII has not been found (16). The mechanism underlying the synergy between PKA and GC is poorly understood. It is not clear which PKA isoform(s) mediates the synergistic effects of cAMP on GC-induced apoptosis, nor is it completely understood upon which pathway(s)/effector(s) PKA and GC converge to exert their effects.
In this study, we used human GC-sensitive and -resistant CEM cells as model systems to investigate mechanisms of PKA potentiated apoptosis. We report that RIIβ subunit of PKA is reduced in GC-resistant cells, and this reduction/condition is partially responsible for the decreased GC sensitivity. We demonstrate that activation of PKA(II), but not PKA(I), recapitulates the synergistic proapoptotic effect of cAMP through the inhibition of the AKT/MCL-1 prosurvival pathway.
MATERIALS AND METHODS
Reagents—Dexamethesome and shRNA plasmids were purchased from Sigma-Aldrich. Forskolin was purchased from Alexis Biochemicals (San Diego, CA). Rp-cAMP and 2me, an Epac activator, 8-piperidinoadenosine-cAMP, 8-hexylaminoadenosinecAMP, N6-mono-tert-butylcarbamoyladenosinecAMP, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole-3′,5′-cyclic monophosphorothioate Sp-isomer, and RP-8-CPTcAMPS were purchased from BIOLOG Life Science Institute (Bremen, Germany). Triciribine (TCN) was obtained from the NCI, National Institutes of Health, Developmental Therapeutics program. AlmarBlue dye was purchased from BIOSOURCE (Camarillo, CA).
Cell Culture—Human CEM cell lines C7–14 and C1–15 cells were grown in RPMI 1640 supplemented with 5% fetal bovine serum (Invitrogen). The human CEM cell line was isolated from a patient with acute lymphoblastic leukemia. Subclone C7–14, sensitive to GC-evoked apoptosis, was derived from the original sensitive C7 clone. Subclone C1–15, which was derived from the original resistant C1 clone, remains resistant to GC.
Stable shRNA Transfection of C7–14 Cells by Lentiviral Infection—Lentiviral supernatant was produced by transient transfection of HEK293T cells (ATCC, Manassas, VA). A total of 1×106 cells were seeded in a 6-well plate 24 h before transfection. Transfection was performed by the Lipofectamine method (Invitrogen), according to the manufacturer's instructions. Plasmids used were the short hairpin RNAs in pLKO.1-puro vector for 1.0 μg/well in a 6-well plate (Sigma-Aldrich). The viral-containing supernatants were harvested 48 h after transfection and filtered through a 0.45-μm filter unit. To transduce CEM cells with lentivirus shRNAs, logarithmically growing C7–14 cells were seeded at a density of 2×105 cells/well in 6-well plates. 0.5 ml of lentivirus suspension and 8 μg/ml of Polybrene were added to RPMI with 5% fetal bovine serum in a total volume of 1 ml. Cells were allowed to incubate at 37 °C for 12 h before removing the medium and replacing with 2 ml of fresh RPMI for expansions of the transductants. 24 h after lentiviral infection, cells were selected with puromycin at 2.5 μg/ml for another 5 days before further experiments.
PKA Activity Assay—A radioisotopic method was used to measure PKA activity in the cell lysate. The kinase reaction mixture (50 μl) contained 50 mm Mops (pH 7.0), 10 mm MgCl2, 0.25 mg/ml bovine serum albumin, 0.1 mm peptide substrate (kemptide), and 0.1 mm ATP at 100 cpm/pmol, cell lysate containing 20 μg total proteins, and various concentrations of cAMP. The reaction was pre-equilibrated at room temperature for 10 min and initiated by adding the peptide substrate. Following a 10-min incubation, aliquots (45 μl) were withdrawn, spotted onto discs of Whatman P81 paper, and immediately immersed in 75 mm phosphoric acid (10–20 ml/sample) to terminate the reaction. After washing three times in phosphoric acid and once with ethanol, the discs were dried under a heating lamp. Radioactivity was measured by liquid scintillation spectrometry. Background counts from reactions that did not contain kemptide were subtracted.
Reverse Transcription-PCR (RT-PCR) Analysis—Cells (5 × 106) were lysed in TRIzol, RNA extraction was performed according to a standard protocol supplied by the manufacturer (Invitrogen, Inc.), and pellets were resuspended in RNase-free water. The cDNA was transcribed with a Superscript RT III based-protocol.
Immunoblotting Analysis—Protein concentration of cell lysates was assayed with the Bradford protein assay (Bio-Rad). Equal amounts of protein (5–30 μg) were loaded onto 10% SDS-polyacrylamide mini-gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes. After being blocked overnight in 5% milk in Tris-buffered saline-Tween, blots were incubated with primary antibodies for 1.5 h followed by horseradish peroxidase-conjugated secondary antibody (1:4,000) for 45 min. Antigen-antibody complexes were detected by enhanced chemiluminescence.
AlamarBlue Cell Viability Assay—Cell growth was determined by either classic trypan blue assay or AlamarBlue assay. Cells were seeded at a density of 10,000 cells (100 μl) per well in a 96-well plate. The plates were incubated with drugs for 24–72 h.10 μl of AlamarBlue was added to each well and incubated for 4 h at 37 °C. AlamarBlue is a fluorescent substrate reduced by mitochondrial enzyme activity in viable cells. Fluorescence intensity was determined using a Molecular Devices plate reader with an excitation filter centered on 530 nm and an emission filter centered on 590 nm. Error bars in Figs. 1, 2, 3, 4 indicate one standard deviation.
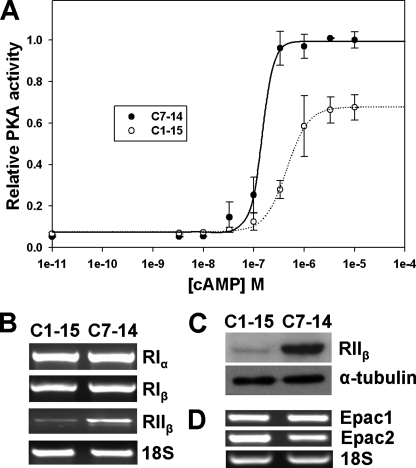
FIGURE 1.
Levels of PKA activity and isoform expression in C7–14 and C1–15 cells. A, cellular PKA activity as a function of cAMP concentration added to cell lysate. B, mRNA expression levels of PKA regulatory subunits measured by RT-PCR. C, RIIβ protein expression levels measured by Western blot. D, Epac1 and Epac2 mRNA expression levels measured by RT-PCR.
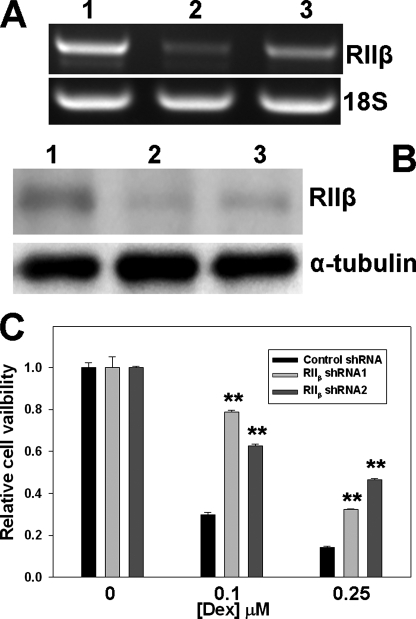
FIGURE 2.
Suppression of PKA RIIβ subunit expression by shRNAs reduces Dex sensitivity in C7–14 cells. A, repression of RIIβ mRNA expression by RIIβ-specific shRNAs as measured by RT-PCR. B, inhibition of RIIβ protein expression levels by RIIβ-specific shRNAs as measured by Western blot. Lane 1, control shRNA; lane 2, RIIβ shRNA1; and lane 3, RIIβ shRNA2. C, cell viability of C7–14 cells transduced with control or RIIβ-specific shRNAs in the presence of various Dex concentrations. p values were indicated by asterisks (relative to control shRNA); two symbols, p < 0.01.
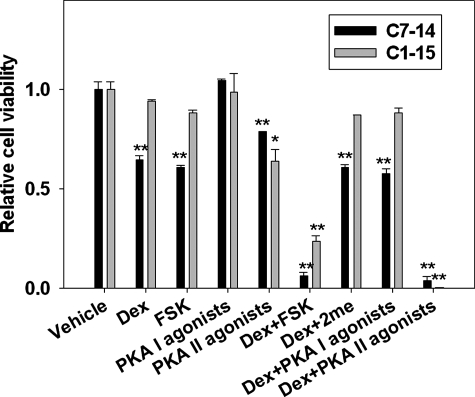
FIGURE 3.
Activation of PKA(II) sensitizes CEM cells to GC treatment. Cell viability of C7–14 and C1–15 cells after treatment with Dex and Dex in combination with cAMP agonists selective for PKA(I), PKA(II), and Epac was measured. FSK, forskolin. p values were indicated by asterisks (relative to vehicle) and pound signs (relative to Dex treatment); single symbol, p < 0.05; two symbols, p < 0.01. Compound concentrations used are as following: Dex, 0.1 μm for C7–14 and 1.00 μm for C1–15; forskolin, 10 μm; PKA agonists, 50 μm; 2me, 100 μm.
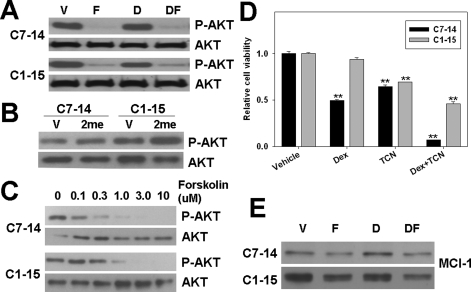
FIGURE 4.
Activation of PKA inhibits AKT activation and MCL-1 levels in CEM cells. A, levels of phosphorylated AKT in response to Dex and/or forskolin treatment measured by Western blot using AKT phospho-specific antibodies. B, levels of phosphorylated AKT in response to 8-CPT-2′-O-Me-cAMP (100μm) treatment. C, levels of phosphorylated AKT as a function of forskolin concentration. D, cell viability of CEM cells after treatment with AKT inhibitor TCN and/or Dex. p values were indicated by asterisks (relative to vehicle) and pound signs (relative to Dex treatment); two symbols, p < 0.01. E, levels of MCL-1 protein expression after Dex (0.1 μm for C7–14 and 1.00 μm for C1–15) and/or forskolin treatment as measured by Western blot using MCL-1-specific antibodies. D, dexamethasone; F, forskolin; and DF, dexamethasone + forskolin. Total Akt level (as shown in panel A) was used as a loading control. Compound concentrations used are as following unless specifically indicated: Dex, 0.1 μm for C7–14 and 1.00 μm for C1–15; forskolin, 10 μm; 2me, 100 μm; TCN, 5 μm.
RESULTS
PKA Is Less Sensitive to cAMP-induced Activation in the GC-resistant Leukemia Clone—We previously reported that total cellular PKA activity under saturating cAMP concentration was lower in GC resistance C1–15 cells than in GC-sensitive C7–14 cells (9). To explore the mechanism of the apparent differential PKA activities in CEM cells, we measured cellular PKA activity as a function of cAMP concentration in vitro using a sensitive radioactive method (17). In the absence of exogenously added cAMP, cellular lysates from C1–15 and C7–14 gave minimal PKA activities, suggesting that that most PKA enzymes exist in the inhibited holoenzyme complex due to the significant dilution of the endogenous basal cAMP during the preparation of cell lysates (Fig. 1A). As expected, cellular PKA activities increased in a sigmoid manner with increasing cAMP concentrations. In addition, the cellular PKA activity under saturating cAMP concentration in GC-resistant C1–15 cells was lower than that in GC-sensitive C7–14 cells, confirming our earlier study (9). Activation of PKA in GC-resistant C1–15 cells was significantly less sensitive to cAMP than that in GC-sensitive C7–14 cells (Fig. 1A). The cAMP concentration required to activate 50% of PKA (AC50) in C1–15 cells was 0.45 ± 0.02 μm, whereas the AC50 in C7–14 cells was 0.15 ± 0.01 μm. It is known that the resting physiological cAMP concentrations are within the range of 0.1–1.0 μm (18). Therefore, we can conclude from Fig. 1A that basal PKA activity in GC-sensitive cells is higher than that in GC-resistant cells at physiological conditions.
Reduced Expression of PKA RIIβ in GC-resistant Leukemia Cell Line—There are four human PKA R subunit isoforms, RIα, RIβ, RIIα and RIIβ, each with distinct cellular localization and cAMP affinity. The observed difference in cAMP sensitivity in CEM cells could arise from the differential expression of PKA R isoforms in these cells. Therefore, we compared expression levels of all four PKA R isoforms by RT-PCR between C1–15 and C7–14 cells. Although the mRNA levels of RIα and RIβ were similar, the expression of RIIβ in C1–15 cells was dramatically reduced as compared with that in C7–14 cells (Fig. 1B). No expression of RIIα was detectable in either C1–15 or C7–14 cells (data not shown). We confirmed the differential expression of RIIβ at the protein level in C1–15 and C7–14 cells using a specific RIIβ antibody (Fig. 1C). In addition to PKA R subunits, the exchange proteins directly activated by cAMP, Epac1 and Epac2, are the other known intracellular cAMP receptors. Their levels of expression may also affect PKA cAMP sensitivity indirectly. We ruled out this possibility. As shown in Fig. 1D, both Epac1 and Epac2 were expressed at similar levels in C1–15 and C7–14 cells.
Decreased RIIβ Expression Enhances GC Resistance—To determine whether reduced expression of RIIβ is a contributing factor for GC resistance, we suppressed the RIIβ expression in C7–14 cells using lentivirus-mediated RIIβ-specific shRNAs. GC sensitivity was examined thereafter. To eliminate the potential off-target effects, two independent RIIβ-specific shRNA constructs, as well as a non-targeting control shRNA construct, were used. RT-PCR showed that RIIβ mRNA levels were significantly reduced by both RIIβ-specific shRNAs (Fig. 2A). RIIβ knockdown in C7–14 cells was further confirmed by Western blot, using RIIβ-specific antibodies (Fig. 2B). When these cells were tested for their responses to GC-mediated cell killing, RIIβ knockdown cells were found to be more resistant to dexamethasone (Dex) than C7–14 cells transduced with a control lentivirus shRNA (Fig. 2C). These results suggest that the level of RIIβ expression is a significant contributing factor for GC sensitivity in CEM cells.
Activation of PKA(II), Not PKA(I), Mediates the Synergistic Effects of cAMP in Potentiating GC-evoked Apoptosis—The finding that RIIβ is differentially expressed in C1–15 and C7–14 cells suggests that RIIβ may be responsible for the observed differences in cAMP sensitivity, as well as the synergistic killing effect of cAMP and GC. To test this hypothesis, selective agonists for cAMP receptors were used in combination treatment with GC. As expected, Epac activation by specific agonist 2me did not promote GC-evoked apoptosis (Fig. 3). Selective PKA I or II agonist pairs were used to activate PKA(I) or PKA(II), respectively (23, 24). As shown in Fig. 3, activation of PKA(II) by N6-mono-tert-butylcarbamoyladenosine 3′, 5′-cyclic monophosphate/5, 6-dichlorobenzimi-dazole riboside 3′, and 5′-cyclic monophosphoro-thioate Sp-isomer, and not PKA(I) by 8-piperidinoadenosine 3′, 5′-cyclic monophosphate/8-hexylaminoadenosine 3′, and 5′-cyclic monophosphate, overcame GC resistance in C1–15 cells and also showed synergistic killing effects with GC in C7–14 cells, a result that recapitulated the effects of forskolin. Similar to forskolin, PKA(II) agonists also showed a slightly growth inhibitory effects on both CEM cells. These results suggest that cAMP acts through PKA(II) to promote GC-evoked apoptosis.
Inhibition of the AKT/MCL-1 Pathway, Downstream of PKA, Mediates the Synergistic Apoptotic Effect between cAMP and GC in CEM Cells—Although the connection between cAMP and GC in synergistic killing of ALL cells has been established for more than a decade, the underlying mechanism is still elusive. Our earlier study indicates that the proapoptotic effect of cAMP is mediated by PKA (9) and that activation of PKA can lead to potent inhibition of AKT/PKB (19), which is known for its antiapoptotic role in cancer drug resistance. To determine whether inhibition of AKT plays a role in mediating the synergistic proapoptotic effect between cAMP and GC, we tested the effects of treating CEM cells with forskolin and/or Dex on AKT activation by monitoring the levels of phosphorylated AKT. Western blot analysis using antibodies specific for phospho-AKT showed that forskolin led to a significant decrease in AKT phosphorylation, whereas Dex alone had no effect (Fig. 4A). Inhibition of AKT phosphorylation by forskolin was mediated by PKA as activation of Epac by an Epac-specific cAMP analog, 2me, enhanced AKT phosphorylation in both CEM cell lines (Fig. 4B). Interestingly, the basal AKT phosphorylation level is higher in GC-resistant C1–15 cells, which is in agreement with the fact that C1–15 has a lower basal PKA activity. Forskolin suppressed AKT phosphorylation in a dose-dependent manner in both CEM clones, and inhibition of AKT phosphorylation was less sensitive to forskolin in C1–15 cells than that in C7–14 cells (Fig. 4C), consistent with our finding that PKA is less sensitive to cAMP activation in C1–15 cells (Fig. 1A). To determine whether AKT inhibition contributes to the synergistic apoptotic effect between cAMP and GC, we treated CEM cells with an AKT-specific inhibitor, TCN, with or without Dex cotreatment. As shown in Fig. 4D, TCN, like forskolin, synergistically promoted GC-evoked apoptosis in both CEM cell lines. These results confirmed that AKT inhibition is critical for the proapoptotic effect of PKA.
Because activation of mTOR/MCL-1, downstream of AKT, has been suggested to be a major contributing factor for GC resistance in ALL, we hypothesized that activation of PKA may overcome GC resistance by down-regulating the mTOR/MCL-1 pathway through inhibiting AKT. To test this hypothesis, we treated both C1–15 and C7–14 cells with forskolin and/or Dex. The expression levels of MCL-1 in response to forskolin/Dex treatments were then examined by Western blot analysis using MCL-1-specific antibodies. Our study showed that C1–15 cells expressed a higher MCL-1 level as compared with C7–14 cells and forskolin but not Dex significantly reduced MCL-1 protein levels in both CEM cell lines (Fig. 4E).
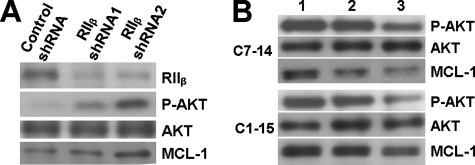
RIIβ/PKA(II) Activation Inhibits AKT/MCL-1 Pathway—Since low expression level of RIIβ is correlated to high AKT/MCL-1 pathway activity, we hypothesize that RIIβ contributes to GC sensitivity by down-regulating AKT/MCL-1 pathway activity. To test this hypothesis, we knocked down RIIβ expression by shRNAs in C7–14 cells and found that AKT phosphorylation and MCL-1 protein levels were increased (Fig. 5A) in response to RIIβ reduction. These results further demonstrate that high levels of RIIβ play an important role in repressing the AKT/MCL-1 pathway activity in GC-sensitive C7–14 cells. Consistent with this notion, when CEM cells were treated with PKA isoform-specific agonists, the levels of phosphorylated AKT and MCL-1 protein expression were reduced by PKA(II)-specific agonists but not PKA(I)-specific agonists (Fig. 5B). Taken together, these results suggest that RIIβ/PKA(IIβ) play a critical role in regulating the AKT/MCL-1 pathway and in determining the GC-sensitivity in CEM cells.
FIGURE 5.
RIIβ/PKA(II), but not PKA(I), regulates AKT/MCL-1 in CEM cells. A, levels of phosphorylated AKT (P-AKT) and MCL-1 protein in C7–14 cells transduced with control or RIIβ-specific shRNAs. B, levels of phosphorylated AKT and MCL-1 protein in CEM cells after treatment with cAMP agonists selective for PKA(I) or PKA(II) Lane 1, vehicle control; lane 2, PKA(I) agonists; and lane 3, PKA(II) agonists.
DISCUSSION
cAMP inhibits cell growth and synergistically promotes GC-dependent apoptosis in lymphoid leukemia cells. Induction of Bcl-2-interacting mediator of cell death (BIM), inhibition of the hedgehog pathway, and activation of p38 MAPK (mitogen-activated protein kinase) have been suggested as playing roles in the synergistic proapoptotic effect between cAMP and GC (9, 20, 21), but the precise mechanism and determinants for the particular cAMP-mediated synergistic effects are not clear. Our previous studies document PKA, and not Epac, as the mediator of the synergistic proapoptotic effects of cAMP (9). We report here that PKA activation in the GC-sensitive C7–14 cells is more sensitive to cAMP than that in GC-resistant C1–15 cells. Among all known intracellular cAMP receptors, we also find a close connection between PKA cAMP sensitivity and levels of RIIβ expression. The expression level of RIIβ in C7–14 cells was substantially higher than that of C1–15. RIIβ is normally present at high levels in adipose, brain, and hematopoietic tissues (22). CEM cell clones are at the early stages of T-lymphocyte maturation (23). CEM C7–14 and C1–15 are subclones of CEM C7 and C1, respectively; these were obtained by soft agar cloning, without selection, from the original CEM line (24). That line was grown from peripheral blood lymphoblasts of a child with ALL, who was in relapse after treatment that included GC. The clones are karyotypically alike. Thus it seems likely that the shift in RIIβ expression and consequent GC resistance had been selected for in vivo, prior to in vitro culturing.
In agreement with our finding of the involvement of RIIβ in regulating GC sensitivity in ALL cells, activation of PKA(II) and not PKA(I) recapitulates the effects of the adenyl cyclase stimulator forskolin; selective PKA(II) agonists inhibit cell growth and strongly synergize with GC to induce cell death in both GC-sensitive and GC-resistant CEM cells, whereas selective PKA(I) agonists have no effects. PKA(I) and PKA(II) have been shown to function distinctly in cell proliferation and differentiation (10). PKA(I) expression is induced when cells undergo proliferation due to physiological stimulation, and overexpression of PKA(I) has been observed in human cancer cell lines and primary tumors. On the contrary, high expression levels of PKA(II), particularly PKA(IIβ) were associated with differentiation and growth arrest. Our study suggests that PKA(II) may also play a role in drug resistance in cancer chemotherapy; PKA(II), when activated, not only inhibits cell growth but also promotes GC-induced cell apoptosis in CEM cells. Resistance in response to GC is a strong prognostic marker in leukemia for general sensitivity to chemotherapy. Thus PKA(II) agonists, as a sensitizer of GC, potentially represent a new therapeutic option for GC-resistant ALL patients.
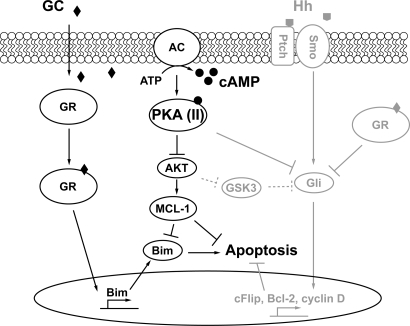
Although PKA(II) activation alone only slows cell growth, it strongly sensitizes cells to GC-dependent apoptosis. Defective apoptosis is a hallmark of cancer (26), whereas the ability to activate the apoptotic program is an important determinant of efficacy for many anticancer drugs (27). We therefore investigated the AKT/mTOR/MCL-1 signaling pathway, which is known to be affected by cAMP/PKA. High level expression of the antiapoptotic MCL-1 is correlated with in vitro GC resistance in primary B-lineage leukemia cells (28). The mTOR/MCL-1 pathway is critical for the GC sensitivity in several leukemia cell lines, including CEM-C1 cells (29). In a recent report concerning the CEM subclone C1–15, the GC-resistant clone we study herein, direct inhibition of mTOR was shown to sensitize the cells to GC (30). In B cells, activation of PKA leads a reduced expression of MCL-1 protein (31). Consistent with these reports, we found that PKA(II) activation inhibits the AKT/MCL-1 pathway, whereas reduced RIIβ expression up-regulates AKT/MCL-1 in CEM cells. Since MCL-1 inhibition has been indicated to be a major factor for GC resistance, these results suggest that down-regulation AKT/MCL-1 by PKA(II) may play a important role for mediating the synergy killing effects between cAMP and GC. Taken together, these studies suggest that PKA(II) may sensitize cells to GC-induced apoptosis by inhibiting the AKT/MCL-1 pathway. These results, combined with our earlier observations (9), suggest a mechanism in which cAMP/PKA exerts its synergism with GC through regulating AKT/MCL-1/Bim and hedgehog signaling in CEM cells (Fig. 6). Although an understanding of the precise mechanism by which cAMP/PKA(II) inhibits the AKT/MCL-1 pathway in CEM cells will require further study, we note that cAMP is capable of inhibiting translocation of PDK1 to the plasma membrane and the lipid kinase activity of phosphatidylinositol 3-kinase (25), both of which could contribute to inhibition of AKT activity.
FIGURE 6.
Mechanism of PKA(II)-mediated synergistic killing effect of cAMP and glucocorticoid. In this study, we reveal a mechanism, represented by the schematic diagram in black, showing that activation PKA(II) by cAMP converges with the GC-mediated pathway eventually affecting downstream Bim expression, to synergistically regulate cellular apoptosis in ALL cells. In addition, our earlier studies (highlighted in gray) show that suppressing hedgehog (Hh) pathway is another important component of the mechanism of synergistic cell killing of lymphoid cells by GCs and cAMP. BIM, Bcl-2-interacting mediator of cell death. GC, glucocorticoid; AC, adenylate cyclase; Ptch, Patched; Smo, Smoothened.
In conclusion, we show that RIIβ/PKA(II) is the major mediator of cAMP to promote GC-induced apoptosis in ALL cells and that activation of RIIβ/PKA(II) inhibits the AKT/mTOR/MCL-1 prosurvival pathway. Therefore, RIIβ/PKA(II) represent a novel therapeutic target for overcoming GC-resistant in ALL patients.
This work was supported, in whole or in part, by National Institutes of Health Grants GM066170 (to X. C.) and CA041407 to (E. B. T.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GC, glucocorticoid; GR, glucocorticoid receptor; ALL, acute lymphoblastic leukemia; Epac, exchange protein activated by cAMP; Dex, dexamethasone; PKA, protein kinase A; RT-PCR, reverse transcription-PCR; Bcl-2, B cell lymphoma 2; 2me, 8-pCPT-2′-O-Me-cAMP; RP-8-CPTcAMPS, 8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate Rp-isomer; mTOR, mammalian target of rapamycin; shRNA, short hairpin RNA; TCN, triciribine.
References
- 1.Kofler, R. (2000) Histochem. Cell Biol. 114 1–7 [DOI] [PubMed] [Google Scholar]
- 2.Kofler, R., Schmidt, S., Kofler, A., and Ausserlechner, M. J. (2003) J. Endocrinol. 178 19–27 [DOI] [PubMed] [Google Scholar]
- 3.Hongo, T., Yajima, S., Sakurai, M., Horikoshi, Y., and Hanada, R. (1997) Blood 89 2959–2965 [PubMed] [Google Scholar]
- 4.Kaspers, G. J., Pieters, R., Van Zantwijk, C. H., van Wering, E. R., Van Der Does-Van Den Berg, and Veerman, A. J. (1998) Blood 92 259–266 [PubMed] [Google Scholar]
- 5.Moalli, P. A., and Rosen, S. T. (1994) Leuk. Lymphoma 15 363–374 [DOI] [PubMed] [Google Scholar]
- 6.Tissing, W. J., Meijerink, J. P., den Boer, M. L., and Pieters, R. (2003) Leukemia (Basingstoke) 17 17–25 [DOI] [PubMed] [Google Scholar]
- 7.Klumper, E., Pieters, R., Veerman, A. J., Huismans, D. R., Loonen, A. H., Hahlen, K., Kaspers, G. J., van Wering, E. R., Hartmann, R., and Henze, G. (1995) Blood 86 3861–3868 [PubMed] [Google Scholar]
- 8.Medh, R. D., Saeed, M. F., Johnson, B. H., and Thompson, E. B. (1998) Cancer Res. 58 3684–3693 [PubMed] [Google Scholar]
- 9.Ji, Z., Mei, F. C., Johnson, B. H., Thompson, E. B., and Cheng, X. (2007) J. Biol. Chem. 282 37370–37377 [DOI] [PubMed] [Google Scholar]
- 10.Cho-Chung, Y. S., Pepe, S., Clair, T., Budillon, A., and Nesterova, M. (1995) Crit. Rev. Oncol.-Hematol. 21 33–61 [DOI] [PubMed] [Google Scholar]
- 11.Taylor, S. S., Buechler, J. A., and Yonemoto, W. (1990) Annu. Rev. Biochem. 59 971–1005 [DOI] [PubMed] [Google Scholar]
- 12.Lee, D. C., Carmichael, D. F., Krebs, E. G., and McKnight, G. S. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 3608–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clegg, C. H., Cadd, G. G., and McKnight, G. S. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 3703–3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott, J. D., Glaccum, M. B., Zoller, M. J., Uhler, M. D., Helfman, D. M., McKnight, G. S., and Krebs, E. G. (1987) Proc. Natl. Acad. Sci. U. S. A. 84 5192–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahnsen, T., Hedin, L., Kidd, V. J., Beattie, W. G., Lohmann, S. M., Walter, U., Durica, J., Schulz, T. Z., Schiltz, E., and Browner, M. (1986) J. Biol. Chem. 261 12352–12361 [PubMed] [Google Scholar]
- 16.Beebe, S. J., Oyen, O., Sandberg, M., Froysa, A., Hansson, V., and Jahnsen, T. (1990) Mol. Endocrinol. 4 465–475 [DOI] [PubMed] [Google Scholar]
- 17.Cheng, X., Phelps, C., and Taylor, S. S. (2001) J. Biol. Chem. 276 4102–4108 [DOI] [PubMed] [Google Scholar]
- 18.Beavo, J. A., Bechtel, P. J., and Krebs, E. G. (1974) Proc. Natl. Acad. Sci. U. S. A. 71 3580–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei, F. C., Qiao, J., Tsygankova, O. M., Meinkoth, J. L., Quilliam, L. A., and Cheng, X. (2002) J. Biol. Chem. 277 11497–11504 [DOI] [PubMed] [Google Scholar]
- 20.Zhang, L., and Insel, P. A. (2004) J. Biol. Chem. 279 20858–20865 [DOI] [PubMed] [Google Scholar]
- 21.Miller, A. L., Webb, M. S., Copik, A. J., Wang, Y., Johnson, B. H., Kumar, R., and Thompson, E. B. (2005) Mol. Endocrinol. 19 1569–1583 [DOI] [PubMed] [Google Scholar]
- 22.Brandon, E. P., Logue, S. F., Adams, M. R., Qi, M., Sullivan, S. P., Matsumoto, A. M., Dorsa, D. M., Wehner, J. M., McKnight, G. S., and Idzerda, R. L. (1998) J. Neurosci. 18 3639–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danel-Moore, L., Kawa, S., Kalmaz, G. D., Bessman, D., and Thompson, E. B. (1993) Leuk. Res. 17 501–506 [DOI] [PubMed] [Google Scholar]
- 24.Norman, M. R., and Thompson, E. B. (1977) Cancer Res. 37 3785–3791 [PubMed] [Google Scholar]
- 25.Kim, S., Jee, K., Kim, D., Koh, H., and Chung, J. (2001) J. Biol. Chem. 276 12864–12870 [DOI] [PubMed] [Google Scholar]
- 26.Hanahan, D., and Weinberg, R. A. (2000) Cell 100 57–70 [DOI] [PubMed] [Google Scholar]
- 27.Fesik, S. W. (2005) Nat. Rev. Cancer 5 876–885 [DOI] [PubMed] [Google Scholar]
- 28.Holleman, A., Cheok, M. H., den Boer, M. L., Yang, W., Veerman, A. J., Kazemier, K. M., Pei, D., Cheng, C., Pui, C. H., Relling, M. V., Janka-Schaub, G. E., Pieters, R., and Evans, W. E. (2004) N. Engl. J. Med. 351 533–542 [DOI] [PubMed] [Google Scholar]
- 29.Wei, G., Twomey, D., Lamb, J., Schlis, K., Agarwal, J., Stam, R. W., Opferman, J. T., Sallan, S. E., den Boer, M. L., Pieters, R., Golub, T. R., and Armstrong, S. A. (2006) Cancer Cell 10 331–342 [DOI] [PubMed] [Google Scholar]
- 30.Miller, A. L., Garza, A. S., Johnson, B. H., and Thompson, E. B. (2007) Cancer Cell Int. 7 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myklebust, J. H., Josefsen, D., Blomhoff, H. K., Levy, F. O., Naderi, S., Reed, J. C., and Smeland, E. B. (1999) J. Cell Physiol. 180 71–80 [DOI] [PubMed] [Google Scholar]