The presence of free-floating hyperechogenic material within the amniotic fluid in close proximity to the uterine cervix (Figures 1 (cover) and 2 and Videoclips S1 and S2) has been described previously in women with an episode of preterm labor1, in women with a history of preterm delivery or threatened preterm labor2, and in asymptomatic women at risk for spontaneous preterm delivery in the mid-trimester of pregnancy3. We have proposed the term amniotic fluid ‘sludge’ to refer to this sonographic finding and provided evidence that ‘sludge’ is an independent risk factor for impending preterm delivery, histological chorioamnionitis and microbial invasion of the amniotic cavity in patients with spontaneous preterm labor and intact membranes1. Moreover, amniotic fluid ‘sludge’ has been identified in asymptomatic women at risk for spontaneous preterm delivery in the mid-trimester of pregnancy and is also an independent risk factor for preterm prelabor rupture of membranes (PROM) and spontaneous preterm delivery3. To determine the nature of amniotic fluid ‘sludge’, the material collected under sonographic guidance was examined under the microscope and microbiological studies were performed.
Figure 1.

Rendered three-dimensional transvaginal ultrasound image demonstrating the presence of amniotic fluid ‘sludge’ in close proximity to the cervix.
Figure 2.
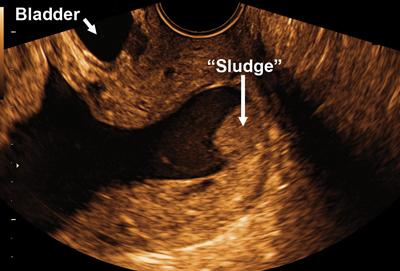
Two-dimensional ultrasound image showing amniotic fluid ‘sludge’ in a patient with a short cervix and a cervical funnel. acute necrotizing chorioamnionitis (Figure 5a) and acute funisitis (Figure 5b). The newborn was admitted to the neonatal intensive care unit and developed metabolic acidosis and respiratory distress syndrome that resolved in the first week of postnatal life. There was no evidence of pneumonia, the result of a neurosonogram was normal, andmicrobial cultures of the cerebrospinal fluid and blood were negative. However, the neonate was treated with ampicillin and gentamycin because of suspected sepsis. After 45 days, the infant was discharged home in good condition.
Case report
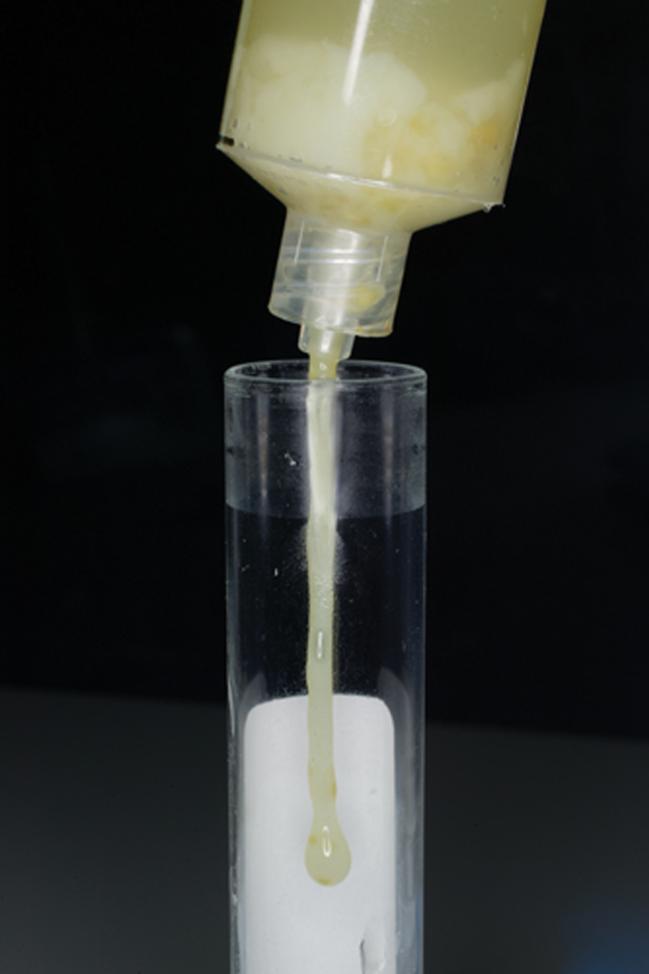
A 31-year-old woman, gravida 5 para 2, with a history of a sonographic short cervix at 25 weeks (8 mm), was admitted at 27 + 2 weeks of gestation because of cervical dilatation (2−3 cm) and bulging membranes. Transvaginal ultrasound revealed a cervical length of 0 mm and the presence of amniotic fluid ‘sludge’ in the portion of the amniotic sac protruding into the cervical canal (Figure 2 and Videoclip S2). A transabdominal amniocentesis to rule out intraamniotic infection/inflammation was performed and the amniotic fluid not used for diagnostic tests was used for research purposes. This was done in accordance with an institutional review board-approved protocol and the patient provided written informed consent at the time of enrollment, prior to the collection of the amniotic fluid samples. The amniotic fluid glucose concentration was 25 mg/dL, white blood cell count was 1 cell/mm3 and Gram stain was negative for bacteria. Amniotic fluid microbial cultures (for aerobic and anaerobic bacteria, genital mycoplasmas and viruses) were negative. Ten days later, the patient complained of cramping and was found to be in labor and dilated (8 cm) with the membranes prolapsed into the vagina. During the course of labor, the diagnosis of clinical chorioamnionitis was made based on the presence of fever, fetal tachycardia and a maternal white blood cell count of 15 600 cells/mm3. The decision was made to augment labor with oxytocin and to perform an amniotomy. After the membranes were cleaned with 10% povidone-iodine (Scrub Care®, Cardinal Health, IL, USA) a needle amniotomy was performed using an 18-gauge spinal needle and the amniotic fluid ‘sludge’ was aspirated under transabdominal ultrasound guidance. The gross characteristics of ‘sludge’ are displayed in Figure 3 and Videoclip 3. Amniotic fluid studies indicated that the glucose concentration was below 10 mg/dL, the white blood cell count was 19 650 cells/mm3, and the Gram stain showed Gram-positive cocci (Figure 4). The patient was treated with ampicillin and gentamycin for the clinical diagnosis of chorioamnionitis and progressed quickly to have a spontaneous vaginal delivery of a female infant weighing 1135 g, with Apgar scores at 1 and 5 min of 8 and 8. The amniotic fluid culture was positive for Mycoplasma hominis, Streptococcus mutans and Aspergillus flavus.
Figure 3.
Photograph showing the appearance of amniotic fluid ‘sludge’, aspirated during a transvaginal needle amniotomy under transabdominal ultrasound guidance.
Figure 4.
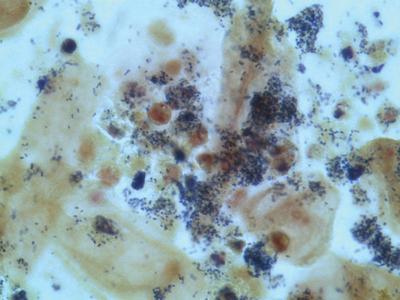
Gram stain of amniotic fluid ‘sludge’ demonstrating the presence of epithelial cells and many Gram-positive cocci; neutrophils are also evident.
Discussion
The observations reported herein describe the sonographic, gross appearance and microscopic findings of amniotic fluid ‘sludge’. At ultrasound examination, ‘sludge’ appears as free-floating hyperechogenic material in close proximity to the cervix (Figures 1 and 2 and Videoclips 1 and 2). The material resembles pus when examined with the naked eye (Videoclip 3). A Gram stain showed Gram-positive bacteria (Figure 4). The amniotic fluid culture was positive for Streptococcus mutans,Mycoplasma hominis and Aspergillus flavus. Collectively, these findings suggest that amniotic fluid ‘sludge’ can be an indicator of microbial invasion of the amniotic cavity and inflammation based on a markedly high amniotic fluid white blood cell count (19 650 cells/mm3, of which 95% were neutrophils). Several details of this case are noteworthy. First, the amniocentesis performed at the time of admission revealed no evidence of microbial invasion of the amniotic cavity or intra-amniotic inflammation. Yet, the second amniotic fluid sample obtained 10 days later, when sludge was retrieved, presented a completely different picture: there was evidence of severe inflammation detectable by the naked eye and infection by microscopic examination. The amniotic fluid was cloudy, thick and similar to pus. The amniotic fluid white blood cell count was markedly elevated and the cultures were positive for bacteria. What does thismean? The patient might have developed a new intra-amniotic infection during the 10 days that had elapsed between the first amniocentesis and the retrieval of the amniotic fluid ‘sludge’. Alternatively, it is possible that the amniotic cavity is compartmentalized so that a sample of the upper compartment (close to the uterine fundus) may not represent the microbiological and inflammatory state of the lower compartment. Indeed, previous studies conducted by our group have indicated that the amniotic fluid concentration of prostaglandins4 and cytokines/chemokines is higher in the lower compartment (which is in close proximity to the cervix) than in the upper compartment 5,6. The precise nature of the particulate material and why it is formed in a fluid cavity are also interesting. Microorganisms are not generally visible unless they form large colonies and this is considered unusual in the amniotic cavity. It is possible that progressive infection induces an intense inflammatory response and that the combination of microorganisms and the inflammatory cells (in this case, neutrophils) leads to the formation of the particulate material observed by sonographic examination. Previously, ultrasound has detected particulate material in the amniotic cavity 7–10 which has been attributed to the presence of meconium7 or vernix8. Free-floating particulate matter has also been described in cases of excessive desquamation of the skin in congenital ichthyosis11. Material that appears similar to what we have called amniotic fluid ‘sludge’ has been attributed to a blood clot in the amniotic cavity by others12, although there is no published evidence that this is the case. Kusanovic et al.3 report in this issue of the Journal that amniotic fluid ‘sludge’ may be observed in asymptomatic patients at risk for spontaneous preterm delivery in the mid-trimester of pregnancy and that such a finding is a risk factor for subsequent spontaneous preterm delivery, preterm PROM, microbial invasion of the amniotic cavity and histological chorioamnionitis. We propose that the detection of amniotic fluid ‘sludge’ represents a sign that microbial invasion of the amniotic cavity and an inflammatory process are in progress. Such an interpretation supports the view that intra-amniotic infection is chronic and subclinical in nature. The observation that the shorter the cervical length, the greater the likelihood of amniotic fluid ‘sludge’3, has been interpreted as an indication that intraamniotic infection/inflammation will eventually lead to a short cervix. Alternatively, patients with a short cervix may be at particularly high risk of developing an ascending infection13. It is possible that effacement leading to a short distance between the ectocervix and the membranes may disrupt the mucus plug and impair other components of the innate and/or adaptive immunity in the lower genital tract. Indeed, we have frequently observed that the mucus plug is disrupted or partially expelled in cases with a short cervix and ‘sludge’. Cervical length may be an important component of innate immunity by separating the microbial population normally present in the vagina and ectocervix from the chorioamniotic membranes. Thus, cervical shortening alone may predispose to intrauterine infection13,14. In addition, the mucus plug is a mechanical as well as a biochemical barrier to infection15−20. In fact, cervical mucus has antimicrobial properties16, 18−20 and the endocervical epithelium can produce antimicrobial peptides17. Thus, loss or disruption of the mucus plug because of cervical shortening may also predispose to intrauterine infection. Microorganisms have the capacity to cross intact membranes21, 22 and, therefore, if a large dose of microorganisms reaches the membranes, the risk of infection may increase. A major question is whether bacteria in the amniotic fluid are in planktonic form (single cells), organized in biofilms or both. In the context of microbial invasion, the host (mother and/or fetus) mounts an inflammatory response to protect her/himself. This process involves the delivery of inflammatory cells (e.g. neutrophils, monocytes) to the site of microbial invasion as well as the production of antimicrobial peptides and other mediators which can kill or injure bacteria. In turn, bacteria can protect themselves from the host response by changing their phenotype, aggregating themselves in building-like structures called ‘bacterial biofilms’ and generating a matrix to maintain them23−25. Biofilms make bacteria more resistant to the attack of white blood cells, natural or synthetic antibiotics and inflammatory mediators23−26. We have proposed that bacteria in the amniotic fluid, which contains natural antimicrobial peptides such as defensins27−29, can generate biofilms1. Such biofilms have been demonstrated in patients with similar sonographic images (particulate matter) in the biliary tract30−32. Bacteria in biofilms are less likely to elicit an inflammatory response33,34. Thus, the formation of biofilms in the amniotic cavity and/or membranes has important implications and would explain, in part, why intra-amniotic infection is chronic in nature. It could also explain why intra-amniotic infections are difficult to treat, since bacteria in biofilms are relatively resistant to antibiotic treatment24−26,35,36. The relative proportions of bacteria in planktonic form and those in a biofilm state may determine the probability of obtaining a positive culture of amniotic fluid. Planktonic bacteria are more likely to grow in culture than are bacteria in biofilm24, a finding that is well established in otitis media37,38.Many cases of otitis media with negative microbial cultures were attributed to viruses or non-microbial processes until the recent development of molecular microbiological techniques39,40 that allow the detection of bacteria using cultivation-independent methods41,42. The relative proportions of planktonic and biofilms will also determine, in part, the magnitude of the inflammatory response and even pregnancy outcome. Planktonic bacteria are more effective at eliciting an intense inflammatory response than are bacteria in biofilm33,34. Other factors determining the intensity of the inflammatory response are under genetic control; in the case of pregnancy, the genome of the fetus and/or the mother may play a role. It is becoming increasingly clear that the uterine cavity in the non-pregnant state is not sterile43; microorganisms are normally present on the surface of the endometrium44−46 and biofilms47−49 have been reported in these locations. Thus, a critical question is: why do bacteria in the endometrium elicit an inflammatory response which can lead to spontaneous miscarriage or preterm birth in some cases, while in others, bacteria and the host develop a pacific coexistence? We believe that the traditional view that the pregnant endometrium (decidua) is sterile also needs revision. It is likely that microorganisms are present at the time of implantation and that they remain on the surface of the endometrial cavity during the first trimester when fusion of the deciduas (capsularis and parietalis) occurs. The presence of bacteria on the endometrial surface may even be important in maintaining the local immunological state required for successful implantation. Only when commensal flora becomes invasive will pathological inflammation occur. Under these circumstances, implantation failure, spontaneous miscarriage, cervical insufficiency, preterm PROM and spontaneous preterm labor with intact membranes may occur43. In conclusion, we provide evidence that amniotic fluid ‘sludge’ detected by ultrasound reflects a severe intraamniotic infection-related inflammatory process. Such a complication of pregnancy may be subclinical and may not be detected without careful examination of the amniotic fluid.
Figure 5.


Histological sections of (a) the fetal membranes, demonstrating marked ‘acute necrotizing chorioamnionitis’ and (b) the umbilical cord, showing acute funisitis.
Acknowledgment
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
References
- 1.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM, Hassan S, Gomez R, Yoon BH, Chaiworapongsa T, Lee W, Mazor M. The prevalence and clinical significance of amniotic fluid “sludge” in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–352. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 2.Bujold E, Pasquier JC, Simoneau J, Arpin MH, Duperron L, Morency AM, Audibert F. Intra-amniotic sludge, short cervix, and risk of preterm delivery. J Obstet Gynaecol Can. 2006;28:198–202. doi: 10.1016/S1701-2163(16)32108-9. [DOI] [PubMed] [Google Scholar]
- 3.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, Khalek N, Camacho N, Hendler I, Mittal P, Friel LA, Gotsch F, Erez O, Than NG, Mazaki-Tovi S, Schoen ML, Hassan SS. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Gonzalez R, Baumann P, Behnke E, Rittenhouse L, Barberio D, Cotton DB, Mitchell MD. Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot Essent Fatty Acids. 1994;50:97–104. doi: 10.1016/0952-3278(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 5.Baumann P, Romero R, Gonzales R, Cotton DB, Mammen E. Evidence of topographic differences in amniotic fluid plasminogen activator/plasminogen activator inhibitor concentrations during spontaneous active labor at term. Am J Obstet Gynecol. 1994;170:270. [Google Scholar]
- 6.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Gonzalez R, Adashi EY. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med. 2003;14:51–56. doi: 10.1080/jmf.14.1.51.56. [DOI] [PubMed] [Google Scholar]
- 7.Benacerraf BR, Gatter MA, Ginsburgh F. Ultrasound diagnosis of meconium-stained amniotic fluid. AmJObstetGynecol. 1984;149:570–572. doi: 10.1016/0002-9378(84)90038-3. [DOI] [PubMed] [Google Scholar]
- 8.DeVore GR, Platt LD. Ultrasound appearance of particulate matter in amniotic cavity: vernix or meconium? J Clin Ultrasound. 1986;14:229–230. doi: 10.1002/jcu.1870140316. [DOI] [PubMed] [Google Scholar]
- 9.Sepulveda WH, Quiroz VH. Sonographic detection of echogenic amniotic fluid and its clinical significance. J PerinatMed. 1989;17:333–335. doi: 10.1515/jpme.1989.17.5.333. [DOI] [PubMed] [Google Scholar]
- 10.Sherer DM, Abramowicz JS, Smith SA, Woods JR., Jr. Sonographically homogeneous echogenic amniotic fluid in detecting meconium-stained amniotic fluid. Obstet Gynecol. 1991;78:819–822. [PubMed] [Google Scholar]
- 11.Vohra N, Rochelson B, Smith-Levitin M. Three-dimensional sonographic findings in congenital (harlequin) ichthyosis. J Ultrasound Med. 2003;22:737–739. doi: 10.7863/jum.2003.22.7.737. [DOI] [PubMed] [Google Scholar]
- 12.Rust O, Atlas R, Rawlinson K, Gaalen JV, Balducci J. Sonographic description of the cervix at risk for preterm birth. Am J Obstet Gynecol. 2001;184:S41. [Google Scholar]
- 13.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, Sorokin Y. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J PerinatMed. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez R, Romero R, Nien JK, Chaiworapongsa T, Medina L, Kim YM, Yoon BH, Carstens M, Espinoza J, Iams JD, Gonzalez R. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192:678–689. doi: 10.1016/j.ajog.2004.10.624. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Gomez R, Araneda H, Ramirez M, Cotton DB. Cervical mucus inhibits microbial growth: a host defense mechanism to prevent ascending infection in pregnant and non-pregnant women. Am J Obstet Gynecol. 1993;168:312. [Google Scholar]
- 16.Helmig R, Uldbjerg N, Ohlsson K. Secretory leukocyte protease inhibitor in the cervical mucus and in the fetal membranes. Eur J Obstet Gynecol Reprod Biol. 1995;59:95–101. doi: 10.1016/0028-2243(94)02023-8. [DOI] [PubMed] [Google Scholar]
- 17.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol. 1997;176:470–475. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 18.Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod. 2000;15:778–784. doi: 10.1093/humrep/15.4.778. [DOI] [PubMed] [Google Scholar]
- 19.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185:586–592. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 20.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 21.Evaldson G, Malmborg AS, Nord CE, Ostensson K. Bacteroides fragilis, Streptococcus intermedius and group B streptococci in ascending infection of pregnancy. An animal experimental study. Gynecol Obstet Invest. 1983;15:230–241. doi: 10.1159/000299415. [DOI] [PubMed] [Google Scholar]
- 22.Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol. 1984;148:915–928. doi: 10.1016/0002-9378(84)90534-9. [DOI] [PubMed] [Google Scholar]
- 23.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003;112:1466–1477. doi: 10.1172/JCI20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J. 2000;46:S47–S52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 27.Buhimschi I, Christner R, Buhimschi C, Chaiworapongsa T, Romero R. Proteomic analysis of preterm parturition: a novel method of identifying the patient at risk for preterm delivery. Am J Obstet Gynecol. 2002;187:S55. [Google Scholar]
- 28.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, Blackwell S, Whitty J, Berman S, Redman M, Yoon BH, Sorokin Y. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal NeonatalMed. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 29.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal NeonatalMed. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung JY, Leung JW, Shaffer EA, Lam K, Olson ME, Costerton JW. Ascending infection of the biliary tract after surgical sphincterotomy and biliary stenting. J Gastroenterol Hepatol. 1992;7:240–245. doi: 10.1111/j.1440-1746.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 31.Sung JY, Leung JW, Shaffer EA, Lam K, Costerton JW. Bacterial biofilm, brown pigment stone and blockage of biliary stents. J Gastroenterol Hepatol. 1993;8:28–34. doi: 10.1111/j.1440-1746.1993.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 32.Sosna J, Kruskal JB, Copel L, Goldberg SN, Kane RA. USguided percutaneous cholecystostomy: features predicting culture-positive bile and clinical outcome. Radiology. 2004;230:785–791. doi: 10.1148/radiol.2303030121. [DOI] [PubMed] [Google Scholar]
- 33.Jensen ET, Kharazmi A, Lam K, Costerton JW, Hoiby N. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect Immun. 1990;58:2383–2385. doi: 10.1128/iai.58.7.2383-2385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen ET, Kharazmi A, Hoiby N, Costerton JW. Some bacterial parameters influencing the neutrophil oxidative burst response to Pseudomonas aeruginosa biofilms. APMIS. 1992;100:727–733. [PubMed] [Google Scholar]
- 35.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 36.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 37.Fergie N, Bayston R, Pearson JP, Birchall JP. Is otitismediawith effusion a biofilm infection? Clin Otolaryngol. 2004;29:38–46. doi: 10.1111/j.1365-2273.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- 38.Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Post JC, Preston RA, Aul JJ, Larkins-Pettigrew M, Rydquist-White J, Anderson KW, Wadowsky RM, Reagan DR, Walker ES, Kingsley LA, Magit AE, Ehrlich GD. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA. 1995;273:1598–1604. [PubMed] [Google Scholar]
- 40.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, Jun JK. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–1137. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 42.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, Jun JK. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am JObstet Gynecol. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Espinoza J, Mazor M. Can endometrial infection/ inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 44.Ansbacher R, Boyson WA, Morris JA. Sterility of the uterine cavity. Am J Obstet Gynecol. 1967;99:394–396. doi: 10.1016/s0002-9378(16)34549-5. [DOI] [PubMed] [Google Scholar]
- 45.Duff P, Gibbs RS, Blanco JD, St Clair PJ. Endometrial culture techniques in puerperal patients. Obstet Gynecol. 1983;61:217–222. [PubMed] [Google Scholar]
- 46.Moller BR, Kristiansen FV, Thorsen P, Frost L, Mogensen SC. Sterility of the uterine cavity. Acta Obstet Gynecol Scand. 1995;74:216–219. doi: 10.3109/00016349509008942. [DOI] [PubMed] [Google Scholar]
- 47.Marrie TJ, Costerton JW. A scanning and transmission electron microscopic study of the surfaces of intrauterine contraceptive devices. Am J Obstet Gynecol. 1983;146:384–394. doi: 10.1016/0002-9378(83)90818-9. [DOI] [PubMed] [Google Scholar]
- 48.Bank HL, Williamson HO. Scanning electron microscopy of Dalkon Shield tails. Fertil Steril. 1983;40:334–339. doi: 10.1016/s0015-0282(16)47296-1. [DOI] [PubMed] [Google Scholar]
- 49.Jacques M, Olson ME, Costerton JW. Microbial colonization of tailed and tailless intrauterine contraceptive devices: influence of the mode of insertion in the rabbit. Am J Obstet Gynecol. 1986;154:648–655. doi: 10.1016/0002-9378(86)90624-1. [DOI] [PubMed] [Google Scholar]