Abstract
Two recent reports provide new physical information on how the XPA protein recruits the ERCC1-XPF heterodimer to the site of damage during the process of mammalian nucleotide excision repair (NER). Using chemical shift perturbation NMR experiments, the contact sites between a central fragment of ERCC1 and a XPA fragment have been mapped. While both studies agree with regard to the XPA binding site, they differ on whether the ERCC1-XPA complex can simultaneously bind DNA. These studies have important implications for both the molecular process and the design of potential inhibitors of NER.
1. Introduction
In this Hot Topics review, we describe the results of two recent papers that report the interaction of a fragment of human XPA with human ERCC1, [1,2]. Prior to discussing these two studies, we will briefly review the process of nucleotide excision repair and the pertinent literature leading up to these papers.
DNA is the hereditary molecule of life and cells have evolved elaborate strategies to protect it from damage. Nucleotide excision repair (NER) is one pathway utilized by cells to repair endogenous and exogenous DNA damage [3]. In mammalian cells, NER involves 11 factors composed of over 30 proteins whose combined activities find and excise DNA damage from the genome, for a recent review see Gillet and Scharer [4].
There are two distinct pathways for NER: transcription-coupled repair (TCR), which preferentially recognizes and repairs DNA lesions in actively transcribed genes and global genome repair (GGR), which removes lesions from the remainder of the genome. NER is an ATP-dependent multistep process. The reaction pathway has been defined based on biochemical assays in vitro and local UV-irradiation combined with immunofluorescence localization of repair proteins in vivo. Together these techniques reveal that NER proceeds through the sequential ordered assembly of protein complexes instead of repair taking place within a pre-organized “repairosome” [5,6].
The basic steps of the NER reaction are DNA distortion recognition, damage verification, repair complex assembly, dual incision and damage excision, DNA resynthesis and ligation. While the precise order of addition of the damage recognition factors remains somewhat controversial [7], the favored working model for GGR is described below and see Fig. 1. DNA distortion recognition is initiated by the XPC-HR23B-centrin complex. DDB1-DDB-2 (XPE) also participates in recognition of some lesions especially cyclobutane pyrimidine dimers. The DNA damage is verified by opening of the DNA strands surrounding the lesion by the transcription factor TFIIH. The activities of two components of TFIIH, XPB and XPD, are essential for this strand opening and repair complex assembly. XPA and RPA are then recruited to the opened structure surrounding the lesion. XPG, the nuclease responsible for incision 4-8 nucleotides 3′ to the lesion, is engaged by interactions with TFIIH and XPA. XPC-HR23B is believed to be released upon entry of XPG. The last complex to be recruited to the lesion prior to incision is the ERCC1-XPF endonuclease. The heterodimer associates with the complex by its interactions with XPA, RPA and XPB. ERCC1-XPF is recruited independently of XPG and is required for the 5′ single strand cut ∼20 nucleotides from the lesion. Addition of XPF induces the dual strand incisions on the damaged strand, allowing damage excision and release of TFIIH and XPA. Finally, pol δ/ε, pol κ, RFC, PCNA, RPA, DNA ligase I and DNA ligase III conduct repair synthesis and ligation of the nascent DNA to the parental strands (see Gillet and Scharer and references site therein [4,8,9]).
Fig. 1. Model of Mammalian General Genome Repair.
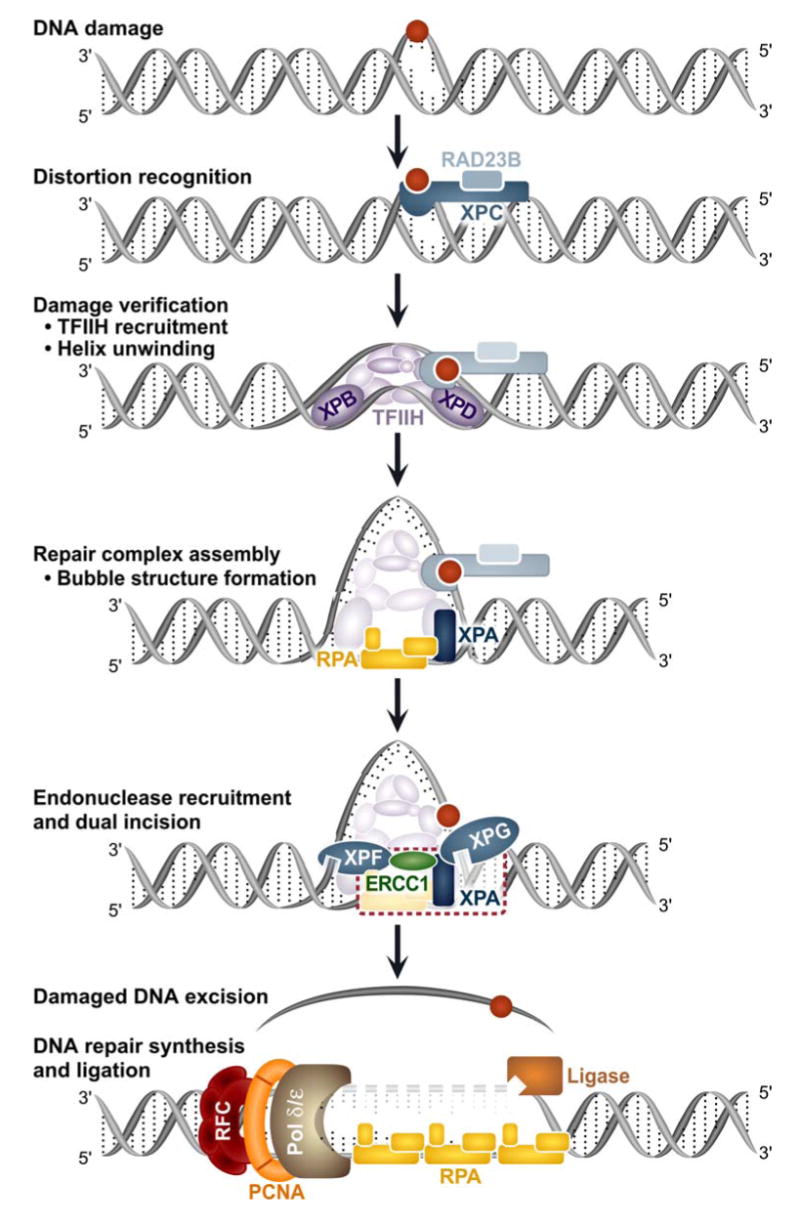
Mammalian NER proceeds through the sequential addition or exit of repair factors. See text for more details. The dashed red box in the penultimate step emphasizes the ERCC1-XPA interaction under investigation by Tripsianes et al. [1] and Tsodikov et al. [1,2].
Recent NMR and X-ray crystallography studies are beginning to define the protein-DNA and protein-protein interactions between and among NER factors. During the past five years several exciting structures of eukaryotic NER proteins, or fragments thereof, have appeared and a detailed structural picture is beginning to emerge that more clearly defines the physical interactions between the NER components and DNA at atomic resolution, see Table 1 [1,2,10-23].
Table I. Eukaryotic NER Structures (as of 12/2007).
| Protein | Source | PDB | Year | Method | Reference |
|---|---|---|---|---|---|
| XPA-MDB domain | human | 1XPA | 1998 | NMR | [10] |
| XPA-MDB domain | human | 1D4U | 1999 | NMR | [11] |
| hHR23A XPCB domain | human | 1TP4 | 2004 | NMR | [12] |
| XPC-Centrin 2 domains | human | 2A4J | 2005 | NMR | [13] |
| hHR23B XPCB domain | human | 1PVE | 2005 | NMR | [14] |
| central ERCC1 | human | 2A1I | 2005 | X-ray | [15] |
| XPF-ERCC1 HhH | human | 1Z00 | 2005 | NMR | [16] |
| XPF | A. pernix | 2BHN | 2005 | X-ray | [17] |
| XPF with DNA | A. pernix | 2BGW | 2005 | X-ray | [17] |
| XPF-ERCC1 HhH | human | 2A1J | 2005 | X-ray | [15] |
| CT XPB | A. fulgidus | 2FZL | 2006 | X-ray | [18] |
| NT XPB | A. fulgidus | 2FZ4 | 2006 | X-ray | [18] |
| XPB | A. fulgidus | 2FWR | 2006 | X-ray | [18] |
| DDB1-Cul4 | human | 2HYE | 2006 | X-ray | [19] |
| Centrin 2-XPC peptide | human | 2GGM | 2006 | X-ray | [20] |
| XPF HhH | human | 2AQ0 | 2007 | NMR | [21] |
| ERCC1-XPA peptide | human | 2JNW | 2007 | NMR | [2] |
| central ERCC1 | human | 2JPD | 2007 | NMR | [1] |
| Centrin 2-XPC peptide | human | 2OBH | 2007 | X-ray | [22] |
| Rad4-Rad23 | S. cerevisiae | 2QSF | 2007 | X-ray | [23] |
| Rad4-Rad23 UV DNA | S. cerevisiae | 2QSG | 2007 | X-ray | [23] |
| Rad4-Rad23 MM DNA | S. cerevisiae | 2QSH | 2007 | X-ray | [23] |
ERCC1-XPF Focus
ERCC1-XPF is a structure specific endonuclease that prefers to cleave at the ssDNA/dsDNA junction of DNA structures such as hairpins, splayed arms and bubbles [15,16,24]. XPF is the subunit that contains the active nuclease center, Fig. 2 and 3A. In addition, XPF contains an N-terminal helicase-like fold, and a C-terminus tandem helix-hairpin-helix (HhH2) domain. The helicase-like fold of human XPF is not conserved among the XPF family members and in vitro it is dispensable for nuclease activity [15]. However, the efficiency and specificity of the incision reaction declined upon deletion of this domain.
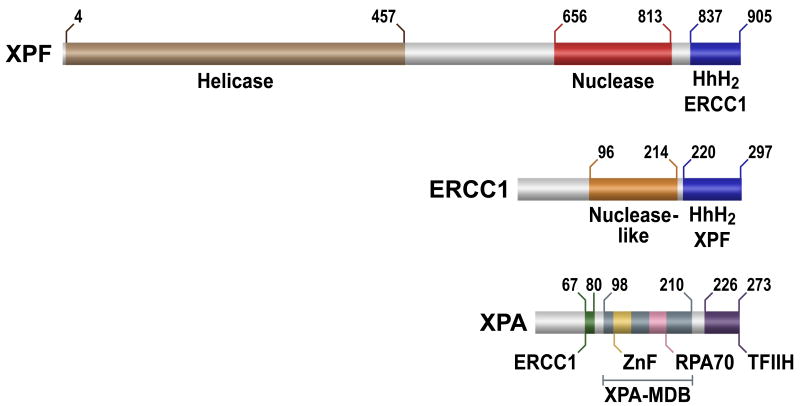
Fig. 2. The domain organization of XPF, ERCC1 and XPA.
The homology-based domains of human proteins are shown in different boxes with the boundary residue numbers. Known protein-protein interactions are denoted beneath the graphic representations.
Fig. 3. The structures of the nuclease domain of A. pernix XPF (PDB ID: 2BGW), the nuclease-like domain of human ERCC1 (PDB ID: 2JPD) and the C-terminal HhH2 domain of human ERCC1-XPF complex (PDB ID: 2A1J).
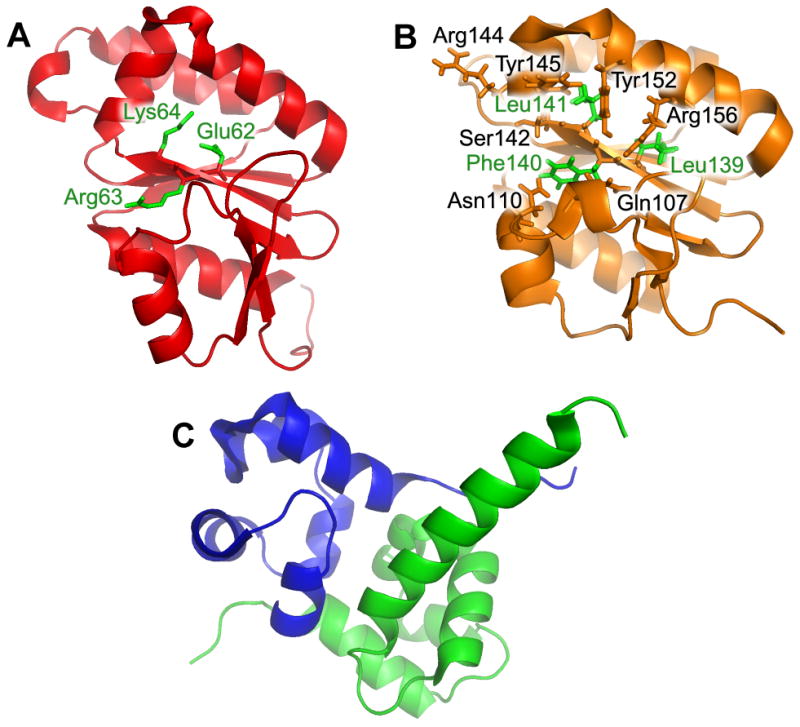
(A) The crystal structure of the nuclease domain of A. pernix XPF. The important catalytic residues, Glu62, Arg63 and Lys64, are labeled green. (B) The NMR structure of the nuclease-like domain of human ERCC1. The non-catalytic residues in ERCC1's nuclease-like fold are Leu139, Leu141 and Phe140 and are noted in green. Those residues in black are proposed to interact with residues 67-80 in XPA (C) The crystal structure of the human C-terminal HhH2 domain complex between the XPF and ERCC1. ERCC1 C-terminal domain is shown as green and XPF C-terminal domain as blue.
ERCC1 contains a XPF-like nuclease fold, Fig. 3B, it is catalytically inactive, and has been hypothesized to have arisen from a gene duplication of XPF [25]. ERCC1 also contains a C-terminal HhH2 fold which is essential for dimerization with XPF [16]. ERCC1 and XPF mutually stabilize one another through interactions between their HhH2 domains [16,26-29]. ERCC1's nuclease fold has been solved by NMR and X-ray crystallography ([1,2,15], PDB records 2JPD, 2JNW and 2A1I, respectively) and the nuclease domain of Aeropyrum pernix XPF-like protein has been solved with and without DNA ([17], PDB files 2BHN, 2BGW). The structures revealed a conserved architecture of 6 stranded β-sheet flanked on either side by α-helixes. This domain organization was also seen in another nuclease, Pyrococcus furiosus Hef [30]. All of these proteins share structural homology with the type II family of restriction endonucleases [30-32]. While ERCC1 shares the nuclease fold with the other XPF family members, the conserved catalytic amino acids have diverged [25]. In archaea, the XPF-like proteins form a homodimer, and the two active nuclease centers interact [30,33]. In contrast, human ERCC1-XPF form heterodimers and the nuclease centers do not form a stable complex [15].
The HhH2 domains of human ERCC1 and XPF have been solved by X-ray crystallography and NMR, Fig. 3C (2A1J, [1,2,15]; 1Z00, [16]; 2AQO, [21]). In addition, the same domain was crystallized from the archaea A. pernix XPF in the presence and absence of DNA [17]. The all α-helix structure is the dimerization domain between ERCC1 and XPF and it revealed an extensive network of hydrophobic interactions that are shared between the two proteins [15,17,29]. Thus explaining how the two proteins mutually stabilize one another. This dimer interface between ERCC1 and XPF homologs is not conserved in lower eukaryotes [28].
The helix-hairpin-helix domain is present in numerous DNA repair proteins in part because it mediates nonspecific DNA binding by making electrostatic interactions with the DNA phosphate backbone [34,35]. The domains in ERCC1 and XPF are structurally similar to the tandem HhH2 found in the prokaryotic proteins UvrC and RuvA [16,36-39]. The DNA binding properties of the isolated HhH2 domains of ERCC1 and XPF have been evaluated [16]. The authors showed that the DNA binding affinity of the HhH2 heterodimer was weaker than that observed for full-length ERCC1-XPF; however the DNA substrate binding preference was preserved. To map the amino acids that might be involved in the HhH2 heterodimer-DNA interaction Tripsianes et al. performed amide chemical shift perturbation experiments [16]. Specific shifts were observed in the hairpin of ERCC1's monomer whereas no shifts were observed in the corresponding hairpin region of the XPF subunit. From these observations the authors suggested that only ERCC1's HhH2 domain participates in DNA binding.
The results and model, from Tripsianes et al. above, differ from data obtained from the crystal structure of A. pernix homodimeric XPF with DNA [17] and the proposed model by Tsodikov et al. [15] of how the HhH2 domains of ERCC1-XPF interact with DNA. In the A. pernix XPF, both HhH2 subunits were shown to interact with DNA. Since no experimental evidence exists to support the role of XPF's HhH2 in DNA binding, perhaps this region of XPF has evolved another function; maybe it provides a protein-protein interaction site.
ERCC1 and XPA
Previously, deletion mutagenesis and the yeast two-hybrid system were used to identify the region of XPA that was responsible for the interaction with ERCC1, and vise versa [40-42]. The research led to the identification of regions on XPA, residues 58-114, and ERCC1, residues 91-118, that are important for the interaction. Furthermore, Li et al. discovered that deletion of three glycine residues of XPA, Gly72-Gly74, reduced the interaction between ERCC1 and XPA [41]. Interestingly, the minimal DNA binding domain of XPA, denoted as XPA-MDB in Fig. 2, is adjacent to the XPA-ERCC1 site of interaction. Future structures of a XPA-ERCC1-XPF-DNA complex may reveal whether DNA flows from XPA's DNA binding domain through ERCC1 to the nuclease center in XPF or whether the ERCC1-XPF heterodimer does not engage the DNA until XPA leaves. The latter of course would be a means of regulating the nuclease's activity.
XPA recruits ERCC1-XPF to repair sites
Using beautiful immunofluorescent labeling techniques, it has been shown that XPA recruits ERCC1-XPF into repair foci [6,43]. Two recent papers have appeared that re-examine how XPA helps recruit the ERCC1-XPF complex to the site of damage thereby facilitating incision [1,2]. These two studies use several biophysical techniques including X-ray crystallography and NMR to probe this interaction.
ERRC1 is believed to have evolved from an ancient XPF [25] and the central domain of ERCC1 shares a XPF nuclease fold consisting of a six-stranded β-sheet flanked by five α -helices on both sides, Fig. 3. [1,2,15]. The study by Tsodikov et al. 2007 [2] created a co-crystal of the central domain of ERCC96-114 and a small synthetic peptide of XPA67-80. The fold of the ERCC1 fragment was obtained using molecular replacement based on their previous X-ray structure of the unliganded ERCC1 fragment [15]. The XPA67-80/ERCC1 complex was built based on the low resolution ERCC1 X-ray structure (4 Å) and distance restraints (n = 109, 18 intermolecular) from NOESY NMR experiments of the labeled ERCC1192-214 fragment and XPA67-80 complex. The refined structure suggested that XPA67-80 bound to the central cleft of ERCC1 which is homologous to the nuclease active site of XPF.
The study by Tripsianes et al. [1] took a completely NMR approach by solving the ERCC1 structure using triple labeled 1H, 15N, and 13C ERCC196-219 and standard triple resonance techniques to assign distance constraints (n= 2669) to create an ensemble of 20 conformers for ERCC196-219. The NMR structure [1] was in good agreement with the previous crystal structure [15] of the central ERCC1 domain (rmsd 1.1 Å for 108 Cα atoms). The study by Tsodikov et al. 2007 [2] used equilibrium sedimentation and fluorescence anisotropy, and Tripsianes et al, 2007 [1] used 3D-NMR experiments to determined the stoichiometry of the interaction to be one XPA per ERCC1 molecule.
Both studies used chemical shift perturbation experiments to map the interaction of an XPA fragment onto the central ERCC1 domain [1,2]. For their analysis, Tsodikov et al. [2] mapped the XPA 67-80 fragment onto 15N-labeled ERCC96-214, and in the Tripsianes et al. [1] case, XPA59-99 was mapped onto a similar, but not identical, central fragment of 15N labeled ERCC196-219 (as we will see this becomes important later see discussion below regarding DNA binding).
Chemical shift perturbation is a powerful NMR technique that in these two cases examined the 1H-15N HSQC spectra of the ERCC1 fragment unbound versus bound to a small unlabeled XPA fragment. Specifically, 15N HSQC is a 2D spectroscopic technique that looks at the correlations between the nitrogen atom of an amide group with the bound proton. The amide chemical shift is sensitive to the local environment in both nitrogen and proton dimension. Since there is only one backbone HN per amino acid, each residue should have one HSQC signal (except proline), thus an HSQC spectrum is like a “fingerprint” of the protein. When a protein binds a protein or nucleic acid, the nuclei at the binding interface will experience the local chemical environment changes, which lead to chemical shift perturbation. The perturbation can be due to two effects: residues close to the interface of the complex will be perturbed by the partner close by; or, the global conformational changes of the protein itself occur upon adding the partner. Therefore, the specific 1H-15N resonances associated with particular amino acid residues will potentially change if bound by a specific ligand, which in this case is the XPA fragment. The one limitation of this approach is the proper assignment of the 1H-15N cross-peaks to the correct amino acid. Comparison of the two spectra reported in these two studies showed very similar chemical shifts, albeit a few were differently assigned. Despite these differences the two data sets clearly showed significant perturbations around ERCC1 residues 139 -157, and a secondary perturbation, albeit less dramatic, between residues 107-112. Both studies agreed that these regions define a deep V-shaped cleft on ERCC1 that is the binding site for XPA see figure 3 in [1] and figure 2 in [2]. Thus, confirming the ERCC196-214-XPA67-80 co-crystal structure and assignments by Tsodikov et al. 2007 [2].
The interaction of the XPA fragment with this cleft implicated several critical side-chains that may mediate the interaction between the XPA fragment and the central domain of ERCC1. These included the highly conserved residues in XPA, T71GGGFI76, that are hypothesized to make critical contacts with Q107, N110, L139, F140, L141, S142, R144, Y145, Y152 and R156 in ERCC1, Fig 3B. The important role of F75 in XPA's interaction with ERCC1 has not been previously described, and Tsodikov et al. 2007 [2] showed that the F75A mutation in full length XPA lacked the ability to complement an XPA deficient cell extract for performing dual incisions around a 1,3 intrastrand cisplatin adduct, but the mutant protein bound tightly to a three-way junction substrate. This group also explored whether the XPA67-80 fragment was capable of interfering with the repair reaction and/or the nuclease activity of the ERCC1-XPF heterodimer. They showed that while the XPA peptide is sufficient to block dual incision of a 1,3 intrastrand cisplatin adduct, incision of a 12 base pair stem 22 base loop substrate is unaffected. From these data, they concluded that recruitment of ERCC1-XPF to the damaged site is blocked by the XPA peptide, thus blocking the dual incision. Direct molecular proof of such inhibition could be confirmed using damaged DNA substrates attached to beads in conjunction with pull-down experiments, such as those used by Egly and coworkers [43].
The last topic that both studies addressed is whether the cleft in ERCC1 that binds XPA is also capable of binding DNA. While the ERCC1-XPF heterodimer has been proposed to make contact with DNA through its HhH2 domain based on NMR chemical shift perturbation experiments [15,16], further experimental proof awaits site directed mutagenesis. Tripsianes et al. [1] used chemical shift perturbation experiments in which the chemical shifts of 1H-15N HSQC spectra of the ERCC1 fragment is monitored in the absence and presence of a 20 base DNA bubble substrate consisting of duplex with a 10 base internal bubble. They found three regions that were perturbed in 15N-labeled ERCC196-219 upon binding to this DNA substrate, including: N99, I102, L132, K213, A214 and the side chain amide of Q134. While residues K213 and A214 are very near the C-terminal region of this fragment, which could be unstructured, inhibition of DNA binding by the presence of a HIS-tag on the C-terminal residue at 219 suggests this region is important for DNA binding. Mapping these residues to the surface of the ERCC1-fragment showed that the putative DNA binding site is very different than the XPA binding cleft. This model is further substantiated by NMR chemical shift perturbations showing that both ligands can be in simultaneous contact with the 15N-labeled ERCC196-219 fragment.
It is interesting to note that these data are inconsistent with those data by Tsodikov et al. [2] who did not perform such a DNA perturbation experiment, but used a fluorescence anisotropy experiment with 6-carboxyfluorescein labeled XPA fragment bound to ERCC1 to explore whether the cleft in ERCC1 is also capable of binding DNA. In this experiment the fluorescently labeled XPA when bound to ERCC1 exhibited relatively slow rotation and high fluorescence anisotropy. Addition of a competing single-stranded 40 mer DNA showed a decrease in XPA fluorescence anisotropy which is consistent with both DNA and XPA competing for the same site. These disparate data sets could be due to the different DNA substrates used and/or the slightly different ERCC1 fragment size used in these two experiments.
Reconciliation of these two experimental strategies has important implications into the precise molecular dance between ERCC1-XPF, XPA and DNA. Previous work has suggested that XPA is released from the incision complex upon the dual cleavage event [43], but it is not entirely clear whether all three proteins can be bound simultaneously before incision occurs. To our knowledge a direct test of this model using a nuclease defective XPF in this type of experiment has not been performed. A further caution is warranted in that while structural biology often necessitates the use of fragments of specific proteins, the very use of these fragments can also lead to a “Heisenberg biological uncertainly principle” in that these fragments might have new or different properties that are not present in the complete protein. Future studies will clearly define the precise DNA binding sites on ERCC1 and whether ERCC1-XPF can simultaneously bind DNA and XPA on its path to mediating the 5′ incision at damaged sites during the process of NER.
Finally it is important to remember that ERCC1-XPF has other functions within the cell, namely it participates in interstrand DNA cross-link repair [44-46], homologous recombination [47] and telomere maintenance [48,49]. Curiously, an active XPF nuclease is not required for telomere maintenance [50]. It is interesting to speculate whether ERCC1's non-nuclease active site cleft has evolved to physically interact with other protein partners such that it will target XPF to work in these other cellular processes.
Acknowledgments
This research was supported by the Intramural Research Program of the NIEHS, NIH. We would like to thank Alan Clark and Dr. Geoff Mueller for critically reading this manuscript. We would also like to especially thank Sue Edelstein for assistance with the graphics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tripsianes K, Folkers GE, Zheng C, Das D, Grinstead JS, Kaptein R, Boelens R. Analysis of the XPA and ssDNA-binding surfaces on the central domain of human ERCC1 reveals evidence for subfunctionalization. Nucleic Acids Res. 2007;35:5789–5798. doi: 10.1093/nar/gkm503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsodikov OV, Ivanov D, Orelli B, Staresincic L, Shoshani I, Oberman R, Scharer OD, Wagner G, Ellenberger T. Structural basis for the recruitment of ERCC1-XPF to nucleotide excision repair complexes by XPA. Embo J. 2007;26:4768–4776. doi: 10.1038/sj.emboj.7601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedberg EC, Walker GW, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. ASM Press; Wahsington, DC: 2006. [Google Scholar]
- 4.Gillet LC, Scharer OD. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev. 2006;106:253–276. doi: 10.1021/cr040483f. [DOI] [PubMed] [Google Scholar]
- 5.Houtsmuller AB, Rademakers S, Nigg AL, Hoogstraten D, Hoeijmakers JH, Vermeulen W. Action of DNA repair endonuclease ERCC1/XPF in living cells. Science. 1999;284:958–961. doi: 10.1126/science.284.5416.958. [DOI] [PubMed] [Google Scholar]
- 6.Volker M, Mone MJ, Karmakar P, van Hoffen A, Schul W, Vermeulen W, Hoeijmakers JH, van Driel R, van Zeeland AA, Mullenders LH. Sequential assembly of the nucleotide excision repair factors in vivo. Mol Cell. 2001;8:213–224. doi: 10.1016/s1097-2765(01)00281-7. [DOI] [PubMed] [Google Scholar]
- 7.Kesseler KJ, Kaufmann WK, Reardon JT, Elston TC, Sancar A. A mathematical model for human nucleotide excision repair: Damage recognition by random order assembly and kinetic proofreading. J Theor Biol. 2007;249:361–375. doi: 10.1016/j.jtbi.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogi T, Lehmann AR. The Y-family DNA polymerase kappa (pol kappa) functions in mammalian nucleotide-excision repair. Nat Cell Biol. 2006;8:640–642. doi: 10.1038/ncb1417. [DOI] [PubMed] [Google Scholar]
- 9.Moser J, Kool H, Giakzidis I, Caldecott K, Mullenders LH, Fousteri MI. Sealing of chromosomal DNA nicks during nucleotide excision repair requires XRCC1 and DNA ligase III alpha in a cell-cycle-specific manner. Mol Cell. 2007;27:311–323. doi: 10.1016/j.molcel.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Ikegami T, Kuraoka I, Saijo M, Kodo N, Kyogoku Y, Morikawa K, Tanaka K, Shirakawa M. Solution structure of the DNA- and RPA-binding domain of the human repair factor XPA. Nat Struct Biol. 1998;5:701–706. doi: 10.1038/1400. [DOI] [PubMed] [Google Scholar]
- 11.Buchko GW, Daughdrill GW, de Lorimier R, Rao BK, Isern NG, Lingbeck JM, Taylor JS, Wold MS, Gochin M, Spicer LD, Lowry DF, Kennedy MA. Interactions of human nucleotide excision repair protein XPA with DNA and RPA70 Delta C327: chemical shift mapping and 15N NMR relaxation studies. Biochemistry. 1999;38:15116–15128. doi: 10.1021/bi991755p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamionka M, Feigon J. Structure of the XPC binding domain of hHR23A reveals hydrophobic patches for protein interaction. Protein Sci. 2004;13:2370–2377. doi: 10.1110/ps.04824304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang A, Miron S, Mouawad L, Duchambon P, Blouquit Y, Craescu CT. Flexibility and plasticity of human centrin 2 binding to the xeroderma pigmentosum group C protein (XPC) from nuclear excision repair. Biochemistry. 2006;45:3653–3663. doi: 10.1021/bi0524868. [DOI] [PubMed] [Google Scholar]
- 14.Kim B, Ryu KS, Kim HJ, Cho SJ, Choi BS. Solution structure and backbone dynamics of the XPC-binding domain of the human DNA repair protein hHR23B. Febs J. 2005;272:2467–2476. doi: 10.1111/j.1742-4658.2005.04667.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsodikov OV, Enzlin JH, Scharer OD, Ellenberger T. Crystal structure and DNA binding functions of ERCC1, a subunit of the DNA structure-specific endonuclease XPF-ERCC1. Proc Natl Acad Sci U S A. 2005;102:11236–11241. doi: 10.1073/pnas.0504341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tripsianes K, Folkers G, Ab E, Das D, Odijk H, Jaspers NG, Hoeijmakers JH, Kaptein R, Boelens R. The structure of the human ERCC1/XPF interaction domains reveals a complementary role for the two proteins in nucleotide excision repair. Structure. 2005;13:1849–1858. doi: 10.1016/j.str.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Newman M, Murray-Rust J, Lally J, Rudolf J, Fadden A, Knowles PP, White MF, McDonald NQ. Structure of an XPF endonuclease with and without DNA suggests a model for substrate recognition. Embo J. 2005;24:895–905. doi: 10.1038/sj.emboj.7600581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan L, Arvai AS, Cooper PK, Iwai S, Hanaoka F, Tainer JA. Conserved XPB core structure and motifs for DNA unwinding: implications for pathway selection of transcription or excision repair. Mol Cell. 2006;22:27–37. doi: 10.1016/j.molcel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JR, Ryan ZC, Salisbury JL, Kumar R. The structure of the human centrin 2-xeroderma pigmentosum group C protein complex. J Biol Chem. 2006;281:18746–18752. doi: 10.1074/jbc.M513667200. [DOI] [PubMed] [Google Scholar]
- 21.Das D, Tripsianes K, Jaspers NG, Hoeijmakers JH, Kaptein R, Boelens R, Folkers GE. The HhH domain of the human DNA repair protein XPF forms stable homodimers. Proteins. 2007 doi: 10.1002/prot.21635. [DOI] [PubMed] [Google Scholar]
- 22.Charbonnier JB, Renaud E, Miron S, Le Du MH, Blouquit Y, Duchambon P, Christova P, Shosheva A, Rose T, Angulo JF, Craescu CT. Structural, thermodynamic, and cellular characterization of human centrin 2 interaction with xeroderma pigmentosum group C protein. J Mol Biol. 2007;373:1032–1046. doi: 10.1016/j.jmb.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 23.Min JH, Pavletich NP. Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature. 2007;449:570–575. doi: 10.1038/nature06155. [DOI] [PubMed] [Google Scholar]
- 24.de Laat WL, Appeldoorn E, Sugasawa K, Weterings E, Jaspers NG, Hoeijmakers JH. DNA-binding polarity of human replication protein A positions nucleases in nucleotide excision repair. Genes Dev. 1998;12:2598–2609. doi: 10.1101/gad.12.16.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaillard PH, Wood RD. Activity of individual ERCC1 and XPF subunits in DNA nucleotide excision repair. Nucleic Acids Res. 2001;29:872–879. doi: 10.1093/nar/29.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biggerstaff M, Szymkowski DE, Wood RD. Co-correction of the ERCC1, ERCC4 and xeroderma pigmentosum group F DNA repair defects in vitro. Embo J. 1993;12:3685–3692. doi: 10.1002/j.1460-2075.1993.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Vuuren AJ, Appeldoorn E, Odijk H, Humbert S, Moncollin V, Eker AP, Jaspers NG, Egly JM, Hoeijmakers JH. Partial characterization of the DNA repair protein complex, containing the ERCC1, ERCC4, ERCC11 and XPF correcting activities. Mutat Res. 1995;337:25–39. doi: 10.1016/0921-8777(95)00009-9. [DOI] [PubMed] [Google Scholar]
- 28.de Laat WL, Sijbers AM, Odijk H, Jaspers NG, Hoeijmakers JH. Mapping of interaction domains between human repair proteins ERCC1 and XPF. Nucleic Acids Res. 1998;26:4146–4152. doi: 10.1093/nar/26.18.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi YJ, Ryu KS, Ko YM, Chae YK, Pelton JG, Wemmer DE, Choi BS. Biophysical characterization of the interaction domains and mapping of the contact residues in the XPF-ERCC1 complex. J Biol Chem. 2005;280:28644–28652. doi: 10.1074/jbc.M501083200. [DOI] [PubMed] [Google Scholar]
- 30.Nishino T, Komori K, Ishino Y, Morikawa K. X-ray and biochemical anatomy of an archaeal XPF/Rad1/Mus81 family nuclease: similarity between its endonuclease domain and restriction enzymes. Structure. 2003;11:445–457. doi: 10.1016/s0969-2126(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal AK. Structure and function of restriction endonucleases. Curr Opin Struct Biol. 1995;5:11–19. doi: 10.1016/0959-440x(95)80004-k. [DOI] [PubMed] [Google Scholar]
- 32.Aravind L, Walker DR, Koonin EV. Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acids Res. 1999;27:1223–1242. doi: 10.1093/nar/27.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts JA, White MF. An archaeal endonuclease displays key properties of both eukaryal XPF-ERCC1 and Mus81. J Biol Chem. 2005;280:5924–5928. doi: 10.1074/jbc.M412766200. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- 35.Doherty AJ, Serpell LC, Ponting CP. The helix-hairpin-helix DNA-binding motif: a structural basis for non-sequence-specific recognition of DNA. Nucleic Acids Res. 1996;24:2488–2497. doi: 10.1093/nar/24.13.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishino T, Ariyoshi M, Iwasaki H, Shinagawa H, Morikawa K. Functional analyses of the domain structure in the Holliday junction binding protein RuvA. Structure. 1998;6:11–21. doi: 10.1016/s0969-2126(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 37.Ariyoshi M, Nishino T, Iwasaki H, Shinagawa H, Morikawa K. Crystal structure of the holliday junction DNA in complex with a single RuvA tetramer. Proc Natl Acad Sci U S A. 2000;97:8257–8262. doi: 10.1073/pnas.140212997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, Folkers GE, Bonvin AM, Boelens R, Wechselberger R, Niztayev A, Kaptein R. Solution structure and DNA-binding properties of the C-terminal domain of UvrC from E.coli. Embo J. 2002;21:6257–6266. doi: 10.1093/emboj/cdf627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karakas E, Truglio JJ, Croteau D, Rhau B, Wang L, Van Houten B, Kisker C. Structure of the C-terminal half of UvrC reveals an RNase H endonuclease domain with an Argonaute-like catalytic triad. Embo J. 2007;26:613–622. doi: 10.1038/sj.emboj.7601497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Elledge SJ, Peterson CA, Bales ES, Legerski RJ. Specific association between the human DNA repair proteins XPA and ERCC1. Proc Natl Acad Sci U S A. 1994;91:5012–5016. doi: 10.1073/pnas.91.11.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Peterson CA, Lu X, Legerski RJ. Mutations in XPA that prevent association with ERCC1 are defective in nucleotide excision repair. Mol Cell Biol. 1995;15:1993–1998. doi: 10.1128/mcb.15.4.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg E, Taher MM, Kuemmerle NB, Farnsworth J, Valerie K. A truncated human xeroderma pigmentosum complementation group A protein expressed from an adenovirus sensitizes human tumor cells to ultraviolet light and cisplatin. Cancer Res. 2001;61:764–770. [PubMed] [Google Scholar]
- 43.Riedl T, Hanaoka F, Egly JM. The comings and goings of nucleotide excision repair factors on damaged DNA. Embo J. 2003;22:5293–5303. doi: 10.1093/emboj/cdg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busch DB, van Vuuren H, de Wit J, Collins A, Zdzienicka MZ, Mitchell DL, Brookman KW, Stefanini M, Riboni R, Thompson LH, Albert RB, van Gool AJ, Hoeijmakers J. Phenotypic heterogeneity in nucleotide excision repair mutants of rodent complementation groups 1 and 4. Mutat Res. 1997;383:91–106. doi: 10.1016/s0921-8777(96)00048-1. [DOI] [PubMed] [Google Scholar]
- 45.Kuraoka I, Kobertz WR, Ariza RR, Biggerstaff M, Essigmann JM, Wood RD. Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease. J Biol Chem. 2000;275:26632–26636. doi: 10.1074/jbc.C000337200. [DOI] [PubMed] [Google Scholar]
- 46.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niedernhofer LJ, Essers J, Weeda G, Beverloo B, de Wit J, Muijtjens M, Odijk H, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required for targeted gene replacement in embryonic stem cells. Embo J. 2001;20:6540–6549. doi: 10.1093/emboj/20.22.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu XD, Niedernhofer L, Kuster B, Mann M, Hoeijmakers JH, de Lange T. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol Cell. 2003;12:1489–1498. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]
- 49.Munoz P, Blanco R, Flores JM, Blasco MA. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nat Genet. 2005;37:1063–1071. doi: 10.1038/ng1633. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Zacal NJ, Rainbow AJ, Zhu XD. XPF with mutations in its conserved nuclease domain is defective in DNA repair but functions in TRF2-mediated telomere shortening. DNA Repair (Amst) 2007;6:157–166. doi: 10.1016/j.dnarep.2006.09.005. [DOI] [PubMed] [Google Scholar]