Abstract
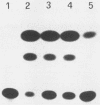
The X gene product of the hepatitis B virus (HBV) has been expressed transiently in HepG2 cells, and the 17-kilodalton protein has been detected by Western (immuno-) blot analysis. Cotransfection of the X gene with the long terminal repeat of human immunodeficiency virus type 1 or 2 results in a stimulation of long terminal repeat-directed expression that is higher than the X-induced stimulation of the HBV enhancer linked to either autologous promoter or to the heterologous simian virus 40 promoter. A frameshift mutation abolished this transactivation. In vitro nuclear transcription assays revealed that HBV X acts at the transcriptional level. The carboxy terminus of the HBV X protein does not seem to be necessary for its transactivating activity, as demonstrated by using HBV X protein deletion mutants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Elfassi E. Broad specificity of the hepatitis B enhancer function. Virology. 1987 Sep;160(1):259–262. doi: 10.1016/0042-6822(87)90069-9. [DOI] [PubMed] [Google Scholar]
- Elfassi E., Haseltine W. A., Dienstag J. L. Detection of hepatitis B virus X product using an open reading frame Escherichia coli expression vector. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2219–2222. doi: 10.1073/pnas.83.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfassi E., Romet-Lemonne J. L., Essex M., Frances-McLane M., Haseltine W. A. Evidence of extrachromosomal forms of hepatitis B viral DNA in a bone marrow culture obtained from a patient recently infected with hepatitis B virus. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3526–3528. doi: 10.1073/pnas.81.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Guyader M., Montagnier L., Baltimore D., Muesing M. A. The specificity of the human immunodeficiency virus type 2 transactivator is different from that of human immunodeficiency virus type 1. EMBO J. 1987 Dec 1;6(12):3755–3760. doi: 10.1002/j.1460-2075.1987.tb02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hauber J., Perkins A., Heimer E. P., Cullen B. R. Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6364–6368. doi: 10.1073/pnas.84.18.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J., Stemler M., Will H., Schröder C. H., Kühn J., Braun R. Frequent detection of antibodies to hepatitis B virus x-protein in acute, chronic and resolved infections. Med Microbiol Immunol. 1988;177(4):195–205. doi: 10.1007/BF00211219. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Smith D. H., Jakobovits E. B., Capon D. J. A discrete element 3' of human immunodeficiency virus 1 (HIV-1) and HIV-2 mRNA initiation sites mediates transcriptional activation by an HIV trans activator. Mol Cell Biol. 1988 Jun;8(6):2555–2561. doi: 10.1128/mcb.8.6.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. Y., Calman A. F., Luciw P. A., Peterlin B. M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987 Dec 3;330(6147):489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Kay A., Mandart E., Trepo C., Galibert F. The HBV HBX gene expressed in E. coli is recognised by sera from hepatitis patients. EMBO J. 1985 May;4(5):1287–1292. doi: 10.1002/j.1460-2075.1985.tb03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Wells F., Tennant B. C., Yoakum G. H., Purcell R. H., Gerin J. L. Hepadnavirus infection of peripheral blood lymphocytes in vivo: woodchuck and chimpanzee models of viral hepatitis. J Virol. 1986 Apr;58(1):1–8. doi: 10.1128/jvi.58.1.1-8.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laure F., Zagury D., Saimot A. G., Gallo R. C., Hahn B. H., Brechot C. Hepatitis B virus DNA sequences in lymphoid cells from patients with AIDS and AIDS-related complex. Science. 1985 Aug 9;229(4713):561–563. doi: 10.1126/science.2410981. [DOI] [PubMed] [Google Scholar]
- Meyers M. L., Trepo L. V., Nath N., Sninsky J. J. Hepatitis B virus polypeptide X: expression in Escherichia coli and identification of specific antibodies in sera from hepatitis B virus-infected humans. J Virol. 1986 Jan;57(1):101–109. doi: 10.1128/jvi.57.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. Antibodies to pre-S and X determinants arise during natural infection with ground squirrel hepatitis virus. J Virol. 1986 Oct;60(1):177–184. doi: 10.1128/jvi.60.1.177-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin B. M., Luciw P. A., Barr P. J., Walker M. D. Elevated levels of mRNA can account for the trans-activation of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9734–9738. doi: 10.1073/pnas.83.24.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Seto E., Yen T. S., Peterlin B. M., Ou J. H. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Patarca R., Rosen C., Wong-Staal F., Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–77. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tognoni A., Cattaneo R., Serfling E., Schaffner W. A novel expression selection approach allows precise mapping of the hepatitis B virus enhancer. Nucleic Acids Res. 1985 Oct 25;13(20):7457–7472. doi: 10.1093/nar/13.20.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Chu K., Robinson W. S. Hepatitis B virus X gene activates kappa B-like enhancer sequences in the long terminal repeat of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5168–5172. doi: 10.1073/pnas.86.13.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcriptional trans-activating function of hepatitis B virus. J Virol. 1987 Nov;61(11):3448–3453. doi: 10.1128/jvi.61.11.3448-3453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice J. L., Levinson A. D. Properties of the human hepatitis B virus enhancer: position effects and cell-type nonspecificity. J Virol. 1988 Apr;62(4):1305–1313. doi: 10.1128/jvi.62.4.1305-1313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm P., Hofschneider P. H., Koshy R. The HBV X-ORF encodes a transactivator: a potential factor in viral hepatocarcinogenesis. Oncogene. 1988 Aug;3(2):169–177. [PubMed] [Google Scholar]