Abstract
In HIV-1 infected people, the accumulation of macrophages in the brain correlates with encephalitis and dementia. We hypothesized that a pattern of blood monocyte surface marker expression may serve as marker for CNS disease. Using the SIV/rhesus monkey model, we analyzed functionally relevant surface markers on monocytes and macrophages from blood and brain in animals that did or did not develop SIV encephalitis. At necropsy, multiple markers (CD44v6, CCR2, and CCR5 on blood monocytes and brain microglia/macrophages, and CX3CR1 on blood monocytes) allowed the separation of animals with encephalitis from those without. Furthermore, the level of expression of CD44v6 on the two main populations of blood monocytes: those that express either low or high levels of CD16, was significantly increased in animals with encephalitis. A longitudinal analysis of blood monocyte markers revealed that, as early as 28 days after inoculation, CD44v6 staining could distinguish the two groups. This provides a potential peripheral biomarker to identify individuals who may develop the HIV-induced CNS disease. Furthermore, given its role in cellular adhesion and as an osteopontin receptor, CD44v6 upregulation on monocytes offers functional clues to the pathogenesis of such complications, and provides a target for preventative and therapeutic measures.
Keywords: SIV, Encephalitis, Macrophage, Monocytes, NeuroAIDS, CD44v6
Introduction
Simian immunodeficiency virus (SIV) and Human Immunodeficiency Virus (HIV) are retroviruses that disturb the immune system integrity in their respective hosts, and induce variable levels of Central Nervous System (CNS) dysfunctions. The most severe CNS disease is characterized pathologically by the presence of encephalitis, dominated by macrophages in the brain, and clinically by a debilitating dementia. In the brain, virus is found predominantly in macrophages as well as microglia. Although the etiopathogenesis of HIV-induced CNS disorders are still under active investigation, resident microglia and infiltrating brain macrophages can secrete harmful products [1-3], which are detrimental to CNS function. In addition, viral proteins themselves have been demonstrated to have numerous neuroinjurious properties.
Although in developed countries efficacious therapy is now available, resulting in a reduced incidence of CNS and other complications of HIV infection, the prevalence of CNS-related abnormalities has not decreased, and in fact appears to be increasing [4, 5]. In addition, the CNS can still serve as a reservoir for the virus.
The discovery of biomarkers to aid in the identification of individuals at risk for, or in the process of developing, CNS disease in the setting of HIV infection has been a challenge. So far, markers have not been found to correlation with encephalitic outcome. The accumulation of macrophages in the brain has been the best correlate of CNS disease, however this is identified post-mortem. Therefore, the detection of blood-monocyte markers modulated by the disease state in correlation with brain pathological findings can be relevant for diagnosis. Monocytes are a heterogeneous population of cells, based on the expression of surface markers [6]. One major subdivision of this cell population, monocytes that are CD16+ (as opposed to CD16low), correspond to 5-10% of monocytes, but this population is expanded in several pathological conditions [6]. For instance, patients and monkeys having symptoms of HIV/SIV-induced CNS were found to have increased CD16+ monocytes in the circulation [7-9]. Furthermore, CD16+ monocytes were suggested to be the cell type that infiltrates the CNS in correlation with HIV dementia, since the perivascular macrophages in the brain are CD16+ [10-12].
Altered monocyte migration into the brain leads to the encephalitis caused by lentiviruses, including HIV and SIV. In the SIV/monkey model, subpopulations of monocytes are expanded during acute SIV infection, but this expansion was not unique or greater in macaques that developed encephalitis [13]. Furthermore, the proportion of monocytes expressing markers such as CD62L, HLA-DR, CD64 and CD40 varied among animals that eventually developed encephalitis [14]. Although no correlation was found, but this neither rules out the importance of activated monocytes in the development of CNS inflammation, nor the possibility that a correlation to CNS disease could be found on monocytes. Therefore the search for predictors remains a challenge.
Similar to humans with dementia and HIV encephalitis, SIV-infected monkeys with neurological symptoms frequently prove to have encephalitis. Therefore, the use of the monkey/SIV model can aid in the detection of a pattern of markers that can be evaluated prior to the development of disease. Here, we compared surface markers expressed on blood monocytes and tissue macrophages and identified patterns that characterize animals with encephalitis. We report the identification of a candidate biomarker, CD44v6, whose expression on peripheral monocytes is correlated with the development of encephalitis.
Material and Methods
Monkeys
Rhesus macaques were infected with a microglia-passaged stock of SIVmac251 [15]), and sacrificed at 6 months post-infection (or earlier if exhibiting symptoms of disease, using criteria including progressive weight loss, marked hematological abnormalities, persistent anorexia, diarrhea not responsive to treatment, and the presence of neurological or behavioral signs). Necropsies were performed following the administration of receiving ketamine (50 mg/kg), xylazine (4 mg/kg) and pentobarbital (10 mg/kg), and animals were extensively perfused with sterile PBS containing 1 U/ml heparin to flush blood-borne cells from the organs. All organs were examined grossly and microscopically. Encephalitis was diagnosed by the presence of multinucleated giant cells, perivascular and infiltrating macrophages, and expression of viral RNA (by in situ hybridization) and/or protein (by immunohistochemistry) in the brain. Two groups of animals were thus identified histopathologically: those that developed encephalitis (n=6) and those that did not (n=9). All animal procedures had the approval of the TSRI Animal Care and Use Committee and followed NIH guidelines.
Viral Infection
A cell-free inoculum stock of SIV, obtained after serial passage of SIV-infected microglia [16], was used to inoculate animals by intravenous injection into the saphenous vein. Animals received an amount of virus containing 5 ng/ml of p27 (Gag) antigen.
Viral Quantitation
To measure viral load, the concentration of SIV RNA in plasma and CSF was calculated by using the quantitative branched DNA (bDNA) signal amplification assay. Bayer Reference Testing Laboratory (Emeryville, CA) performed this assay.
Cells
Mononuclear cells were isolated from serial blood samples, or at necropsy from brain, as previously described [17]. Briefly, buffy coats obtained from centrifugation of EDTA-anti-coagulated blood, and cell suspensions from organs passed through a 70 μm nylon mesh, were subjected to a Histopaque (Sigma-Aldrich, Saint Louis, MO) gradient centrifugation for isolation of the mononuclear fraction. Brain cell suspensions were additionally subjected to a digestion using 28 U/ml DNAse I (Sigma-Aldrich) and 50 U/ml Collagenase II (Sigma-Aldrich) prior to a gradient in 1.033 g/ml Percoll solution (Sigma-Aldrich). After washing, cells were enumerated in a Coulter Z2 (Coulter Co., Miami, FL).
Flow cytometry
Cells isolated as described (2 - 10 × 105) were stained with 50 μl mixtures of antibodies diluted according to a previous titration in staining buffer (HBSS with 2% FCS and 0.01% NaN3). The antibodies used for the staining were against CD11b (clone M1/70(9), BMB, Indianapolis, IN), CD14, CD16, CCR5, CCR2, CX3CR1, CD62L (BD Biosciences, San Jose, CA), CD80 (BD Biosciences), and CD44v6 (clone 2F10, Zymed, San Francisco, CA). Isotype controls (BD Biosciences) were also used, in association with CD14 and CD16, in order to identify differential background on specific populations, and to determine background levels for the setting of gate. In order to minimize daily variations and make results comparable between animals, FACS (Fluorescence-Activated Cell Sorter) controls were calibrated using Spherobeads (Spherotech, Libertyville, IL) prior to each acquisition, for keeping consistent baseline levels. The cells were then processed through a FACSalibur flow cytometer device (BD Biosciences) before analysis of data with FlowJo software (Tree Star Inc., Ashland, OR). Background gating was performed with isotype control antibodies to determine percent positive for a given marker. The geometric mean fluorescence was used to compare relative density of surface marker expression.
Histopathology and immunohistochemistry
Tissues were fixed in 10% formalin, embedded in paraffin, and cut into 5 μm thick sections. After hematoxylin and eosin staining, sections from each tissue block were examined microscopically. Representative sections of brain (plus lymph node, liver and spleen controls) were immunohistochemically stained for CD163. Sections mounted on slides were deparaffinized and hydrated. Endogenous peroxidase was blocked by a 30 minute treatment with 0.3% H2O2 in 100% methanol. Antigen was recovered by immersing slides in 10 mM citrate, pH 6.4, in a steamer for 45 minutes. Nonspecific binding was blocked with a casein solution, in PBS humidified chamber. The slides were incubated overnight with the primary antibody, anti-CD163 (Vector Labs, Burlingame, CA) in casein block solution, at 4° C. Indirect immunoperoxidase detection, using the VIP NovaRed (Vector Labs) chromogen, was followed by Gill’s hematoxylin counterstaining. The slides were mounted and evaluated microscopically.
Statistical Analysis
Measurements for a series of seven time points were analyzed: day 0 (preinfection), days 10, 14, 28, 42, 59 and 120 days (or termination point if earlier). In order to compare levels of expression in terminal blood and brain cells, two-way repeated measures ANOVA (rmANOVA). For longitudinal analysis, a one-way rmANOVA was used across time points to determine overall differences. If a significant rmANOVA was obtained, a post hoc Dunnett’stest was used to identify the day(s) that differed in each group. In addition, the Student’s t test, by day between groups, was applied to identify the differences between the groups. For correlation analysis, a linear regression and calculation of Pearson’s correlation coefficient was performed. Statistical testing was performed, and graphs prepared, using Excel 2003 SP1 (Microsoft Corporation, Seattle, WN), Prism 4 (GraphPad Software, San Diego CA), DeltaGraph5 (Salt Lake City, UT) or StatgraphicsPlus 5.1 Professional edition software (StatPoint, Herndon, VA).
Results
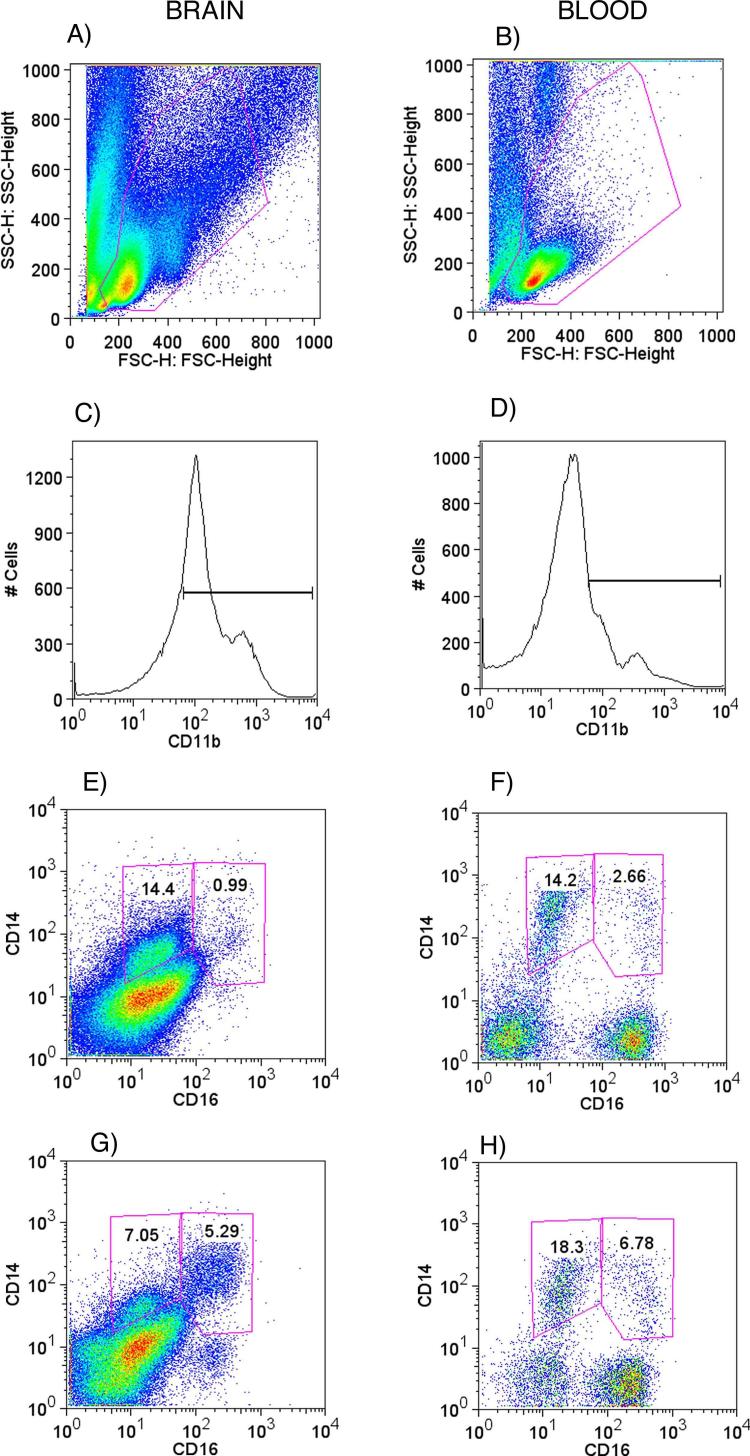
Since macrophages play a prominent role in the pathogenesis of HIV dementia and encephalitis, we used the SIV-infected rhesus monkey model to analyze myeloid cells from blood and brain, searching for molecules with prognosis potential. Mononuclear cells were isolated from the blood and brain from animals with and without encephalitis, and analyzed by FACS to determine surface marker expression on mononuclear myeloid cells. Such cells (monocytes in the blood, macrophage and microglia in brain) were first identified by forward and side-scatter properties (Figures 1A and 1B) using a wide gate to include lymphocyte-like cells, since small monocytes and recently migrating tissue macrophages can be localized in those regions. Upon differential identification of background using isotype controls, monocyte/macrophages/microglia were selected for analysis by positively gating CD11b- expressing cells (Figures 1C and 1D), followed by CD14. This procedure eliminated lymphocytes from the analysis, as such cells were negative when stained for CD3 (T cell marker) or CD19 (B cell marker) (data not shown). Different subsets were further distinguished based on CD16 expression, as illustrated in Figures 1 for brain (left side panels) and blood (right side panels). A higher level of CD16 expression on monocyte-lineage cells has been found to indicate an inflammatory phenotypic state, and an increase of CD14+ CD16+ cells in the brains correlated with SIV encephalitis as opposed to SIV-infected animals without encephalitis (Figure 1G in comparison to Figure 1E).
Figure 1.
Brain and blood CD11b-gated CD14/CD16 expressing cells. On the left side panels (A, C, E, and G) are brain cell suspensions, and on the right side panels (B, D, F, and H) correspond to blood samples. A and B show the gate on total leukocytes, excluding granulocytes. C and D show CD11b+ cell gates. E and F are brain and blood cells respectively, from an animal without encephalitis, segregated according to CD14 and CD16 expression, delimiting our target sub-populations. G and H are brain and blood from an animal with encephalitis.
In order to further assess the CD14+CD16low and CD14+CD16+ cells, a variety of markers were analyzed on their surface (CCR2, CX3CR1, CCR5, CD80, CD62L and CD44v6), covering a range of molecules with potential links to pathogenic processes involved in HIV-induced CNS disease. The percentage of mononuclear myeloid cells expressing surface molecules in blood and brain from SIV infected animals with or without encephalitis was first analyzed for the termination time points using two-way rmANOVA. The percentage of blood monocytes expressing CCR2 (p=0.0415), CX3CR1 (p=0.0021), CCR5 (p=0.0284) and CD44v6 (p=0.0037) distinguished between animals with and without encephalitis. In addition, the percentage of brain-derived cells (microglia and macrophages) expressing CCR2 (p=0.0290), CCR5 (p=0.0049) and CD44v6 (p=0.0064) also differed between animals of different groups.
In addition to the percentage of cells expressing surface molecules, the geometric mean fluorescence was also evaluated to assess’ density of expression of a given marker, since in addition to differences in the percentage of cells staining above the negative control background level, differences in the amount and pattern of expression could also be appreciated (Figure 2). In blood monocytes, the mean fluorescence of CD44v6 increased significantly in animals with encephalitis compared to those without encephalitis (two-way rmANOVA, p=0.0006), and post-hoc testing revealed significant differences on both cell subsets (Bonferroni’s, p<0.01 for CD16low and p<0.05 for CD16+) (Figures 3A and 3B). In the brain, CCR5 could distinguish the groups with and without encephalitis, by a significant increase of mean fluorescence in animals with encephalitis (two-way rmANOVA, p=0.0021); post-hoc testing revealed significant differences in the CD14+CD16+ cells (Bonferroni’s, p<0.01) (Figure 3D). Examination of the mean fluorescence for the other markers did not reveal significant differences.
Figure 2.

Expression intensity of CD44v6 and CCR5 on monocytes and macrophages, from blood and brain. Representative CD44v6 (upper panel) and CCR5 (lower panel) expression histograms in different organs. Cells from different sites were analyzed on FACS for the expression of CD44v6 and CCR5 on CD14+CD16low cells (gray lines) and on CD14+CD16+ cells (black lines), in animals without encephalitis (SIVE-) and in animals with encephalitis (SIVE+).
Figure 3.

CCR5 and CD44v6 on CD14+ cells from blood and brain at termination time point. CD14+ cells from animals without encephalitis (SIVE-) and from animals with encephalitis (SIVE+) were further divided in subpopulations with CD16+ and CD16low expression, for evaluation of levels of CD44v6 as well as CCR5 on cell surface. Results expressed in Geometric Mean Fluorescence, corresponding to average ± SEM of 6 SIVE- and 4 SIVE+, were compared by two-way rmANOVA followed by Bonferroni’s test. *p<0.05 Bonferroni’s between groups.
The markers that distinguished animals with and without encephalitis in the blood (CCR2, CCR5, CD44v6 and CX3CR1) were then analyzed longitudinally in the two populations of monocytes (Figure 4). We found that CD44v6 expression in animals destined to develop encephalitis changed as early as 14 days after SIV infection in CD14+CD16low (one-way ANOVA, p<0.0001, Dunnett’s multiple comparison test, p<0.05 compared to day 0 versus all days except day 10) monocytes in comparison with pre-infection values. Changes were also found in CD14+CD16+ monocytes (one-way ANOVA, p=0.0111), but specific differences from baseline could not be identified by post-hoc testing. The comparison between the staining patterns of an animal before, and 28 days after SIV inoculation, reveals the distinct pattern of CD44v6 expression (Figure 4C). The expression of CD44v6 also distinguished between animals with and without encephalitis, especially on CD14+ CD16+ (Figure 4B), which differed on days 28 (Student’s t test, p=0.0282), 77 (p=0.0017), and 126 (p=0.0003). In the CD14+ CD16low subpopulation, CD44v6 expressing cells differed on day 126 (p=0.0008). The expression of both CX3CR1 and CCR2 did not change either from baseline after infection nor between the groups of animals along infection in either of the CD14+ subsets (Figures 4D, 4E and 4F; 4G, 4H and 4I). CCR5 within CD14+ CD16+, but not the CD14+CD16lo, cells changed from baseline (one-way ANOVA p=0.0157), with differences identified on days 14, 28, 42 and 77 (Dunnett’s, p<0.05). The percentage of CCR5-positive cells differed between the groups in only a single, different time point in the CD16low and CD16+ subpopulations (Figures 4J and 4K).
Figure 4.
Longitudinal expression of CD44v6, CX3CR1, CCR2 and CCR5 on subpopulations of monocytes in animals without encephalitis (SIVE-, open squares) and animals with encephalitis (SIVE+, black circles). Results represent the average ± SEM of 9 SIVE- and 6 SIVE+ animals. Expression of CD44v6 on CD14+ CD16low (A) and CD14+CD16+ (B) cells along infection; C) represents one animal from SIVE+ group, of which the expression of CD44v6 on CD14+ cells is shown at day 0 (gray dots) and at day 28 (red dots) after SIV infection. D) Expression of CX3CR1 on CD14+ CD16low (D) and CD14+ CD16+ (E) cells along infection; F) represents an animal from SIVE+ group, of which the expression of CX3CR1 on CD14+ cells is shown at day 0 (gray dots) and at day 28 (red dots) after SIV infection. Expression of CCR2 on CD14+ CD16low (G) and CD14+ CD16+ (H) cells along infection; I) represents an animal from SIVE+ group, of which the expression of CCR5 on CD14+ cells is shown at day 0 (gray dots) and at day 28 (red dots) after SIV infection. Expression of CCR5 on CD14+ CD16low (J) and CD14+ CD16+ (K) cells along infection; L) represents an animal from SIVE+ group, of which the expression of CCR5 on CD14+ cells is shown at day 0 (gray dots) and at day 28 (red dots) after SIV infection. *p<0.05 Student’s t test between groups; #p<0.05 Dunnett’s test in comparison to baseline within group.
As stated above, CCR5 distinguished encephalitic cells in the brain, based not only on the percentage of positive cells, but also on the density (geometric mean fluorescence) of cell-surface molecules on the CD16+ cells. Therefore we determined whether there is a correlation between CCR5 expression and the high viral load that occurs in encephalitis. The percentage of cells expressing CCR5 within the CD16+ compartment and the brain viral burden were used to derive an inflammatory index [%CCR5+ in CD14+CD16+ gate/(%CD14+CD16low cells + %CD14+CD16+ cells)]. This index takes into account the percentage of CCR5+ cells within the CD16+ subpopulation and normalizing it for the total macrophage population, in order to put it in perspective with non-inflammatory infiltrating cells. Higher values were obtained for SIV-encephalitis animals, and when analyzed versus the brain viral load, a strong correlation was found (Figure 5D, Pearson’s correlation coefficient, r2=0.9025). The high inflammatory index correlated with the histological detection of abundant inflammatory macrophages in SIVE+ animals, identified by the presence focal or diffuse infiltrating cells, which stained positive for CD163 by immunohistochemistry, characterizing inflammatory macrophages in both parenchyma and perivascular domains (Figures 5A and 5B). These cells were rare in the brains of SIV-infected animals without encephalitis (Figures 5C and 5D).
Figure 5.

Inflammation in the SIV-infected brain. SIVE+ (A, B) and SIVE- (C, D) brains were immunostained for CD163 to detect of inflammatory macrophage accumulation. CD163+ cells (*) can be found in abundance in SIVE+ animals in the parenchyma and in perivascular domains, while they are not frequent in SIVE-animals (40x magnification). (E). The percentage of CCR5 expressing cells in the CD16+ compartment in the brain was used to derive an inflammatory index linearized against the brain viral load obtained by bDNA. The Pearson’s correlation coefficient (r2= 0.9025) demonstrated the linear correlation between CCR5-expressing inflammatory cells and viral burden, which segregated SIVE- (open circles) from SIVE+ animals (filled circles).
In regard to the overall values for blood mononuclear phagocytic cells, SIV-infected animals that did not develop encephalitis presented a significant increase in the percentage of both monocyte subpopulations at 10 and 28 days after inoculation in relation to the baseline values (repeat measures ANOVA, p<0.0001 for both CD16+ and CD16low; Dunnett’s post hoc test, p<0.01 for day 10 and p<0.05 for day 28, in both cell subpopulations as compared to baseline values within group) (Figures 6A and 6B, respectively); this was not observed in SIV-encephalitis animals. In addition, the percentage of CD14+ CD16low and CD14+ CD16+ cells was significantly different between groups at day 10 (Student’s t test, p=0.0001 and p=0.0001), respectively), and CD14+CD16low cells differed between groups at day 42 (p= 0.0116). However, in both groups absolute monocyte counts were unchanged in relation to baseline at 10 days pi, thus the difference in percentage was due to a temporary decrease in blood lymphoid cells in the group that did not develop encephalitis.
Figure 6.
Percentage of monocytic subpopulations along SIV infection. CD14+ CD16 low (A) and CD14+ CD16+ (B) were longitudinally analyzed among total gated leukocytes in animals without encephalitis (SIVE-, open squares) and in animals with encephalitis (SIVE+, black circles). Nine SIVE- and 6 SIVE+ animals were compared by one-way rmANOVA for identification of changes along the course of infection, followed by Dunnett’s posthoc within groups to compare to baseline (pre-infection) values. Results represent the average ± SEM. *p<0.05 Student’s t test between groups; # p<0.05 Dunnett’s test in comparison to baseline within group.
Discussion
In the present work, a relationship between changes in monocyte expression of CD44v6 and the development of encephalitis was observed. CD44v6 staining intensity distinguished the SIV-encephalitis animals from those that did not develop encephalitis as early as 28 days post infection in CD16+ monocytes, representing remarkable prognostic power. Although CD44v6 expression did not differ on myeloid cells in the brain tissue itself, CCR5 expression was increased on inflammatory CD14+CD16+ cells at that site, and this expression was found to positively correlate with brain viral load.
Our work also suggests a mechanism for encephalitis development. CD44v6 is a receptor on monocytes and macrophages for osteopontin, a pleiotropic molecule that we found to be upregulated in the brains as well as in plasma and CSF of monkeys with SIV encephalitis [18, 19]. Thus in the setting of disease, both the ligand and receptor are elevated. Osteopontin can also regulate the expression of its receptors [20], leading to a positive feedback loop. Recently, we have reported that osteopontin protects against monocyte apoptosis, and reduces the reverse transmigration (i.e. leaving tissues) of recently immigrated monocytes, both of which can contribute to the development of encephalitis [19]. Together, these results confirm the importance of monocytes/macrophages in SIV/HIV-inducted CNS disease.
The accumulation of mononuclear phagocytes into the brain is a critical step in CNS pathologies. The entrance of blood-derived macrophages, as well as activation of cells in the CNS contribute to several neuronal disruption pathways, through the secretion of inflammatory products, such as nitric oxide, quinolinic acid, or cytokines such as TNF-α. In HIV and SIV infection, the ability to examine peripheral markers, such as monocyte surface antigen expression, that can correlate to CNS disease are extremely valuable given the lack of known biomarkers for CNS disease.
Curiously, the percentage of monocytes peaked in the blood during the acute phase in SIV-infected monkeys that did not develop encephalitis. Although this peak in the SIV-encephalitis group may result from a temporary decrease in blood lymphoid cells in the group that did not develop encephalitis, as absolute monocyte counts were statistically unchanged in relation to baseline at those time points, it still suggests that the ratio between myeloid and lymphoid cells can be an important aspect of early viral control. Alterations in the ratio could interfere with the quality of priming of these same T cells, and affect properties such as specificity and avidity. However our data indicate that the relevant changes on monocytic populations are functional, since surface molecules such as CD44v6 in periphery and CCR5 in the brain tissue distinguished the groups and the brain inflammatory condition.
Interestingly CCR2 distinguished groups only at termination time points, suggesting the involvement of CCL2, which has been described to increase in the CSF in correlation with SIV encephalitis [21], may happen at later stages. However, as changes in blood monocytes during the course of infection were not detectable, this marker did not exhibit predictive power.
The increase on CD44v6 levels appeared at 28 days after infection in the animals that went on to develop encephalitis. However, we still do not know whether macrophage accumulation in the brain occurs synchronously with CD44v6. The CD44 isoform that contains the exon 6, has been described to be generated from the interaction of CD44 with hyaluronan, which leads to modifications of the splice patterns of CD44 mRNA, inducing variants including the CD44 variant 6 [22]. In addition, other components of extracellular matrix, fibronectin and collagen can bind to CD44 [23-25], and can contribute to the migration of cells expressing these molecules towards inflamed tissue. Interestingly, CD44v6 is one of the major receptors for osteopontin [26]. In vitro, osteopontin has been shown to induce an increase of CD44v6 at the protein level [27]. Thus the changes on CD44v6 expression on mononuclear phagocytes observed in SIV encephalitis in rhesus macaques may in part reflect changes of levels of osteopontin, which we find elevated, in terminal samples, in the plasma and CSF of monkeys with SIV encephalitis [19]. Both osteopontin and CD44v6 have been extensively implicated in cell motility related to inflammatory processes [28]. In addition to our findings in SIV encephalitis, osteopontin has been shown to be implicated in other CNS pathologies such as experimental autoimmune encephalomyelitis, multiple sclerosis [29, 30], Tay-Sachs and Sandhoff diseases [31].
The expression of CCR5 was high on CD14+CD16low peripheral monocytes from SIVE+ animals at terminal time points. In the brain, however, SIVE+ animals showed a dramatic increase in the expression of CCR5 on CD14+CD16+ cells, which are the predominant infected cell type in SIV/HIVinfected brains [32]. In addition, the expression of CCR5 on CD14+CD16+ macrophages showed a strong correlation with brain viral load. Given that CCR5-tropic virus predominate in the brain, the increased expression of CCR5 on macrophages provides a mechanism for increased infection and increased virus production. Adhesion, transmigration and chemoattraction markers on monocytes are all affected during SIV infection. In addition to increasing infectivity, increased expression of CCR5 would increase chemotactic responsiveness to its ligands, MIP-1α, -1β and RANTES [33, 34].
Taken together, the present results indicate that qualitative changes are detectable on monocytic populations after SIV infection that can predict the onset of encephalitis. The expression of CD44v6 on monocytes can be used as a peripheral correlate of CNS pathology. In addition, the levels of CCR5 were particularly high on macrophages in inflamed brains, enabling the amplification of virus infection during encephalitis. These findings enable an early detection of a likely susceptible physiological phenotype, which can be potentially altered by adjustments of therapeutic approach. A longitudinal study of CD44v6 expression on monocytes in HIV-infected individuals would be invaluable to assess whether this can serve as a biomarker to predict the development of CNS disease in humans.
Table 1.
Percentage of monocyte/macrophages expressing surface functional markers in the blood and brain of SIV-infected macaques at necropsy. Cells obtained as described were stained for flow cytometry and gated on CD14+, CD16low and CD16+ cells. The results are expressed in percentage within gated cells, and represent the average ± SD of 6 infected animals without encephalitis (SIVE-) and 4 infected animals with encephalitis (SIVE+) monkeys in blood and brain cells obtained at necropsy time-points. Each acquisition was calibrated to assure equivalent brightness and allow result comparison. * Indicates significant difference in blood, ^ indicates significant difference in brain, p values are given in the text.
| Blood | Brain | ||||
|---|---|---|---|---|---|
| CD16low | CD16+ | CD16low | CD16+ | ||
| CCR2*^ | SIVE- | 60.3±26.3 | 31.21±6.8 | 29.4±38.1 | 42.98±31.9 |
| SIVE+ | 80.7±33.8 | 72.2±19.0 | 80.6±12.6 | 86.15±7.5 | |
| CX3CR1* | SIVE- | 23.1±14.9 | 31.8±16.4 | 40.6±24.4 | 60.1±24.9 |
| SIVE+ | 72.2±38.7 | 70.4±33.4 | 68.1±25.8 | 55.9±12.44 | |
| CCR5*^ | SIVE- | 37.1±29.5 | 46.2±29.53 | 45.3±26.0 | 51.2±22.5 |
| SIVE+ | 72.5±18.3 | 84.6±16.9 | 91.3±7.3 | 79.8±19.7 | |
| CD80 | SIVE- | 36.6±34.6 | 49.7±32.6 | 45.9±28.1 | 63.3±12.1 |
| SIVE+ | 33.0±31.2 | 57.6±34.1 | 62.3±29.4 | 65.2±16.4 | |
| CD62L | SIVE- | 59.7±19.8 | 36.4±21.8 | 41.3±28.1 | 64.2±35.6 |
| SIVE+ | 82.6±16.4 | 56.4±38.6 | 74.4±27.5 | 66.5±20.4 | |
| CD44v6*^ | SIVE- | 26.1±21.5 | 35.6±14.1 | 42.2±21.5 | 34.4±26.7 |
| SIVE+ | 69.6±26.2 | 71.9±17.3 | 73.5±16.0 | 78.9±3.6 | |
Acknowledgements
We thank Dr. Momtchilo Russo (Immunology Department, Biomedical Sciences Institute, Universidade de Sao Paulo, Brazil) for insights, Dr. Aluisio Augusto Cotrim Segurado (School of Medicine, Universidade de Sao Paulo, Brazil) for very helpful and interesting discussions, Nancy Delaney for administrative assistance and Cathie York-DeFalco for technical assistance. The authors declare no conflict of interests.
This work was funded by NIH grants NS045534, MH073490, and MH062261. MCGM designed and performed research, collected, analyzed and interpreted data, and drafted this manuscript; CMSL assisted with data and statistical analysis; THB performed experiments and joined discussions, DDW performed experiments and analyzed data; HSF obtained funding, designed research, interpreted data, and drafted this manuscript. This is manuscript # 18994 of The Scripps Research Institute.
References
- 1.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 2.Gendelman HE, Persidsky Y, Ghorpade A. The neuropathogenesis of the AIDS dementia complex. Aids. 1997;11(Suppl A):S35–45. [PubMed] [Google Scholar]
- 3.Koenig S, Gendelman HE, Orenstein JM. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–93. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SA. Anti-HIV drug distribution to the central nervous system. Curr Pharm Des. 2004;10:1313–24. doi: 10.2174/1381612043384835. [DOI] [PubMed] [Google Scholar]
- 5.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. Aids. 2004;18(Suppl 1):S75–8. [PubMed] [Google Scholar]
- 6.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 7.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 8.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–24. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 9.Kim WK, Avarez X, Williams K. The role of monocytes and perivascular macrophages in HIV and SIV neuropathogenesis: information from non-human primate models. Neurotox Res. 2005;8:107–15. doi: 10.1007/BF03033823. [DOI] [PubMed] [Google Scholar]
- 10.Fischer-Smith T, Croul S, Sverstiuk AE. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–41. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 11.Lane JH, Sasseville VG, Smith MO. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;2:423–32. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- 12.Simpson DM, Tagliati M. Neurologic manifestations of HIV infection. Ann Intern Med. 1994;121:769–85. doi: 10.7326/0003-4819-121-10-199411150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Bissel SJ, Wang G, Trichel AM, Murphey-Corb M, Wiley CA. Longitudinal analysis of monocyte/macrophage infection in simian immunodeficiency virus-infected, CD8+ T-cell-depleted macaques that develop lentiviral encephalitis. Am J Pathol. 2006;168:1553–69. doi: 10.2353/ajpath.2006.050240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bissel SJ, Wang G, Trichel AM, Murphey-Corb M, Wiley CA. Longitudinal analysis of activation markers on monocyte subsets during the development of simian immunodeficiency virus encephalitis. J Neuroimmunol. 2006;177:85–98. doi: 10.1016/j.jneuroim.2006.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane TE, Buchmeier MJ, Watry DD, Jakubowski DB, Fox HS. Serial passage of microglial SIV results in selection of homogeneous env quasispecies in the brain. Virology. 1995;212:458–65. doi: 10.1006/viro.1995.1503. [DOI] [PubMed] [Google Scholar]
- 16.Watry D, Lane TE, Streb M, Fox HS. Transfer of neuropathogenic simian immunodeficiency virus with naturally infected microglia. Am J Pathol. 1995;146:914–23. [PMC free article] [PubMed] [Google Scholar]
- 17.Marcondes MC, Burudi EM, Huitron-Resendiz S. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167:5429–38. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- 18.Roberts ES, Zandonatti MA, Watry DD. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162:2041–57. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdo TH, Wood MR, Fox HS. Osteopontin prevents monocyte recirculation and apoptosis. J Leukoc Biol. 2007 doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan SA, Cook AC, Kappil M. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: novel post-transcriptional, post-translational regulation. Clin Exp Metastasis. 2005;22:663–73. doi: 10.1007/s10585-006-9007-0. [DOI] [PubMed] [Google Scholar]
- 21.Zink MC, Coleman GD, Mankowski JL. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–21. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- 22.Stern R, Shuster S, Wiley TS, Formby B. Hyaluronidase can modulate expression of CD44. Exp Cell Res. 2001;266:167–76. doi: 10.1006/excr.2001.5206. [DOI] [PubMed] [Google Scholar]
- 23.Miyake K, Underhill CB, Lesley J, Kincade PW. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990;172:69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter WG, Wayner EA. Characterization of the class III collagen receptor, a phosphorylated, transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem. 1988;263:4193–201. [PubMed] [Google Scholar]
- 25.Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992;116:817–25. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katagiri YU, Sleeman J, Fujii H. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–26. [PubMed] [Google Scholar]
- 27.Gao C, Guo H, Downey L, Marroquin C, Wei J, Kuo PC. Osteopontin-dependent CD44v6 expression and cell adhesion in HepG2 cells. Carcinogenesis. 2003;24:1871–8. doi: 10.1093/carcin/bgg139. [DOI] [PubMed] [Google Scholar]
- 28.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabas D, Baranzini SE, Mitchell D. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–5. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 30.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–9. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 31.Myerowitz R, Lawson D, Mizukami H, Mi Y, Tifft CJ, Proia RL. Molecular pathophysiology in Tay-Sachs and Sandhoff diseases as revealed by gene expression profiling. Hum Mol Genet. 2002;11:1343–50. doi: 10.1093/hmg/11.11.1343. [DOI] [PubMed] [Google Scholar]
- 32.Ellery PJ, Tippett E, Chiu YL. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–9. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 33.Westmoreland SV, Rottman JB, Williams KC, Lackner AA, Sasseville VG. Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am J Pathol. 1998;152:659–65. [PMC free article] [PubMed] [Google Scholar]
- 34.Zou W, Lackner AA, Simon M. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol. 1997;71:1227–36. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]