Summary
Protein sumoylation has emerged as an important regulatory mechanism for the transcriptional machinery. Sumoylation is a highly dynamic process that is regulated in response to cellular stimuli or pathogenic challenges. Altered activity of the SUMO conjugation system is associated with human cancers and inflammation. Thus, understanding the regulation of protein sumoylation is important for the design of SUMO-based therapeutic strategies for the treatment of human diseases. Recent studies indicate that the sumoylation system can be regulated through multiple mechanisms, including the regulation of the expression of various components of the sumoylation pathway, and the modulation of the activity of SUMO enzymes. In addition, extracellular stimuli can signal to the nucleus to trigger the rapid promoter recruitment of SUMO E3 ligases, resulting in the immediate repression of transcription. Finally, the sumoylation system can also be regulated through crosstalk with other posttranslational modifications, including phosphorylation, ubiquitination, and acetylation.
Introduction
The covalent attachment of small ubiquitin-like modifier (SUMO) to protein substrates is defined as SUMOylation [1–3]. The mammalian SUMO family consists of four members: SUMO-1, -2, -3, and -4. Protein sumoylation proceeds in three steps that resemble ubiquitination, but the enzymes involved in sumoylation and ubiquitination are different. SUMO is first activated in an ATP-dependent process by forming a thioester bond with the catalytic cysteine of the E1-activating enzyme, which is a heterodimer consisting of two proteins SAE1 and SAE2 (also known as Aos1 and Uba2, respectively). Next, SUMO is transferred to the catalytic cysteine of the E2-conjugating enzyme Ubc9, which directly binds to substrates. Subsequently, SUMO is conjugated to the protein substrate by forming an isopeptide bond between SUMO and the ε-amino group of a lysine side chain on the substrate. Unlike ubiquitination, sumoylation can occur without E3 ligases in vitro. However, SUMO E3 ligases can enhance the efficiency and provide substrate specificity to the sumoylation process. SUMO E3 ligases include the protein inhibitor of activated STAT family (PIAS), the polycomb group protein Pc2, and Ran binding protein 2 (RanBP2) [4,5]. The list of SUMO E3 ligases is growing as additional proteins with SUMO E3 ligase activity have been recently identified. For example, histone deacetylase 4 (HDAC4) can promote the sumoylation of MEF2, independent of its deacetylase activity [6]. The human topoisomerase I- and p53-binding protein Topors can function as both a ubiquitin and a SUMO E3 ligase for p53 [7,8]. Interestingly, a recent study indicates that the PHD domain of the KAP1 corepressor functions as an intramolecular E3 ligase that promotes the sumoylation of its adjacent bromodomain, which is required for KAP1-mediated gene silencing [9]. SUMO conjugation is reversible, and the SUMO-specific protease (SENP) family is responsible for rapid removal of SUMO from sumoylated protein substrates [10].
SUMO conjugation participates in various cellular processes, and the most recognized function of sumoylation is to regulate transcription. In fact, most sumoylation substrates identified so far are involved in transcriptional regulation. Sumoylation can either positively or negatively regulate transcription, although a majority of studies has identified a functional role of sumoylation in transcriptional repression.
Sumoylation can regulate transcription through multiple mechanisms, a topic which has been extensively reviewed [1,11,12]. Various components of the transcriptional machinery can be subjected to SUMO modification, such as transcription factors themselves, transcriptional co-regulators, and chromatin-remodeling proteins. Sumoylation can regulate transcription at multiple steps, including the DNA binding activity of transcription factors, the subcellular localization of transcription factors, the interaction between transcription factors and co-regulators, and the chromatin structure.
Sumoylation is a dynamic process that is regulated during cellular processes or under pathogenic conditions. Although sumoylation plays an important role in the regulation of transcription, how sumoylation itself is regulated has been poorly understood. This review will highlight the most recent progress made on the understanding of the molecular mechanisms involved in the regulation of the SUMO conjugation process.
Regulation of the expression of the Sumoylation System
The sumoylation system can be regulated at the level of expression. It has been shown that the expression of various components of the sumoylation system is regulated under certain physiological or pathogenic conditions.
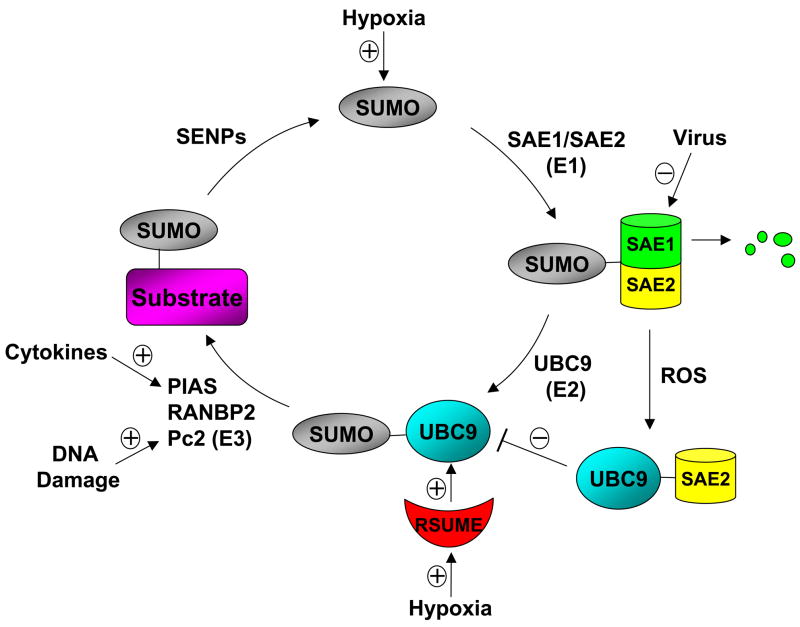
During keratinocyte differentiation, the sumoylation system was transiently upregulated by Ca2+ signaling [13]. Ca2+ induced the transcriptional activation of the genes encoding several components of the sumoylation system, including SAE1/SAE2, Ubc9, SUMO2/3, and PIASx. In human endometrial stromal cells, cAMP and progesterone signaling induced significant changes in the expression of PIAS SUMO E3 ligases and SENPs [14]. In addition, hypoxia can induce the expression of SUMO-1 [15] and an RWD-containing sumoylation enhancer (RSUME) that functions to promote protein sumoylation (see the next section for details) [16]. The elevated expression of Ubc9 has been observed in a number of human malignancies, such as lung adenocarcinoma, melanoma, breast and ovarian carcinoma [17]. Despite these interesting findings, little is known about the molecular mechanisms responsible for the regulation of the expression of the sumoylation system.
Recently, it has been reported that the increased expression of a 30-kDa dominant-negative isoform (C/EBPαp30) of CCAAT/enhancer-binding protein α (C/EBPα), which is present in acute myeloid leukemia (AML) patients with mutations in Cebpα gene, can induce the transcriptional induction of Ubc9 [18]. But initial analysis failed to detect the direct association of C/EBPαp30 with a promoter region of Ubc9. How C/EBPαp30 regulates the transcription of Ubc9 remains to be understood.
Recent studies suggest that the stability of SUMO enzymes can be regulated by ubiquitin E3 ligases. It was shown that human homologues of seven in absentia 2 (hSiah2), a known ubiquitin E3 ligase, targeted PIAS1 for degradation through the ubiquitination-proteasome pathway [19]. The ubiquitin E3 ligase Trim32, which is mutated in human limb-girdle muscular dystrophy type 2H and Bardet-Biedl syndrom, can promote the ubiquitination and degradation of PIASy [20]. In addition, the adenoviral protein Gam1 recruits two cellular cullin RING ubiquitin ligases to target the degradation of the SUMO E1 enzyme [21]. These studies suggest the involvement of the ubiquitination system in the regulation of protein sumoylation.
Regulation of the Activity of the SUMO Enzymes
In addition to regulate the expression levels of the components of the SUMO conjugation system, the intrinsic activity of the enzymes in the SUMO pathway can also be modulated by cellular stimuli (Fig. 1). Bossis and Melchior showed that lower concentrations of reactive oxygen species (ROS) caused the formation of a disulfide bond involving the catalytic cysteines of the SUMO E1 subunit Uba2 and the E2 enzyme Ubc9, resulting in the prevention of Ubc9-SUMO thioester formation, and thus the inhibition of SUMO conjugation [22]. ROS such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (·OH), can be produced in response to a variety of stimuli, including DNA-damaging agents, cellular stresses, cytokines and growth factors. Therefore, the inhibition of the activity of SUMO conjugating enzymes by ROS may represent a commonly utilized mechanism to regulate global sumoylation by multiple stimuli.
Figure 1. The regulation of the SUMOylation system.
A schematic representation of the regulation of the SUMO conjugation system at multiple steps. Protein sumoylation is accomplished in three steps involving three groups of enzymes: E1 (SAE1/SAE2), E2 (UBC9), and E3 ligases such as protein inhibitors of activated STAT (PIAS), Ran binding protein 2 (RanBP2), and the polycomb group protein Pc2. The SUMO-specific protease (SENP) family is responsible for the removal of SUMO from substrates. Hypoxia can induce the expression of SUMO-1 and an RWD-containing sumoylation enhancer (RSUME). Viral proteins such as Gam1 can target SAE1 for degradation through the ubiquitin-proteasome pathway. DNA damage can induce the SUMO ligase activity of Pc2. Cytokines such as TNFα signal to the nucleus to target PIAS1 E3 ligase for gene repression (See Figure 2 for details). Reactive oxygen species (ROS) can cause the formation of UBC9-SAE2 complex, which inhibits UBC9-SUMO thioester formation. RSUME binds to UBC9 to promote UBC9-SUMO thioester formation.
Most recently, a protein named RSUME (RWD-containing sumoylation enhancer) has been reported that can enhance overall SUMO-1, -2, and -3 conjugation [16]. RSUME binds to the E2 enzyme Ubc9 and increases the noncovalent association of Ubc9 with SUMO, which leads to the enhanced Ubc9-SUMO thioester formation and SUMO conjugation. Interestingly, RSUME expression is induced by hypoxia, which leads to the enhanced sumoylation of HIF-1α that promotes the stabilization and transcriptional activity of HIF-1α during hypoxia. It should be pointed out that the role of sumoylation in the regulation of HIF-1α stability is controversial. A recent study indicates that the hypoxia-induced sumoylation of HIF-1α targets HIF-1α for degradation through the von Hippel-Lindau (VHL) protein-mediated ubiqutin-proteasome pathway [23]. SENP1 functions to deconjugate sumoylated HIF-1α and prevents the degradation of HIF-1α during hypoxia. Consistently, Senp1 disruption leads to the degradation of HIF-1α, resulting in decreased erythropoietin (Epo) production and severe fetal anemia. Thus, sumoylation of HIF-1α triggers the ubiquitination and the subsequent degradation of HIF-1α. Clearly, further studies are needed to clarify these controversial findings on the role of sumoylation in the regulation of HIF-1α stability during hypoxia. Nevertheless, the studies by Carbia-Nagashima et al. strongly suggest that RSUME is a novel regulator of the sumoylation system.
The activity of a SUMO E3 ligase can be regulated by its sumoylation substrate. For example, the polycomb group protein Pc2 binds to homeodomain interacting protein kinase 2 (HIPK2), and acts as a SUMO E3 ligase for HIPK2 [24]. Upon DNA damage, HIPK2 is activated and then phosphorylates Pc2 on Thr495 as well as several other sites. The phosphorylation of Pc2 enhances its SUMO ligase activity toward HIPK2, resulting in the enhanced sumoylation and thus increased activity of HIPK2 in gene repression. These studies identify an interesting mechanism for the regulation of a SUMO E3 ligase activity by its sumoylation target.
A Signaling Pathway to Recruit SUMO E3 Ligase for Gene Repression
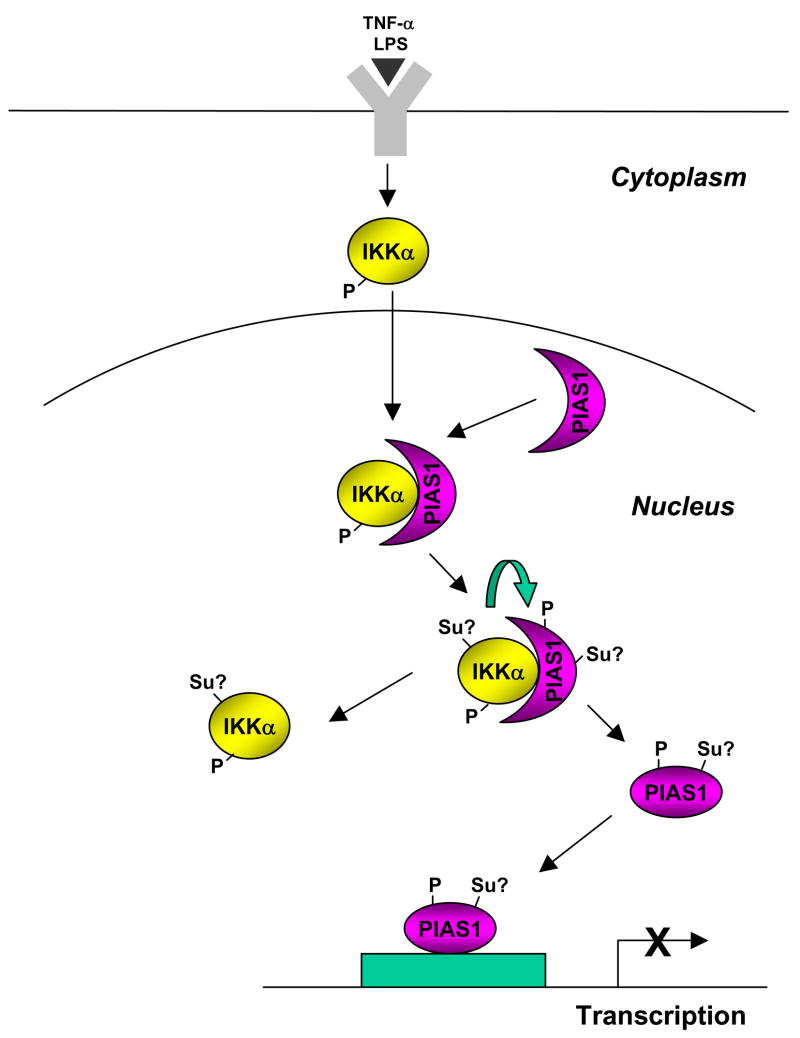
SUMO E3 ligases function to promote the efficiency and substrate specificity of sumoylation. Recent studies indicate that SUMO conjugation can be fine-tuned by regulating the subcellular localization of SUMO E3 ligases (Fig. 2). It has been well documented that extracellular stimuli such as cytokines and growth factors induce rapid changes in gene expression to modulate various cellular functions. One important question in the SUMO research field is to understand how extracellular signals can rapidly mobilize the sumoylation system to regulate specific gene expression in the nucleus. PIAS1 E3 ligase is localized in the nucleus and can regulate the activity of a number of transcription factors such as NF-κB, STAT1, and PPARγ upon ligand stimualtion [25–27]. Gene targeting studies indicate that the removal of PIAS1 does not affect the global sumoylation, and PIAS1 negatively regulates the transcriptional induction of a subset of NF-κB or STAT1-target genes, with a notable preference for cytokines and chemokines [25,26]. Recently, it has been shown that PIAS1 becomes rapidly phosphorylated on Ser90 residue in response to proinflammatory stimuli such as TNFα and LPS [28]. The Ser90 phosphorylation of PIAS1 does not affect its intrinsic SUMO ligase activity, but is required for targeting PIAS1 to the promoters of PIAS1-responsive genes, resulting in the immediate inhibition of inflammatory gene induction. The kinase that mediates PIAS1 Ser90 phosphorylation in response to TNFα and LPS has been identified as IKKα, which is largely localized in the cytoplasm but can rapidly translocate into the nucleus upon ligand stimulation [29]. Interestingly, the ability of IKKα to phosphorylate PIAS1 on Ser90 requires the SUMO ligase activity of PIAS1. Consistently, elevated sumoylation can enhance the IKKα-mediated PIAS1 phosphorylation [28]. It is possible that the sumoylation of IKKα and/or PIAS1 promotes the kinase activity of IKKα toward PIAS1, a hypothesis that can be tested by further experiments. Nevertheless, these studies identify an interesting regulatory pathway in which extracellular stimuli signal to the nucleus to regulate transcription by targeting a SUMO E3 ligase to gene promoters (Fig. 2).
Figure 2. A signaling pathway to recruit PIAS1 SUMO E3 ligase to gene promoters for transcriptional repression.
Inflammatory stimuli such as TNFα and LPS activates the kinase IKKα, which then translocates into the nucleus where it binds to PIAS1 and then phosphorylates PIAS1 on Ser90 residue. The IKKα-mediated PIAS1 phosphorylation is dependent on the SUMO E3 ligase activity of PIAS1, suggesting the possible sumoylation (Su?) of PIAS1 and/or IKKα during this process. Phosphorylated PIAS1 disassociates from IKKα, and is then recruited to the promoters of PIAS1-responsive genes for transcriptional repression.
Crosstalk between Sumoylation and Other Posttranslational Modifications
Sumoylation can be regulated through crosstalk with other posttranslational modification events. Phosphorylation has been shown to regulation SUMO conjugation through a highly conserved motif named PDSM (phosphorylation-dependent sumoylation motif) [30]. The PDSM motif, which contains a SUMO consensus site and an adjacent proline-directed phosphorylation site (ψKxExxSP, where ψ represents large hydrophobic residue and × is any amino acid), regulates phosphorylation-dependent sumoylation of multiple transcription factors, including heat-shock factors (HSFs), myocyte enhancer factor 2 (MEF2), and estrogen-related receptors (ERR) α and γ [31–34]. This phosphorylation-dependent regulation of sumoylation has been referred to as a phospho-sumoyl switch [35]. Similarly, an extended sumoylation motif that contains clusters of acidic residues located downstream of the core SUMO modification sites has been identified in proteins such as the transcription factor Elk-1 [36].
SUMO is conjugated to lysine residues of the substrates. The lysine residue is also a target of several other posttranslational modifications, including ubiquitination, acetylation, and methylation. It has been documented that SUMO conjugation can occur on the same lysine residue as ubiquitination or acetylation in some proteins. For example, the competition between sumoylation and ubiquitination on the same lysine residue regulates the stability of IκBα [37]. It should be noted that sumoylation and ubiquitination do not necessarily compete with each other. In some cases, sumoylation actually acts as a recognition signal for a ubiquitin ligase [23]. The interplay between sumoylation and acetylation has been observed in the regulation of proteins such as MEF2, histone, and hypermethylated in cancer 1 (HIC1) [38–41]. In the case of MEF2, the sumoylation-acetylation switch is regulated by phosphorylation [39]. These studies demonstrate the importance of signaling crosstalk in the regulation of protein sumoylation.
Conclusion
The field of protein sumoylation has advanced rapidly, especially in the area of understanding the molecular mechanisms of sumoylation in transcriptional regulation. Sumoylation is a dynamic process that is regulated during normal cellular processes and under pathogenic conditions. Recent studies have uncovered several mechanisms for the regulation of the sumoylation system. However, many important questions remain to be answered. For example, little is known about how the expression levels of various components of the SUMO system are regulated. In addition, the signaling pathways that regulate the activity of SUMO enzymes in response to various stimuli need to be characterized. Finally, the abnormal regulation of the sumoylation pathway in human diseases such as cancers should be explored. Clearly, the research field on the regulation of sumoylation is exciting and challenging.
Acknowledgments
This work is supported by a grant from the National Institute of Allergy and Infectious Diseases (1R01 AI063286). B.S. is supported by a Research Scientist Development Award from the National Institutes of Health (K01 AR52717-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- 3.Melchior F. SUMO--nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- 4.Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol. 2005;5:593–605. doi: 10.1038/nri1667. [DOI] [PubMed] [Google Scholar]
- 5.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 6.Zhao X, Sternsdorf T, Bolger TA, Evans RM, Yao TP. Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol Cell Biol. 2005;25:8456–8464. doi: 10.1128/MCB.25.19.8456-8464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weger S, Hammer E, Heilbronn R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett. 2005;579:5007–5012. doi: 10.1016/j.febslet.2005.07.088. [DOI] [PubMed] [Google Scholar]
- 8.Rajendra R, Malegaonkar D, Pungaliya P, Marshall H, Rasheed Z, Brownell J, Liu LF, Lutzker S, Saleem A, Rubin EH. Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. J Biol Chem. 2004;279:36440–36444. doi: 10.1074/jbc.C400300200. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov AV, Peng H, Yurchenko V, Yap KL, Negorev DG, Schultz DC, Psulkowski E, Fredericks WJ, White DE, Maul GG, et al. PHD Domain-Mediated E3 Ligase Activity Directs Intramolecular Sumoylation of an Adjacent Bromodomain Required for Gene Silencing. Mol Cell. 2007;28:823–837. doi: 10.1016/j.molcel.2007.11.012. • This paper describes the identification of a domain that acts as an intramolecular SUMO E3 ligase to promote the sumoylation of an adjacent domain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends Biochem Sci. 2007;32:286–295. doi: 10.1016/j.tibs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Gill G. Something about SUMO inhibits transcription. Curr Opin Genet Dev. 2005;15:536–541. doi: 10.1016/j.gde.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Lyst MJ, Stancheva I. A role for SUMO modification in transcriptional repression and activation. Biochem Soc Trans. 2007;35:1389–1392. doi: 10.1042/BST0351389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deyrieux AF, Rosas-Acosta G, Ozbun MA, Wilson VG. Sumoylation dynamics during keratinocyte differentiation. J Cell Sci. 2007;120:125–136. doi: 10.1242/jcs.03317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci U S A. 2006;103:16272–16277. doi: 10.1073/pnas.0603002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comerford KM, Leonard MO, Karhausen J, Carey R, Colgan SP, Taylor CT. Small ubiquitin-related modifier-1 modification mediates resolution of CREB-dependent responses to hypoxia. Proc Natl Acad Sci U S A. 2003;100:986–991. doi: 10.1073/pnas.0337412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell. 2007;131:309–323. doi: 10.1016/j.cell.2007.07.044. •• This paper reports a novel protein regulator of the sumoylation system that acts by binding to Ubc9 to promote SUMO conjugation. [DOI] [PubMed] [Google Scholar]
- 17.Baek SH. A novel link between SUMO modification and cancer metastasis. Cell Cycle. 2006;5:1492–1495. doi: 10.4161/cc.5.14.3008. [DOI] [PubMed] [Google Scholar]
- 18.Geletu M, Balkhi MY, Peer Zada AA, Christopeit M, Pulikkan JA, Trivedi AK, Tenen DG, Behre G. Target proteins of C/EBPalphap30 in AML: C/EBPalphap30 enhances sumoylation of C/EBPalphap42 via up-regulation of Ubc9. Blood. 2007;110:3301–3309. doi: 10.1182/blood-2007-01-071035. [DOI] [PubMed] [Google Scholar]
- 19.Depaux A, Regnier-Ricard F, Germani A, Varin-Blank N. A crosstalk between hSiah2 and Pias E3-ligases modulates Pias-dependent activation. Oncogene. 2007;26:6665–6676. doi: 10.1038/sj.onc.1210486. • References 19–21 suggest the involvement of ubiquitin E3 ligases in the regulation of the sumoylation system. [DOI] [PubMed] [Google Scholar]
- 20.Albor A, El-Hizawi S, Horn EJ, Laederich M, Frosk P, Wrogemann K, Kulesz-Martin M. The interaction of Piasy with Trim32, an E3-ubiquitin ligase mutated in limb-girdle muscular dystrophy type 2H, promotes Piasy degradation and regulates UVB-induced keratinocyte apoptosis through NFkappaB. J Biol Chem. 2006;281:25850–25866. doi: 10.1074/jbc.M601655200. [DOI] [PubMed] [Google Scholar]
- 21.Boggio R, Passafaro A, Chiocca S. Targeting SUMO E1 to ubiquitin ligases: a viral strategy to counteract sumoylation. J Biol Chem. 2007;282:15376–15382. doi: 10.1074/jbc.M700889200. [DOI] [PubMed] [Google Scholar]
- 22.Bossis G, Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell. 2006;21:349–357. doi: 10.1016/j.molcel.2005.12.019. •• This paper reports that the activity of SUMO enzymes E1 and E2 can be inhibited by reactive oxygen species. [DOI] [PubMed] [Google Scholar]
- 23.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. •• This paper demonstrates a physiological role of SENP1 in the regulation of HIF1alpha and erythropoiesis, and suggests that sumoylation can target HIF1alpha for degradation through the ubiquitin-proteasome pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roscic A, Moller A, Calzado MA, Renner F, Wimmer VC, Gresko E, Ludi KS, Schmitz ML. Phosphorylation-dependent control of Pc2 SUMO E3 ligase activity by its substrate protein HIPK2. Mol Cell. 2006;24:77–89. doi: 10.1016/j.molcel.2006.08.004. •• This paper reports a novel regulatory mechanism in which the activity of Pc2 SUMO E3 ligase can be regulated by its sumoylation substrate. [DOI] [PubMed] [Google Scholar]
- 25.Liu B, Mink S, Wong KA, Stein N, Getman C, Dempsey PW, Wu H, Shuai K. PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat Immunol. 2004;5:891–898. doi: 10.1038/ni1104. • References 25–27 report the role of PIAS1 SUMO E3 ligase in the regulation of immunity and inflammation. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Yang R, Wong KA, Getman C, Stein N, Teitell MA, Cheng G, Wu H, Shuai K. Negative regulation of NF-kappaB signaling by PIAS1. Mol Cell Biol. 2005;25:1113–1123. doi: 10.1128/MCB.25.3.1113-1123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, et al. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129:903–914. doi: 10.1016/j.cell.2007.03.056. •• This paper reports a novel signaling pathway in which extracellular stimuli signal to the nucleus to recruit a SUMO E3 ligase onto gene promoters for transcriptional repression. [DOI] [PubMed] [Google Scholar]
- 29.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 30.Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci U S A. 2006;103:45–50. doi: 10.1073/pnas.0503698102. •• This paper together with reference 36 describes a conserved motif that is important for phosphorylation-dependent sumoylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol. 2003;23:2953–2968. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilgarth RS, Hong Y, Park-Sarge OK, Sarge KD. Insights into the regulation of heat shock transcription factor 1 SUMO-1 modification. Biochem Biophys Res Commun. 2003;303:196–200. doi: 10.1016/s0006-291x(03)00312-7. [DOI] [PubMed] [Google Scholar]
- 33.Gregoire S, Tremblay AM, Xiao L, Yang Q, Ma K, Nie J, Mao Z, Wu Z, Giguere V, Yang XJ. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation. J Biol Chem. 2006;281:4423–4433. doi: 10.1074/jbc.M509471200. [DOI] [PubMed] [Google Scholar]
- 34.Tremblay AM, Wilson BJ, Yang XJ, Giguere V. Phosphorylation-Dependent Sumoylation Regulates ERR{alpha} and {gamma} Transcriptional Activity Through a Synergy Control Motif. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XJ, Gregoire S. A recurrent phospho-sumoyl switch in transcriptional repression and beyond. Mol Cell. 2006;23:779–786. doi: 10.1016/j.molcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Yang SH, Galanis A, Witty J, Sharrocks AD. An extended consensus motif enhances the specificity of substrate modification by SUMO. Embo J. 2006;25:5083–5093. doi: 10.1038/sj.emboj.7601383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2:233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 38.Gregoire S, Yang XJ. Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol Cell Biol. 2005;25:2273–2287. doi: 10.1128/MCB.25.6.2273-2287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. • This paper shows that a sumoylation-acetylation switch can be regulated by phosphorylation. [DOI] [PubMed] [Google Scholar]
- 40.Nathan D, Ingvarsdottir K, Sterner DE, Bylebyl GR, Dokmanovic M, Dorsey JA, Whelan KA, Krsmanovic M, Lane WS, Meluh PB, et al. Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 2006;20:966–976. doi: 10.1101/gad.1404206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stankovic-Valentin N, Deltour S, Seeler J, Pinte S, Vergoten G, Guerardel C, Dejean A, Leprince D. An acetylation/deacetylation-SUMOylation switch through a phylogenetically conserved psiKXEP motif in the tumor suppressor HIC1 regulates transcriptional repression activity. Mol Cell Biol. 2007;27:2661–2675. doi: 10.1128/MCB.01098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]