Abstract
Aims
Most lipoprotein-associated phospholipase A2 (Lp-PLA2) studies included mainly white men. We sought to determine whether Lp-PLA2 levels differ according to race and sex.
Methods
Lp-PLA2 mass and activity were measured in 3,332 subjects age 30 to 65 participating in the Dallas Heart Study, a multiethnic, population-based, probability sample. Lp-PLA2 levels were compared between different race and sex groups.
Results
Mean age was 45 ± 9 years and 44% were men; 30% were white, 17% hispanic, and 53% black. Mean Lp-PLA2 activity and mass were 146 ± 40 nmol/min/mL and 191 ± 60 ng/mL, respectively. Lp-PLA2 activity was lower in women compared with men (134 ± 35 vs. 161 ± 40, p=0.001) and was lowest in black (136 ± 38), intermediate in hispanic (151 ± 36), and highest in white subjects (161 ± 39) (trend p=0.0001). In multivariable linear regression models, after adjusting for age, body mass index (BMI), smoking, total, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, triglycerides and high sensitivity C-reactive protein (hsCRP), Lp-PLA2 activity was 19 nmol/min/mL higher in men vs. women (p<0.001); compared with black subjects, adjusted Lp-PLA2 activity was 11 and 20 nmol/min/mL higher in white and hispanic subjects, respectively (both p<0.001). Similar race and sex differences were observed for Lp-PLA2 mass.
Conclusion
Race and sex independently influence Lp-PLA2 activity and mass. Thresholds to define Lp-PLA2 elevation may need to be sex and race specific.
Keywords: lipoprotein-associated phospholipase A2, race, sex factors
1. INTRODUCTION
Lipoprotein-Associated Phospholipase A2 (Lp-PLA2) is an enzyme that circulates in blood, mainly bound to low-density lipoprotein (LDL) cholesterol particles. Lp-PLA2 hydrolyzes oxidized phospholipids, leading to the generation of lysophosphatidylcholine and oxidized free fatty acids, and is increasingly recognized as a novel atherosclerosis risk marker. A recent systematic review of 14 studies showed an adjusted odds ratio of 1.60 for the association between Lp-PLA2 and prevalent cardiovascular disease(1), and higher Lp-PLA2 levels have been associated with an increased incidence of first-ever cardiac events in population-based studies(2–4), and with recurrent cardiovascular events in patients with clinically manifest coronary artery disease(5–8) and with initial(4,9) and recurrent(10) stroke. However, some of these studies have included only men(3,11), and most studies have included mainly white subjects(3–7,11,12), except the Atherosclerosis Risk in Communities study that included 25% black subjects(2) and the Northern Manhattan Stroke Study that included 53% hispanic and 27% black subjects(10). Moreover, independent of race and sex considerations, very little is known about the population distribution of Lp-PLA2, which is important for interpretation of the Lp-PLA2 assay results(13).
We measured Lp-PLA2 mass and activity in a large multiethnic population-based study of adult residents of Dallas, Texas to determine: (a) the population-based range of Lp-PLA2, and (b) whether sex and racial differences in Lp-PLA2 distribution exist.
2. METHODS
2.1 Study population
The Dallas Heart Study is a population-based, multiethnic, probability sample of 6,101 subjects in Dallas County designed to study cardiovascular disease(14–19). Briefly, a stratified random sample of Dallas County residents age 18–65 was obtained from a pool of 841,943 eligible subjects using the U.S. Postal Service Delivery Sequence File, with deliberate oversampling of African Americans. An initial visit for 6,101 participants included a detailed in-home interview for demographic and health-related data, as well as measurements of weight, heart rate, and 5 sequential blood pressure measures. All subjects between the ages of 30–65 who completed the initial visit were invited to participate in a second home visit to collect fasting venous blood and urine samples, and if they completed the second visit, invited to a third detailed clinic visit, consisting of a 12-lead electrocardiogram, cardiac and aortic magnetic resonance imaging, electron beam computed tomography (EBCT) to assess coronary artery calcification, and dual-energy X-ray absorbtometry (DEXA) scanning to evaluate fat distribution and bone density. For the present analyses, we included data from the 3,332 subjects in whom plasma was available for Lp-PLA2 measurement and whose (self-reported) race was white, Hispanic, or black. Study definitions for various covariates have been previously reported(14).
2.2 Lp-PLA2 assay
Blood samples were obtained in EDTA tubes and were stored for ≤ 4 hours at 4°C before processing. Plasma was frozen in aliquots of 100 microliters at −80°C.
Lp-PLA2 activity was measured with a colorimetric activity method provided by Glaxo Smithkline (Research Triangle Park, NC, USA). Samples, standards, or controls were added to wells of a non-binding 96-well microplate, followed by addition of reaction buffer containing substrate. In the presence of Lp-PLA2 enzyme, the substrate is converted upon hydrolysis by the phospholipase enzyme. The change in absorbance was immediately measured at 405 nm over 60–180 seconds. The level of Lp-PLA2 activity in nmol/min/mL was calculated from the slope (OD405/min), based on a standard conversion factor from a p-Nitrophenol calibration curve. The mean duplicate coefficient of variation was 2.94% for the low controls and 3.85% for the high controls. Samples that had activity values outside the range of 2 to 300 nmols/min/ml exceeded the dynamic range of the assay and were reported as such. All samples were assayed in duplicate on consecutive microtiter plates (mean values were reported). The mean duplicate CV% for samples was 3.87%. Analysis of Lp-PLA2 mass was performed by diaDexus, Inc (South San Francisco, CA. USA) using a dual monoclonal antibody immunoassay standardized to recombinant Lp-PLA2, as previously reported(5). The mean duplicate coefficient of variation was 2.89% for the low controls and 3.23% for the high controls. Samples were run in single point.
2.3 Statistical analysis
Categorical data are reported as proportions and continuous data as mean values with standard deviations, or as medians (interquartile range) for non-normally distributed variables. Subjects were divided into 6 groups based on their race and gender. Baseline demographic variables and CV risk factors were compared across the different groups with the χ2 trend test for categorical variables and the test for trend or the Kruskall Wallis test across ordered groups for continuous variables. The association between Lp-PLA2 levels and continuous variables was explored using Spearman correlation coefficients. Multivariable stepwise linear regression analysis was used to determine variables independently associated with Lp-PLA2 levels. The stepwise linear regression model included all baseline covariates with a univariable association of p <0.1 with Lp-PLA2 activity, as well as race and sex, and used backward selection to derive the final models. All analyses were performed using Stata 9.0 (College Station, Texas).
3. RESULTS
3.1 Baseline Characteristics
The baseline characteristics of the study population stratified according to sex and race are shown in Table 1. Overall, mean age was 45 ± 9 years and 44% were men; 30% were white, 17% hispanic, and 53% black. Compared with men, women were more likely to have a family history of myocardial infarction, and less likely to smoke, or take aspirin. Women had higher high-sensitivity C-reactive protein (hsCRP) and high-density lipoprotein (HDL) cholesterol and lower LDL cholesterol and triglycerides. Compared with whites and hispanic subjects, black subjects were more likely to have hypertension and had higher median CRP. Mean and median Lp-PLA2 activity was 146 ± 40 and 144 nmol/min/mL, respectively. Mean and median Lp-PLA2 mass was 191 ± 60 and 187 ng/mL, respectively. There was a strong correlation between Lp-PLA2 mass and activity (Spearman’s rho=0.69, p<0.001, Fig. 1).
Table 1.
Baseline characteristics of the study population stratified by race and sex.
| White Men (n = 480) | White Women (n = 518) | Hispanic men (n=236) | Hispanic women (n=334) | Black Men (n = 743) | Black Women (n=1,021) | p | |
|---|---|---|---|---|---|---|---|
| Age, years* | 45 ± 9 | 46 ± 10 | 41 ± 9 | 41 ± 8 | 46 ± 9 | 45 ± 10 | <0.001 |
| Hypertension, (%) | 21 | 21 | 15 | 16 | 43 | 43 | <0.001 |
| Diabetes, (%) | 7 | 7 | 13 | 12 | 15 | 15 | <0.001 |
| Smoking, (%) | 27 | 28 | 32 | 13 | 41 | 30 | <0.001 |
| Family history of MI, (%) | 34 | 41 | 21 | 25 | 33 | 37 | <0.001 |
| BMI kg/m2* | 28 ± 5 | 29 ± 7 | 29 ± 5 | 30 ± 7 | 29 ± 6 | 32 ± 8 | <0.001 |
| Aspirin, (%) | 15 | 12 | 4 | 3 | 9 | 8 | <0.001 |
| Statin, (%) | 8 | 9 | 4 | 4 | 7 | 6 | 0.03 |
| ACE-I, (%) | 9 | 9 | 6 | 6 | 13 | 12 | <0.001 |
| Beta-blocker, (%) | 6 | 6 | 0 | 3 | 8 | 7 | <0.001 |
| Oral estrogen, (%) | 34 | 9 | 13 | <0.001 | |||
| Total cholesterol, mg/dL* | 184 ± 38 | 184 ± 39 | 189 ± 43 | 178 ± 38 | 177 ± 39 | 179 ± 41 | <0.001 |
| LDL, mg/dL* | 112 ± 35 | 105 ± 34 | 114 ± 35 | 103 ± 32 | 104 ± 37 | 106 ± 36 | <0.001 |
| HDL, mg/dL* | 42 ± 10 | 55 ± 17 | 42 ± 10 | 48 ± 12 | 50 ± 16 | 54 ± 15 | <0.001 |
| Triglycerides, mg/dL* | 156 ± 123 | 123 ± 88 | 168 ± 132 | 141 ± 130 | 122 ± 117 | 98 ± 76 | <0.001 |
| hsCRP, mg/dL† | 1.7 (0.8, 3.5) | 3.1 (1.3, 7.2) | 2.0 (1.1, 3.7) | 4 (1.8, 8.3) | 2.4 (1.1, 6.0) | 4.6 (1.9, 9.9) | <0.001 |
| Lp-PLA2 activity, nmol/min/mL* | 179 ± 38 | 145 ± 33 | 172 ± 33 | 136 ± 30 | 146 ± 38 | 128 ± 36 | <0.001 |
| Lp-PLA2 mass, ng/mL* | 223 ± 61 | 198 ± 48 | 216 ± 55 | 175 ± 50 | 191 ± 63 | 173 ± 57 | <0.001 |
mean ± SD;
median, IQR
MI, myocardial infarction; BMI, body mass index; ACE-I, angiotensin converting enzyme inhibitor; LDL, low density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; Lp-PLA2, lipoprotein-associated phospholipase A2
Figure 1.
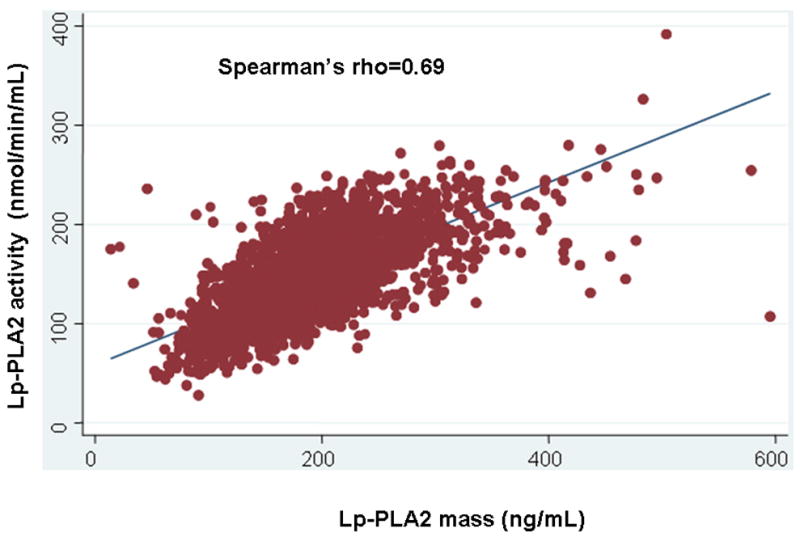
Association between Lp-PLA2 activity and Lp-PLA2 mass in the study population.
3.2 Associations between clinical variables and Lp-PLA2 activity
Lp-PLA2 activity was positively associated with total cholesterol (rho=0.30), LDL cholesterol (rho=0.42) and triglycerides (rho=0.19), and negatively associated with HDL cholesterol (rho= −0.37) and hs-CRP (rho= −0.15) (p<0.001 for each). Lp-PLA2 activity was higher in current smokers vs. non- smokers (150 ± 41 vs. 145 ± 39, p<0.001), and lower in hypertensive vs. normotensive subjects (142 ± 40 vs. 148 ± 39, p<0.001), in subjects taking statins vs. non-users (141 ± 38 vs. 147 ± 39, p=0.03), and in female users of oral estrogen vs. non-users (126 ± 35 vs. 136 ± 34, p<0.001). Lp-PLA2 levels were not associated with the presence of diabetes or with family history of coronary artery disease.
The association of Lp-PLA2 activity with several continuous parameters, stratified by race and sex, is shown in Table 2. Except among black men, Lp-PLA2 activity was not associated with age, body mass index, or creatinine clearance. Lp-PLA2 activity had a modest negative association with hsCRP levels in women but not in men. Data were qualitatively similar for Lp-PLA2 mass (data not shown).
Table 2.
Association (Spearman rank correlation and p-value) of Lp-PLA2 activity with different continuous parameters across various sex/race strata.
| White Men (n = 480) | White Women (n = 518) | Hispanic men (n = 236) | Hispanic women (n = 334) | Black Men (n = 743) | Black Women (n = 1,021) | |
|---|---|---|---|---|---|---|
| Age | 0.07, P=0.10 | −0.07, P=0.12 | 0.05, P=0.41 | 0.11, P−0.04 | 0.09, P=0.02 | −0.01, P=0.73 |
| BMI | 0.00, P=1 | 0.03, P=0.47 | 0.06, P=0.36 | 0.03, P=0.55 | 0.13, P<0.001 | −0.01, P=0.84 |
| Total cholesterol | 0.37, P<0.001 | 0.24, P<0.001 | 0.33, P<0.001 | 0.30, P<0.001 | 0.32, P<0.001 | 0.32, P<0.001 |
| LDL | 0.48, p<0.001 | 0.43, P<0.001 | 0.45, P<0.001 | 0.38, P<0.001 | 0.46, P<0.001 | 0.45, P<0.001 |
| HDL | −0.22, P<0.001 | −0.38, P<0.001 | −0.12, P=0.06 | −0.23, P<0.001 | −0.31, P<0.001 | −0.28, P<0.001 |
| Triglycerides | 0.08, P=0.10 | 0.14, P=0.002 | 0.02, P=0.75 | 0.11, P=0.04 | 0.11, P=0.003 | 0.09, P=0.003 |
| hsCRP | 0.06, P=0.17 | −0.14, P=0.002 | 0.04, P=0.57 | −0.12, P=0.04 | 0.06, P=0.08 | −0.09, P=0.03 |
BMI, body mass index; LDL, low density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; CRP, C-reactive protein; Lp-PLA2, lipoprotein-associated phospholipase A2
3.3 Association of Lp-PLA2 activity and mass with race and sex
Lp-PLA2 activity was significantly lower in women compared with men (134 ± 35 vs. 161 ± 40; p=0.001). Lp-PLA2 activity was lowest in black (136 ± 38), intermediate in hispanic (151 ± 36), and highest in white subjects (161 ± 39) (p=0.0001) (Fig. 2a). Similar sex and race associations were observed for Lp-PLA2 mass (Fig. 2b).
Figure 2.
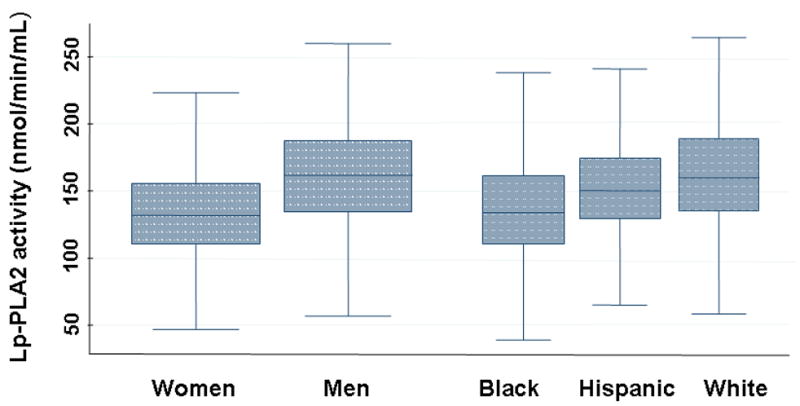
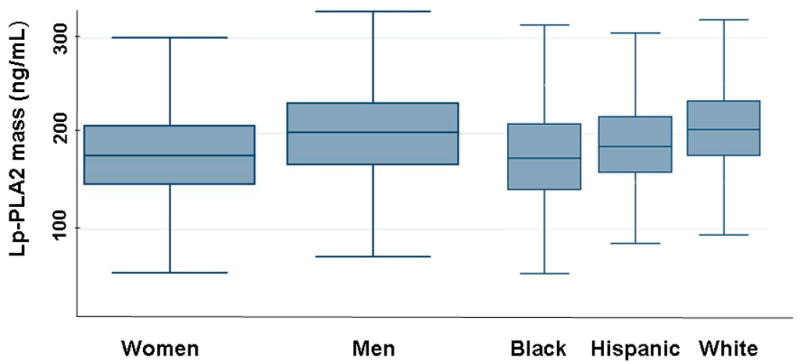
Lp-PLA2 activity (Fig. 2a) and mass (Fig. 2b) in 3,332 subjects classified according to race and sex. The upper, middle, and lower line of the shaded box correspond to the 75th percentile, median, and 25th percentile value, respectively. The upper line at the end of the ‘whisker’ coming out of the box on the upper side is the maximum value of either: a) the maximum value in the data set, or b) the largest value in the dataset that is less than the upper quartile value + 1.5 times the interquartile range (IQR). The lower line at the end of the ‘whisker’ coming out of the box on the lower side is the maximum value of either: a) the minimum value in the data set, or b) the smallest value in the dataset that is more than the lower quartile value − 1.5 times the IQR.
Based on univariable analyses, candidate covariates tested in the multivariable linear regression model for Lp-PLA-2 activity included age, body mass index (BMI), smoking, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides and hsCRP In the final model that included smoking, LDL and HDL cholesterol, and hsCRP, race and sex remained independently associated with Lp-PLA2 activity. Adjusted Lp-PLA2 activity was 19 nmol/min/mL higher in men vs. women (p<0.001); compared with black subjects, adjusted Lp-PLA2 activity was 11 nmol/min/mL higher in hispanic and 20 nmol/min/mL higher in white subjects (both p<0.001).
Similarly, candidate covariates tested in the multivariable linear regression model for Lp-PLA-2 mass included age, BMI, hypertension, smoking, total cholesterol, LDL-cholesterol, HDL cholesterol, triglycerides and hsCRP. In the final model that included smoking, BMI, total and HDL cholesterol, race and sex remained independently associated with Lp-PLA2 mass. Adjusted Lp-PLA2 mass was 19 ng/mL higher in men vs. women (p<0.001); compared with black subjects, adjusted Lp-PLA2 mass was 9 ng/mL higher in hispanic and 24 ng/mL higher in white subjects (both p<0.001).
To determine the contribution of race and sex in the prediction of Lp-PLA2 levels, several multivariable linear regression models were created with Lp-PLA2 activity as the endpoint: (1) a baseline model that included LDL cholesterol, HDL cholesterol, C-reactive protein, and current smoking; (2) the baseline model with sex added; (3) the baseline model with race added, and (4) the baseline model with sex and race added (Figure 3). Addition of race and sex improved the prediction of Lp-PLA2 levels as shown by the increase in adjusted R2 from 0.31 in model 1 to 0.41 in model 4.
Figure 3.
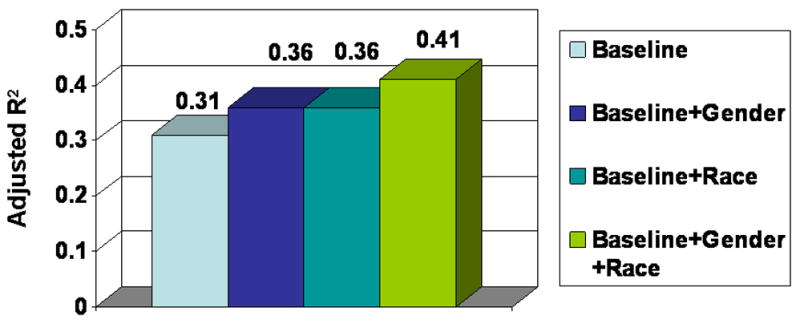
Adjusted R2 in four multivariable linear regression models with Lp-PLA2 activity as the endpoint: (1) baseline model that includes LDL cholesterol, LDL cholesterol, high sensitivity C-reactive protein, and current smoking; (2) baseline model with sex added; (3) baseline model with race added, and (4) baseline model with sex and race added.
4. DISCUSSION
Our study of a multiethnic, population-based cohort shows significant and independent associations of circulating Lp-PLA2 mass and activity with sex (male>female) and race (white>hispanic>black subjects), with white men having the highest and black women the lowest levels.
4.1 Lp-PLA2 and race
Lp-PLA2 has been studied in predominantly Caucasian populations(3–5,11,12,20,21). In an analysis of a random sample of 1348 subjects in the ARIC (Atherosclerosis Risk In Communities) cohort study, mean Lp-PLA2 mass was 388 μg/L in white subjects and 333 μg/L in African Americans(2). In the Northern Manhattan Stroke Study (457 patients), Lp-PLA2 mass (ng/mL) was 327±113 in black, vs. 319±123 in hispanic vs. 379±130 in white patients, p=0.002(10). Multivariable analysis of Lp-PLA2 levels was not done in either study. The large differences in the absolute Lp-PLA2 mass seen between different studies could be due to: (a) use of different versions of the Lp-PLA2 mass assay (generation 1 in the ARIC study vs. generation 2 in the Northern Manhattan Stroke Study and in the Dallas Heart Study), or (b) to differences in the studied populations (stroke patients in the Northern Manhattan Stroke Study vs. a younger and healthier population-based sample in the Dallas Heart Study).
The explanation for the large racial differences in Lp-PLA2 activity and mass seen in our study is unclear, but genetic differences could be partially responsible. Even after adjusting for differences in traditional risk factors and environmental exposures such as tobacco use that are known to influence Lp-PLA2 levels, significant racial differences remained, although we cannot exclude other unknown confounders that were not assessed. No data are currently available identifying specific genetic variants associated with Lp-PLA2 mass or activity among black or hispanic subjects, and few studies overall have evaluated genetic contributions to Lp-PLA2 mass or activity. A loss of function single nucleotide polymorphism (Val-279->Phe) in the Lp-PLA2 gene is seen in approximately 30% of Japanese subjects (4% homozygous) and has been associated with higher risk of CAD and stroke (22,23) though not confirmed in larger studies(24,25). Another polymorphism, Ala-379->Val, is seen in Caucasians and appears to result in a secreted enzyme with reduced catalytic activity and lower risk of CAD(26,27), but this polymorphism would be unlikely to be contributing to the Lp-PLA2 racial differences observed in our population, in which Lp-PLA2 mass and activity were highly correlated.
4.2 Lp-PLA2 and sex
Some early Lp-PLA2 studies only included men(3,11). In other studies including both sexes, Lp-PLA2 levels were consistently higher in men compared with women. In the ARIC study the weighted mean Lp-PLA2 mass was 421 μg/L in men (n=716) and 339 μg/L in women (n=632)(2). In the Rotterdam study Lp-PLA2 activity was higher in men (n=958) than in women (n=1,433): 46.8 vs. 43.0 nmol/min/mL)(4). In the Malmö Diet and Cancer Study, compared with women (n=3,167), men (n=2,235) had higher Lp-PLA2 activity (49.6 ± 13.3 vs. 42.5 ± 12 nmol/min/mL) and mass (287.7 ± 83.1 vs 257.2 ± 86.5 ng/mL)(21). Similar results were found in most(12),(8), (28), (7), (5), but not all(6,10) studies. None of these prior studies assessed the relationship with sex after adjusting for potential confounders, with the exception of (a) the Malmö Diet and Cancer Study in which the Lp-PLA2 levels remained lower in women after adjustment for LDL(21), and (b) the study by Brilakis et al, in which the Lp-PLA2 difference between men and women was no longer significant after adjustment for HDL cholesterol(29).
The association between sex and Lp-PLA2 levels persisted after adjusting for both LDL and HDL levels, which have a known strong association with Lp-PLA2 levels. Another possible explanation for the lower Lp-PLA2 levels in women is that the higher estrogen levels in women down-regulate Lp-PLA2 expression and activity(30)and also decrease LDL-cholesterol. This concept is further supported by the lower Lp-PLA2 levels seen in women taking oral estrogen in our study. In our population, women had lower Lp-PLA2 but higher hsCRP levels(16) compared with men, suggesting that the mechanism of action and the predictive value of those two proatherogenic biomarkers could differ between the two sexes.
4.3 Clinical Implications
Recently the Lp-PLA2 mass 50th percentile cut point (235 ng/mL) has been proposed as a conservative cut point associated with increased risk for cardiovascular disease(13). The significant and independent association of Lp-PLA2 mass and activity with race and sex seen in our study would favor future evaluation of race- and sex-specific cutoffs when Lp-PLA2 is used for risk stratification.
4.4 Limitations
Lp-PLA2 measurements were performed on frozen rather than fresh specimens, but Lp-PLA2 has been shown to be stable in repeated freeze-thaw cycles(5). Because of the cross-sectional design of our study, we could not assess the prognostic implications of the LpPLA2 measurements on CVD risk prediction, nor the clinical implications of the observed race and sex difference in Lp-PLA2. Our population was an urban, multiethnic cohort with high-rates of obesity and standard cardiovascular risk factors, and our findings may not apply to other populations with different composition.
4.5 Conclusion
Lp-PLA2 mass and activity had a strong and independent association with race and sex, in our multiethnic, population-based cohort. Race and sex specific-cutoffs may allow optimum interpretation of Lp-PLA2 levels.
Acknowledgments
Supported in part by a research grant from GlaxoSmithkline. Emmanouil S. Brilakis receives support by the Clark R. Gregg Fund, Harris Methodist Health Foundation 6100 Western Place, Suite 1001, Fort Worth, TX 76107, USA, and by a Veterans Affairs VISN 17 startup award, Waco, Texas, USA. The Dallas Heart Study was funded by the Donald W. Reynolds Foundation (Las Vegas, NE) and was partially supported by USPHS GCRC grant #M01-RR00633 from NIH/NCRR-CR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garza CA, Montori VM, McConnell JP, Somers VK, Kullo IJ, Lopez-Jimenez F. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82:159–65. doi: 10.4065/82.2.159. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–42. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 3.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903–8. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 4.Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111:570–5. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 5.Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J. 2005;26:137–44. doi: 10.1093/eurheartj/ehi010. [DOI] [PubMed] [Google Scholar]
- 6.Gerber Y, McConnell JP, Jaffe AS, Weston SA, Killian JM, Roger VL. Lipoprotein-associated phospholipase A2 and prognosis after myocardial infarction in the community. Arterioscler Thromb Vasc Biol. 2006;26:2517–22. doi: 10.1161/01.ATV.0000240406.89440.0c. [DOI] [PubMed] [Google Scholar]
- 7.May HT, Horne BD, Anderson JL, et al. Lipoprotein-associated phospholipase A2 independently predicts the angiographic diagnosis of coronary artery disease and coronary death. Am Heart J. 2006;152:997–1003. doi: 10.1016/j.ahj.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.O’Donoghue M, Morrow DA, Sabatine MS, et al. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation. 2006;113:1745–52. doi: 10.1161/CIRCULATIONAHA.105.612630. [DOI] [PubMed] [Google Scholar]
- 9.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2005;165:2479–84. doi: 10.1001/archinte.165.21.2479. [DOI] [PubMed] [Google Scholar]
- 10.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med. 2006;166:2073–80. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 11.Packard CJ, O’Reilly DS, Caslake MJ, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–55. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 12.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586–93. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- 13.Lanman RB, Wolfert RL, Fleming JK, et al. Lipoprotein-associated phospholipase A2: review and recommendation of a clinical cut point for adults. Prev Cardiol. 2006;9:138–43. doi: 10.1111/j.1520-037x.2006.05547.x. [DOI] [PubMed] [Google Scholar]
- 14.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 15.de Lemos JA, Zirlik A, Schonbeck U, et al. Associations between soluble CD40 ligand, atherosclerosis risk factors, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:2192–6. doi: 10.1161/01.ATV.0000182904.08513.60. [DOI] [PubMed] [Google Scholar]
- 16.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–9. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 17.Abdullah SM, Khera A, Das SR, et al. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the Dallas Heart Study) Am J Cardiol. 2005;96:1284–9. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 18.Khera A, de Lemos JA, Peshock RM, et al. Relationship between C-reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation. 2006;113:38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 19.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–65. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 20.Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol. 2001;38:1302–6. doi: 10.1016/s0735-1097(01)01554-6. [DOI] [PubMed] [Google Scholar]
- 21.Persson M, Nilsson JA, Nelson JJ, Hedblad B, Berglund G. The epidemiology of Lp-PLA(2): Distribution and correlation with cardiovascular risk factors in a population-based cohort. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Yoshida H, Ichihara S, Imaizumi T, Satoh K, Yokota M. Correlations between plasma platelet-activating factor acetylhydrolase (PAF-AH) activity and PAF-AH genotype, age, and atherosclerosis in a Japanese population. Atherosclerosis. 2000;150:209–16. doi: 10.1016/s0021-9150(99)00385-8. [DOI] [PubMed] [Google Scholar]
- 23.Hiramoto M, Yoshida H, Imaizumi T, Yoshimizu N, Satoh K. A mutation in plasma platelet-activating factor acetylhydrolase (Val279-->Phe) is a genetic risk factor for stroke. Stroke. 1997;28:2417–20. doi: 10.1161/01.str.28.12.2417. [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, Izawa H, Ichihara S, et al. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med. 2002;347:1916–23. doi: 10.1056/NEJMoa021445. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Metoki N, Yoshida H, et al. Genetic risk for ischemic and hemorrhagic stroke. Arterioscler Thromb Vasc Biol. 2006;26:1920–5. doi: 10.1161/01.ATV.0000229694.97827.38. [DOI] [PubMed] [Google Scholar]
- 26.Ninio E, Tregouet D, Carrier JL, et al. Platelet-activating factor-acetylhydrolase and PAF-receptor gene haplotypes in relation to future cardiovascular event in patients with coronary artery disease. Hum Mol Genet. 2004;13:1341–51. doi: 10.1093/hmg/ddh145. [DOI] [PubMed] [Google Scholar]
- 27.Abuzeid AM, Hawe E, Humphries SE, Talmud PJ. Association between the Ala379Val variant of the lipoprotein associated phospholipase A2 and risk of myocardial infarction in the north and south of Europe. Atherosclerosis. 2003;168:283–8. doi: 10.1016/s0021-9150(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 28.Oldgren J, James SK, Siegbahn A, Wallentin L. Lipoprotein-associated phospholipase A2 does not predict mortality or new ischaemic events in acute coronary syndrome patients. Eur Heart J. 2007;28:699–704. doi: 10.1093/eurheartj/ehl565. [DOI] [PubMed] [Google Scholar]
- 29.Brilakis E, McConnell J, Lennon R, Meyer J, Berger P. Lipoprotein-Associated Phospholipase A2 levels are lower in women compared to men because of a significant negative correlation with HDL cholesterol. Circulation. 2003;107:45. [Google Scholar]
- 30.Miyaura S, Maki N, Byrd W, Johnston JM. The hormonal regulation of platelet-activating factor acetylhydrolase activity in plasma. Lipids. 1991;26:1015–20. doi: 10.1007/BF02536494. [DOI] [PubMed] [Google Scholar]


