Abstract
A major obstacle in understanding the evolution of Cenozoic climate has been the lack of well dated terrestrial evidence from high-latitude, glaciated regions. Here, we report the discovery of exceptionally well preserved fossils of lacustrine and terrestrial organisms from the McMurdo Dry Valleys sector of the Transantarctic Mountains for which we have established a precise radiometric chronology. The fossils, which include diatoms, palynomorphs, mosses, ostracodes, and insects, represent the last vestige of a tundra community that inhabited the mountains before stepped cooling that first brought a full polar climate to Antarctica. Paleoecological analyses, 40Ar/39Ar analyses of associated ash fall, and climate inferences from glaciological modeling together suggest that mean summer temperatures in the region cooled by at least 8°C between 14.07 ± 0.05 Ma and 13.85 ± 0.03 Ma. These results provide novel constraints for the timing and amplitude of middle-Miocene cooling in Antarctica and reveal the ecological legacy of this global climate transition.
Keywords: climate change, tundra biota, Dry Valleys, diatoms, ostracods
The Earth's climate has been cooling for the last 50 million years in a trend punctuated by three steps. Higher rates of cooling occurred during the late Eocene–early Oligocene (c. 34 Ma), the middle Miocene (c. 14 Ma), and the late Pliocene (c. 3 Ma) (1). The step at 34 Ma is associated with the onset of continental glaciation in Antarctica, and that centered on 3 Ma registers the expansion of Northern Hemisphere ice sheets. The mid-Miocene step, often referred to as the middle-Miocene climatic transition (MMCT), is the least understood, with significant questions surrounding its timing, style, and fundamental cause. Marine isotopic studies imply significant ice volume expansion in Antarctica and coeval cooling of the Southern Ocean (2). Because of the paucity of dated terrestrial archives from the continent, little evidence for climate change has been available to constrain directly the nature and timing of the MMCT. This has resulted in a number of unanswered questions. To what extent did the continent cool during the MMCT, and was this cooling gradual or abrupt? What effect did the MMCT have on the thermal regime of Antarctic glaciers? And from a biological perspective, what was the ecological legacy of the MMCT for organisms whose ancestors had survived the cooling step at 34 Ma? Did the extinction of a shrub tundra occur during the middle Miocene, or was it delayed until the Pliocene (3, 4)?
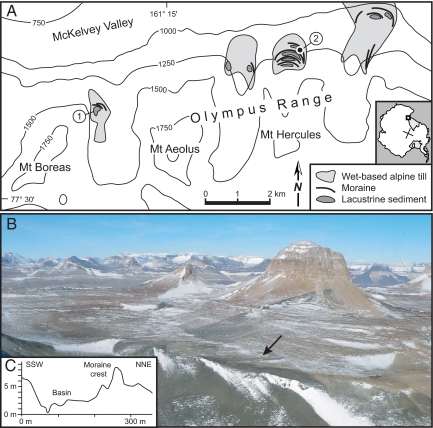
The fossiliferous glacigenic sediments reported here are from the western Olympus Range in the McMurdo Dry Valleys sector of the Transantarctic Mountains. The fossils and their glacial geologic context permit analyses of the MMCT from three broad perspectives: paleoecological, glaciological, and geomorphological. The in situ fossil-bearing strata occur within several north-facing valleys that open into McKelvey Valley, one of the major ice-free troughs of the McMurdo Dry Valleys (Fig. 1). Prior work has shown that surficial deposits in the Range include a basal group of unconsolidated tills deposited from wet-based alpine glaciers and an overlying group of stacked sublimation tills from cold-based alpine glaciers; on the basis of dated ashfall covering the oldest (stratigraphically lowest) sublimation till, the transition from wet- to cold-based glaciation occurred before 13.85 ± 0.03 Ma (5). Results from our recent fieldwork show that lacustrine deposits interfinger with wet-based tills deposited just before the transition, indicating that a series of small lakes developed where recessional moraines impounded meltwater (Fig. 1). It is within these lacustrine sediments that we find well preserved freshwater fossils and pristine, bedded ashfall. A date of 14.07 ± 0.05 Ma for an in situ ashfall layer, 3 cm thick, within an extensive sequence of locally fossiliferous lake deposits provides a direct, unambiguous age for the last phase of wet-based alpine glaciation and an age for tundra plants and animals that had colonized the locally deglaciated terrain [Fig. 1, supporting information (SI) Table S1, and Fig. S1].
Fig. 1.
Location of the fossiliferous deposits in the western Olympus Range. (A) Sketch map showing alpine glacier retreat sequences, moraines, and lacustrine deposits. 1, fossiliferous deposits at the Boreas site; 2, location of dated ash layer (Table S1). For clarity, mapped deposits from cold-based alpine glaciers are not provided, although in most valleys such deposits occur to the south (in the up-valley direction) and in places rest directly on till and lake sediments registering the final retreat of wet-based alpine glaciers. (B) Mount Boreas in center right, with the general location of the lacustrine deposits shown by an arrow; the view is to the southwest. (C) Topographic profile across the Boreas fossil site, showing the moraine to the north and the circular basin to the south; the maximum estimated water depth of 8 m for the lake at this site is based on the elevation difference between the moraine crest and lowermost lake sediments in this profile.
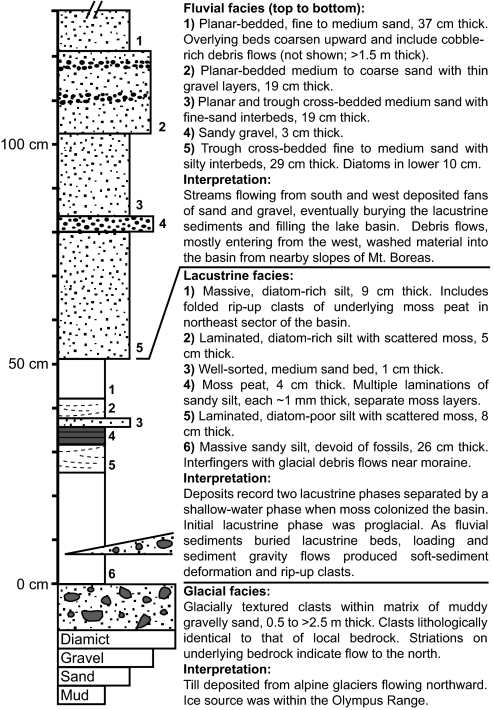
The best-studied fossil assemblage comes from a small (14,000 m2) moraine-dammed basin at 1,425 m near Mount Boreas in the western Olympus Range (Fig. 1). Deflation by wind scour has exposed lacustrine beds, which are unconsolidated, planar, and near-horizontal except where locally disturbed by the development of polygonal patterned ground. At the basin center, the stratigraphy records infilling, first by glaciolacustrine silt and sand, later by diatom- and moss-rich muds, and finally by fluvial sands and debris flows (Fig. 2). The diatom stratigraphy suggests that the lake persisted for thousands of years, during which time water levels fell at least once and the floor of the basin became a bryophyte-rich mire. The small alpine lake continued to support obligate freshwater organisms even during the low water phase.
Fig. 2.
Stratigraphic column showing sedimentary succession at the Boreas fossil site.
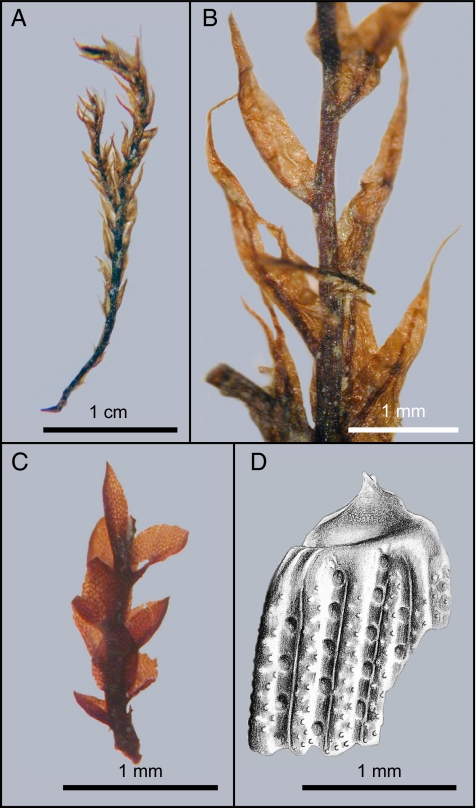
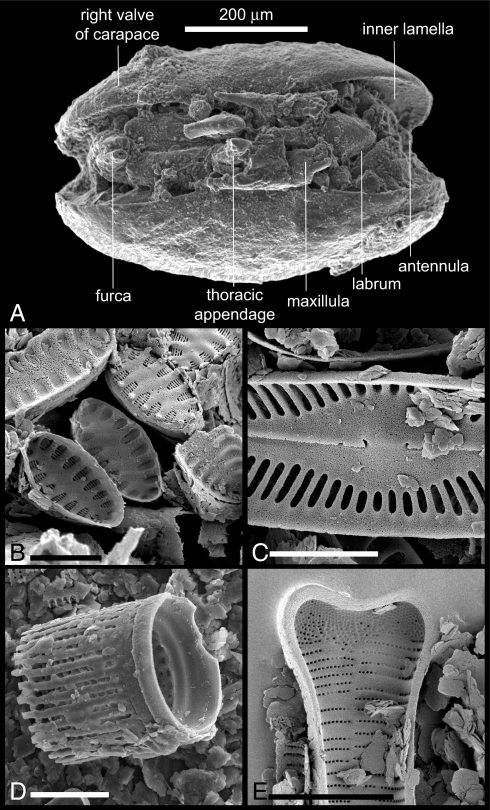
Many of the fossils from the Mount Boreas lacustrine beds are preserved in exquisite detail. The majority represent aquatic taxa that inhabited the lake during an early shallow-water and later deep-water phase. The shallow-water assemblage is represented by layered mosses, benthic diatoms, and ostracodes. The moss tissues are freeze-dried and, like dried museum specimens, can be rehydrated. Comparison with herbarium specimens showed that the dominant moss species is indistinguishable from the extant Drepanocladus longifolius, a bryophyte within the semiaquatic Amblystegiaceae (Fig. 3 A and B). Between stems and leaves of the mosses, we have found ostracode carapaces representing several instars of a single species. Remarkably, soft anatomy of appendages and mouth parts is preserved in a few specimens as a result of replacement by an iron oxide mineral, most likely goethite (Fig. 4A). The absence of key morphological characteristics presently does not allow the ostracodes to be assigned to a species, but their anatomy places them in the superfamily Cypridoidea. The numbers of specimens and the generations represented indicate that moss mats provided a favorable habitat for these benthic crustaceans to complete their life cycles.
Fig. 3.
Fossil mosses and a beetle. (A) Stem and leaves of the semiaquatic moss Drepanocladus longifolius (Mitt.) Broth. ex. Paris. (B) Enlargement of stem and leaves. The diagnostic features of the fossils are long lanceolate leaves up to 2.2 mm in length, strong percurrent or excurrent costae, elongate lamina cells, and enlarged alar cells that are quadrate-rectangular across the leaf base. Short, broad pseudoparaphyllia or juvenile leaves with single, long, sharp apical cells are visible in some leaf axils, and the stem has thick-walled outer cells and a central strand with thin-walled cells. (C) Stem and leaves of an unknown species of a terrestrial haplolepidous moss with large isodiametric cells. (D) Illustration of the base of the left elytron of a beetle.
Fig. 4.
Fossil ostracode and diatoms. (A) Soft anatomy preserved between the valves of an ostracode in the superfamily Cypridoidea. (B) Disarticulated colony of the benthic alkaliphilous diatom Staurosirella pinnata. (C) Pinnularia sp., a benthic periphytic form. (D) Aulacoseira sp., a tychoplanktonic filamentous diatom. (E) The planktonic form Asterionella formosa. (Scale bars: in B–E, 5 μm.)
The shift to deeper water and associated changes in water chemistry are recorded by changing assemblages of diatoms (Fig. 4 B–E). The shallow-water flora comprises colonial benthic fragilarioid genera (Staurosira, Staurosirella, and Pseudostaurosira spp.), which occur in profusion and with remarkable preservation in association with the mosses. In overlying sediments, these taxa are progressively replaced by the genera Encyonema, Eunotia, Gomphonema, Navicula, Pinnularia, and Stauroneis. Toward the top of the diatom sequence, planktonic diatoms including Asterionella formosa and Aulacoseira spp. appear with increasing regularity, likely a consequence of increased water depth to a maximum of 8 m (Fig. 1C). The uppermost lacustrine strata contain only diatoms of the genera Eunotia and Aulacoseira.
The diatom assemblages bear little resemblance to extant Antarctic floras (6, 7), instead they have a far greater overall floristic similarity to low ionic-strength lakes of the Arctic (8, 9). The floristic succession from benthic alkaliphilous fragilarioids to planktonic acidophilous taxa, culminating with Aulacoseira and Eunotia, denotes a pattern of progressive lake acidification that has striking parallels to postglacial lakes in the northern hemisphere, including the Arctic (10–12). Because natural lake acidification occurs on time scales of several millennia, whether driven by edaphic (13) or climatic processes (12), a comparable interval of deposition is envisaged for this diatom sequence. Moreover, the development of a diversified diatom flora containing both benthic and planktonic components implies that the water-body was both permanent and seasonally ice-free. The algal flora of modern perennially ice-covered lakes in Antarctica includes planktonic forms that are either neutrally buoyant or motile (flagellate). The planktonic diatoms reported here (Aulacoseira and Asterionella) do not fulfill these ecological prerequisites and therefore mandate circulation within the water column to survive. Organic carbon isotope composition (mean δ13C = −23.3‰) and organic carbon concentration (up to 3.3%) of bulk sediment suggests that the lake was productive, mainly from algae with some aquatic mosses based on fossil content (Table S2).
In addition to fossils of organisms that inhabited the lake, we have recovered pollen and spores, and a few macroscopic remains of plants and insects that inhabited the lake margins. The pollen and spore assemblage is the least diverse that has been recovered from Cenozoic sediments in the Ross Sea area and suggests a habitat near the climatic limits for plant growth (14, 15). The assemblage is dominated by Leiosphaeridia spp. and other algal cysts of uncertain provenance. Angiosperm pollen is represented by abundant grains of Nothofagidites lachlaniae from a species of Nothofagus (southern beech), rare grains of Periporopollenites sp., probably from a species of Caryophyllaceae (pink family), and two tricolpate pollen types whose natural affinity is less well known. Gymnosperm pollen is absent, and the remainder of the palynoflora is comprised of spores of Belgisporis sp. (liverwort, possibly Marchantiaceae) and Coptospora spp. (mosses, possibly Bartramiaceae), and more rarely of Retitriletes sp., a club moss in the Lycopodium fastigiatum group. Macrofossils of terrestrial plants are rare, consisting of stems and attached leaves of two species of small haplolepidous mosses. The affinity of one of the mosses is unknown, whereas the other is either a species of Dicranaceae (Dicranella) or Bruchiaceae (Fig. 3C). Insect remains are also rare. Most are submillimeter fragments of larval head capsules, probably of a nematoceran Diptera (midge) species. The largest insect specimen is the base of a left elytron of a small beetle with highly distinctive ornamentation (Fig. 3D). There are too few characteristics to enable it to be taxonomically assigned, but size, ornamentation, and cuticle thickness suggest that it belongs to a weevil. From the palynoflora and the rare terrestrial macroscopic remains, we infer that the slopes were mostly barren with patches of bryophytes. Although no wood or leaves of Nothofagus (southern beech) were found within the sediments, its pollen indicates that it was growing within the region, possibly at lower elevations. Our interpretation of a sparse lake-marginal vegetation is supported by isotopic data, which indicate minimal carbon input to the lake from terrestrial sources (Table S2). The fossil biota, which included vascular plants, insects, ostracods, and diatom species that do not currently occur in Antarctica, is completely different from the existing biota of the McMurdo Dry Valleys. The modern biota is dominated by prokaryotes, eukaryotes only in protected locations, and small numbers of species of bryophytes, rotifers, tardigrades, nematodes, collembolans, and mites (16, 17).
The existence of wet-based glaciers, proglacial lakes, tundra vegetation, and insect remains all indicate that the climate of the western Olympus Range at ≈14.07 Ma was warmer and wetter than that of today. We are able to estimate summer warmth from the modern autecologies of several of the recovered taxa. The closest locations to the McMurdo Dry Valleys inhabited by the moss D. longifolius today is on James Ross and Vega islandsn at the northern tip of the Antarctic Peninsula (64°S) where the mean summer temperature (MST) is −1.7°C.o D. longifolius is such a widely distributed moss in both the northern and southern hemispheres (18) that we assume it would have colonized farther south in continental Antarctica if there were sufficient warmth and moisture to support its growth. Whereas the moss D. longifolius provides an estimate for the minimum thermal environment at the site, the paleoecology of fossil ostracodes and beetle indicate a warmer MST. The nearest lakes with cypridoidean ostracodes today occur in the South Orkney Islands (60°S) where MST is approximately 1°C (19, 20). Likewise, the southernmost records for indigenous beetles are for the island of South Georgia (55°S), where the MST is approximately 5°C;p lathridiid beetle species have been recorded farther south on South Orkney and South Shetland islands (62°S), but they are considered to be recent human introductions (21). Together, the fossil data suggest that MST was ≈5°C. In comparison, the MST at the site today is approximately −12°C (22).
To estimate the amount of cooling that occurred between the wet-based glaciation at 14.07 ± 0.05 Ma and the onset of cold-based glaciation at 13.85 ± 0.03 Ma, we used a steady-state thermomechanically coupled glaciological model with higher-order stress treatments (see SI Text). The model provides a test of basal-ice sensitivity for local alpine glaciers to varying atmospheric temperatures. Assumptions include minimal change in the elevation of the western Olympus Range (23) and that the former wet-based alpine glaciers were similar in size and shape to modern cold-based counterparts throughout the McMurdo Dry Valleys region; the latter assumption is borne out by detailed mapping of recessional moraines from wet-based alpine glaciers in the western Olympus Range. Model results show that the maximum atmospheric temperature consistent with cold-based alpine glaciation in the western Olympus Range is −3°C (Fig. S2). Given that maximum atmospheric temperatures are achieved during summer, the value can be seen as a conservative proxy for MST. If the landscape during the mid-Miocene were at a lower elevation, as some models of landscape evolution imply (24), then an even lower MST would be required to maintain cold-based glaciation. The combined results from paleoecology and glaciological modeling indicate a minimum decrease in MST of 8°C between 14.07 and 13.85 Ma. This interpretation is consistent with the timing of a sea-surface cooling of 6–7°C between 14.2 and 13.8 Ma inferred from Mg/Ca ratios of Southern Ocean planktonic foraminifera (2).
An ongoing debate centers on when hyperarid, polar-desert conditions became established in Antarctica. At question is the long-term stability of the East Antarctic Ice Sheet and whether global warming during the Pliocene (≈3 Ma) (25) resulted in deglaciation of the continental interior and permitted recolonization of tundra organisms as far south as lat. 85°S (4, 26, 27). Our combined paleoglaciological and paleoecological results clarify several of these issues. First, the glacial stratigraphy and geomorphology of the McMurdo Dry Valleys indicate that the transition to cold-based, alpine glacial regimes at 13.85 Ma was never subsequently reversed (5). Second, the exquisite preservation of several fossil groups recovered from Miocene lake sediments (Figs. 3 and 4) indicates minimal postdepositional degradation, which is best reconciled by these assemblages having remained in a perennially dry and frozen state. Third, glass shards from the dated ash deposits show no evidence of either hydration or authigenic clay mineral formation (5, 28), suggesting essentially no chemical weathering. Taken together, these observations constitute robust arguments against the return to warmer and wetter conditions at any time after the MMCT. Accordingly, we surmise that polar desert climate has persisted uninterrupted in the western Olympus Range, and by inference all regions in the Transantarctic Mountains south of 77°S, since at least 13.85 Ma. This climate reconstruction is incompatible with either a marked reduction in the volume of the East Antarctic Ice Sheet inland of the McMurdo Dry Valleys region, or with the recolonization by tundra biota during the middle Pliocene.
The evidence presented here suggests that the climate of East Antarctica cooled abruptly between 14.07 ± 0.05 and 13.85 ± 0.03 Ma. The ultimate cause of the climate change is uncertain. A decline in global pCO2, based on changes in stomatal density on fossil leaves, has been proposed as the primary driver (29), although marine geochemical evidence suggests that pCO2 declined several million years earlier than the MMCT (30). Alternative explanations focus on tectonically driven changes to oceanic circulation resulting in a reorganization of the ocean–atmosphere–cryosphere system (2, 31, 32). Regardless of the ultimate cause, this shift was sufficient to initiate the transition from wet- to cold-based alpine glaciation in the central and southern Transantarctic Mountains, and to induce the regional extinction of tundra plants and animals. The only possible survivors from this earlier biota are microscopic, mostly soil-dwelling organisms (33). The climatic and environmental changes reported here were likely the most significant to have impacted Antarctica since the initial onset of Cenozoic continental glaciation. These results provide challenging but testable targets for climate models, as well as new constraints for Antarctic biogeography.
Supplementary Material
Acknowledgments.
We thank P. Wilby for his work on ostracode taphonomy that recognized the preservation by iron oxide; D. Horne and D. Siveter for advice on ostracode anatomy; B. Vandenheuvel, H. Margerison, S. Burns, E. Klingler, D. Kowalewski, D. Shean, K. Swanger, K. Gorz, and A. Podoll for help in excavating and mapping the lacustrine deposits; R. Thomasson, T. Kummer, and A. Pannem for their help in sample processing; and R. Mink for her illustration of the beetle fossil. This study was supported by National Science Foundation Grants ANT 0440711 (to D.R.M.), ANT 9811877 (to D.R.M.), and ANT-0230696 and ANT 0440761 (to A.C.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802501105/DCSupplemental.
British Antarctic Survey Plant Database, www.antarctica.ac.uk/bas_research/data/access/plantdatabase/index.php. Last updated 2007; last accessed January 8, 2008.
Antarctic Climate Data: Results from the SCAR Reader Project. www.antarctica.ac.uk/met/gjma/temps.html. Last updated June 3, 2007; last accessed January 8, 2008.
Antarctic CRC and Australian Antarctic Division Climate Data Sets. www.antarctica.ac.uk/met/READER/temperature.html. Last updated February 29, 2000; last accessed January 8, 2008.
References
- 1.Zachos J, Pagani M, Stone L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 2.Shevenell AE, Kennett JP, Lea DW. Middle Miocene Southern Ocean cooling and Antarctic cryosphere expansion. Science. 2004;305:1766–1770. doi: 10.1126/science.1100061. [DOI] [PubMed] [Google Scholar]
- 3.Webb P-N, Harwood DM, McKelvey BC, Mercer JH, Stott LD. Cenozoic marine sedimentation and ice-volume variation on the East Antarctic craton. Geology. 1984;12:287–291. [Google Scholar]
- 4.Hill RS, Harwood DM, Webb P-N. Nothofagus beardmorensis (Nothofagaceae), a new species based on leaves from the Pliocene Sirius Group, Transantarctic Mountains, Antarctica. Rev Palaeobot Palynol. 1996;94:11–24. [Google Scholar]
- 5.Lewis AR, Marchant DR, Ashworth AC, Hemming SR, Machlus ML. Major middle Miocene global change: Evidence from East Antarctica and the Transantarctic Mountains. Geol Soc Am Bull. 2007;119:1449–1461. [Google Scholar]
- 6.Spaulding SA, McKnight DM. In: The Diatoms: Applications to the Environmental and Earth Sciences. Stoermer E, Smol J, editors. Cambridge, UK: Cambridge Univ Press; 1999. pp. 245–263. [Google Scholar]
- 7.Kellogg TB, Kellogg DE. In: Non-Marine and Littoral Diatoms from Antarctic and Subantarctic Regions: Distribution and Updated Taxonomy. Witkowski A, editor. Vol 1. Königstein, Germany: Gantner, Ruggell, Liechtenstein, Koeltz Scientific; 2002. Diatom Monographs. [Google Scholar]
- 8.Joynt EH, Wolfe AP. Paleoenvironmental inference models from sediment diatom assemblages in Baffin Island lakes (Nunavut, Canada) and reconstruction of summer water temperature. Can J Fish Aquat Sci. 2001;58:1222–1243. [Google Scholar]
- 9.Smol JP, et al. Climate-driven regime shifts in the biological communities of arctic lakes. Proc Natl Acad Sci USA. 2005;102:4397–4402. doi: 10.1073/pnas.0500245102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehead DR, Charles DF, Jackson ST, Smol JP, Engstrom ER. The developmental history of Adirondack (N.Y.) lakes. J Paleolimnol. 1989;2:185–206. [Google Scholar]
- 11.Renberg I. A 12,600 year perspective of the acidification of Lilla Oresjon, Southwest Sweden. Philos Trans R Soc London. 1990;327:357–361. [Google Scholar]
- 12.Michelutti N, Wolfe AP, Briner JP, Miller GH. Climatically controlled chemical and biological development in Arctic lakes. J Geophys Res. 2007;112:G03002. [Google Scholar]
- 13.Engstrom DR, Fritz SC, Almendinger JE, Juggins S. Chemical and biological trends during lake evolution in recently deglaciated terrain. Nature. 2000;408:161–166. doi: 10.1038/35041500. [DOI] [PubMed] [Google Scholar]
- 14.Prebble JG, Raine JI, Barrett PJ, Hannah MJ. Vegetation and climate from two Oligocene glacioeustatic sedimentary cycles (31 and 24 Ma) cored by the Cape Roberts Project, Victoria Land Basin, Antarctica. Palaeogeogr Palaeoecl. 2006;231:41–57. [Google Scholar]
- 15.Raine JI. Terrestrial palynomorphs from Cape Roberts Project Drillhole CRP-1, Ross Sea, Antarctica. Terra Antarctica. 1998;5:539–548. [Google Scholar]
- 16.Morrhead DL, Priscu JC. In: Ecosystem Dynamics in a Polar Desert: The McMurdo Dry Valleys, Antarctica. Priscu JC, editor. Washington, DC: Am Geophys Union; 1998. pp. 351–369. AGU Antarctic Research Series. [Google Scholar]
- 17.Stevens MI, Hogg ID. Expanded distributional records of Collembola and Acari in southern Victoria Land, Antarctica. Pedobiologia. 2002;46:485–495. [Google Scholar]
- 18.Hedenäs L, editor. The Drepanocladus s. str. species with excurrent costae (Musci: Amblystegiaceae) Nova Hedwigia. 1997;64:535–547. [Google Scholar]
- 19.Pugh PJA, Dartnall HJG, McInnes SJ. The non-marine Crustacea of Antarctica and the Islands of the Southern Ocean: Biodiversity and biogeography. J Nat Hist. 2002;36:1047–1103. [Google Scholar]
- 20.Peck LS, Convey P, Barnes KA. Environmental constraints on life histories in Antarctic ecosystems: Tempos, timings and predictability. Biol Rev. 2006;81:75–109. doi: 10.1017/S1464793105006871. [DOI] [PubMed] [Google Scholar]
- 21.Gressitt JL. In: Entomology of Antarctica. Gressitt J, editor. Vol 10. Washington, DC: Am Geophys Union; 1967. pp. 1–33. AGU Antarctic Research Series. [Google Scholar]
- 22.Doran PT, et al. Valley floor climate observations from the McMurdo Dry Valleys, Antarctica, 1986–2000. J Geophys Res. 2002 doi: 10.1029/2001JD002045. [DOI] [Google Scholar]
- 23.Sugden DE, Denton GH, Marchant DR. Landscape evolution of the McMurdo Dry Valleys, Transantarctic Mountains: Tectonic implications. J Geophys Res. 1995;100:9949–9967. [Google Scholar]
- 24.Stern TA, Baxter AK, Barrett PJ. Isostatic rebound due to glacial erosion within the Transantarctic Mountains. Geology. 2005;33:221–224. [Google Scholar]
- 25.Naish T, et al. A record of Antarctic climate and ice sheet history recovered. Eos. 2007;88:557–558. [Google Scholar]
- 26.Ashworth AC, Cantrill DJ. Neogene vegetation of the Meyer Desert formation (Sirius Group) Transantarctic Mountains, Antarctica. Palaeogeogr Palaeoecol. 2004;213:65–82. [Google Scholar]
- 27.Ashworth AC, Thompson FC. A fly in the biogeographic ointment. Nature. 2003;423:135. doi: 10.1038/423135a. [DOI] [PubMed] [Google Scholar]
- 28.Marchant DR, Denton GH, Sugden DE, Swisher CC. Miocene glacial stratigraphy and landscape evolution of the western Asgard Range, Antarctica. Geogr Ann. 1993;75A:303–330. [Google Scholar]
- 29.Kürschner WM, Kvaček Z, Dilcher DL. The impact of Miocene atmospheric carbon dioxide fluctuations on climate and the evolution of terrestrial ecosystems. Proc Natl Acad Sci USA. 2008;105:449–453. doi: 10.1073/pnas.0708588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagani M, Freeman KH, Arthur MA. Late Miocene Atmospheric CO2 Concentrations and the Expansion of C4 Grasses. Science. 1999;285:876–879. doi: 10.1126/science.285.5429.876. [DOI] [PubMed] [Google Scholar]
- 31.Kennett JP. Cenozoic evolution of Antarctic glaciation, the Circum-Antarctic Ocean and their impact on global paleoceanography. J Geophys Res. 1975;82:3843–3860. [Google Scholar]
- 32.Woodruff F, Savin SM. Mid-Miocene isotope stratigraphy in the deep sea: High resolution correlations, paleoclimatic cycles, and sediment preservation. Paleoceanography. 1991;6:755–806. [Google Scholar]
- 33.Convey P, Stevens MI. Antarctic biodiversity. Science. 2007;317:1877–1878. doi: 10.1126/science.1147261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.