Abstract
Pyramidal neurons in the deep layers of the cerebral cortex can be classified into two major classes: callosal projection neurons and long-range subcortical neurons. We and others have shown that a gene expressed specifically by subcortical projection neurons, Fezf2, is required for the formation of axonal projections to the spinal cord, tectum, and pons. Here, we report that Fezf2 regulates a decision between subcortical vs. callosal projection neuron fates. Fezf2−/− neurons adopt the fate of callosal projection neurons as assessed by their axonal projections, electrophysiological properties, and acquisition of Satb2 expression. Ctip2 is a major downstream effector of Fezf2 in regulating the extension of axons toward subcortical targets and can rescue the axonal phenotype of Fezf2 mutants. When ectopically expressed, either Fezf2 or Ctip2 can alter the axonal targeting of corticocortical projection neurons and cause them to project to subcortical targets, although Fezf2 can promote a subcortical projection neuron fate in the absence of Ctip2 expression.
Keywords: callosal, cell fate, zinc finger transcription factor, corticospinal tract, axon guidance
The mammalian cerebral cortex is organized into six layers, in which projection neurons within a layer tend to share similar morphologies, functional properties, and connectivity (1, 2). During development, neurons within a layer are generated at similar times: cells destined for deep layers arise at early times, whereas those destined for more superficial positions are generated later (3–5). Transplantation studies demonstrate that early progenitor cells are multipotent and can produce neurons of any layer, whereas late progenitors are restricted to producing upper-layer neurons (1, 6–8). Although much previous work has emphasized differences between neurons in different layers and commonalities of those within a layer, a variety of phenotypes can exist side-by-side within a layer (9–12). For example, layer 5 contains large pyramidal neurons that extend axons to subcortical targets and callosal neurons that project to the contralateral hemisphere (9–12). From the earliest stages of axon outgrowth, these two classes of neurons show distinct behaviors: the axons of subcortical projection neurons descend toward the internal capsule, whereas those of callosal projection neurons steer toward the midline (2, 11, 13, 14). Callosal and subcortical projection neurons also exhibit distinctive electrophysiological properties. Callosal neurons show strong spike frequency adaptation in response to intracellular current injection, whereas subcortically projecting cells fire actions potentials without adaptation (2, 10, 13–15). Finally, these classes of neurons differ in dendritic morphology: subcortical projection neurons extend apical dendrites into layer 1, whereas those of callosal neurons are shorter (2, 10, 13, 14, 16). Although cell birthday predicts the eventual fates of many classes of cortical neurons, layer 5 subcortical and callosal projection neurons are generated from telencephalic progenitors at the same time during development, raising the question of how their distinct fates are determined.
Recent studies have begun to unravel the molecular mechanisms that underlie the development of deep layer neurons and the formation of callosal vs. subcortical projections. For example, the chromatin remodeling protein Satb2 is required for the development of callosal projection neurons (17, 18). Conversely, subcortical projection neurons express the zinc finger transcription factors Fezf2 (formerly known as Fezl or Zfp312) and Ctip2, and mutation of either gene disrupts the formation of the corticospinal tract (CST) (19–22). Ctip2 is expressed in many brain regions, but its expression is prominent in corticospinal motor neurons (CSMNs) (22). These neurons fail to extend axons into the spinal cord in Ctip2−/− mice (22). Fezf2 expression is detected in early forebrain progenitors and in their postmitotic progeny in cortical layers 5 and 6 (19–21, 23). In Fezf2−/− mice, deep-layer neurons are generated and migrate into appropriate positions but fail to express Ctip2 and other markers of layers 5 and 6 (19, 20). To assess axonal projections in mutants, we replaced the Fezf2 ORF with the axonal marker PLAP. These studies revealed that Fezf2−/− layer 5 neurons fail to form the CST and instead project aberrantly across the midline through the anterior commissure (19).
The extension of PLAP-labeled axons through the anterior commissure to the contralateral hemisphere in Fezf2−/− mice raises the possibility that mutant subcortical projection neurons may adopt an alternative fate. Callosal projection neurons normally reach the opposite hemisphere by traversing the corpus callosum. However, this commissure is missing in Fezf2 mutants (19), and the absence of the callosum in Fezf2 mutants precludes axons from taking this route. The anterior commissure may serve as an alternative route by which axons can reach the opposite hemisphere. Here, we examine the hypotheses that Fezf2 regulates the choice between subcortical and callosal projection neuron fates and that Fezf2 acts upstream of Ctip2 and Satb2 in regulating this decision.
Results
Fezf2 Regulates a Fate Switch Between Subcortical and Callosal Projection Neurons.
To examine the possibility that subcortical projection neurons in Fezf2 mutants alter their fate and become callosal projection neurons, we attempted to rescue corpus callosum development by generating aggregation chimeras. We predicted that if the formation of the corpus callosum could be restored by the presence of wild-type cells, axons from Fezf2 mutant neurons might extend across this structure.
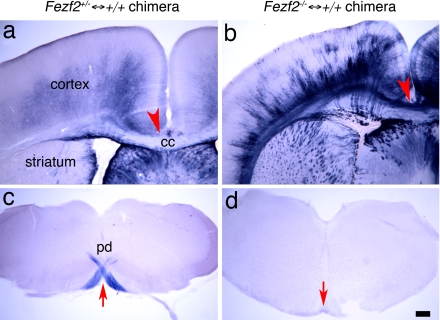
Chimeric mice were generated by aggregating wild-type embryos with either Fezf2+/− or Fezf2−/− embryos. The resulting chimeric brains contained both wild-type cells and PLAP+ Fezf2+/− cells (Fezf2+/− ↔ +/+ chimeric mice) or Fezf2−/− cells (Fezf2−/− ↔ +/+ chimeric mice) (Fig. 1 a and b). Analysis of Fezf2−/− ↔ +/+ chimeric brains revealed that the wild-type cells restored the development of corpus callosum (Fig. 1b). We visualized the axonal projections of neurons derived from Fezf2+/− cells or Fezf2−/− cells by using the PLAP marker that was knocked into the Fezf2 locus (19). Consistent with previous studies of Fezf2+/− mice, PLAP-labeled axons from Fezf2+/− cells in control chimeric mice projected into the CST (Fig. 1c, arrow), with few labeled axons visible in the corpus callosum (Fig. 1a). In contrast, Fezf2−/− neurons in Fezf2−/− ↔ +/+ chimeras extended PLAP+ axons that failed to project into CST (Fig. 1d), confirming previous results from Fezf2 mutants (19) and suggesting that this defect may be cell-autonomous.
Fig. 1.
Fezf2 mutant neurons project across the corpus callosum in Fezf2−/− ↔ +/+ chimeric mice. (a and c) PLAP-labeled axons from Fezf2+/− neurons in Fezf2+/− ↔ +/+ chimeras are largely absent from the corpus callosum (a, arrowhead) and striatum. PLAP+ axons project into the pyramidal decussation (c, arrow). (b and d) PLAP-labeled axons from Fezf2−/− neurons in Fezf2−/− ↔ +/+ chimeric mice project across the corpus callosum to the contralateral cortical hemisphere (b, arrowhead). Extensive collaterals are also seen in the striatum. Labeled axons do not extend into the pyramidal decussation (d, arrow). All sections were from P5 chimeric mouse brains. cc, corpus callosum; pd, pyramidal decussion. (Scale bar, 200 μm.)
The restoration of callosal development in Fezf2−/− ↔ +/+ chimeras enabled us to ascertain whether Fezf2−/− neurons adopted a callosal identity. Consistent with this hypothesis, PLAP-labeled axons entered the callosum and crossed the midline into the contralateral hemisphere (Fig. 1b). We do not know whether these axons originate exclusively from Fezf2−/− neurons in layer 5 or are also derived from layer 6 (as seems likely in light of studies discussed below). Interestingly, although few PLAP-labeled axons were observed in the dorsal striatum in control chimeras (Fig. 1a), PLAP+ axons formed patchy projections to the dorsal striatum in Fezf2−/− ↔ +/+ chimeras (Fig. 1b). Previous studies demonstrated that a subset of callosal projection neurons called intratelencephalic-type corticostriatal cells send contralateral projections to striatum and cortex (24). Because PLAP-labeled axons from Fezf2+/− cortical neurons do not form callosal projections, the observation that PLAP+ axons in Fezf2−/− ↔ +/+ chimeras form both callosal and striatal projections suggests that some mutant cells adopt a callosal projection neuron fate and extend axon collaterals to the striatum.
Fezf2−/− Neurons Display the Electrophysiological Properties of Callosal Projection Neurons.
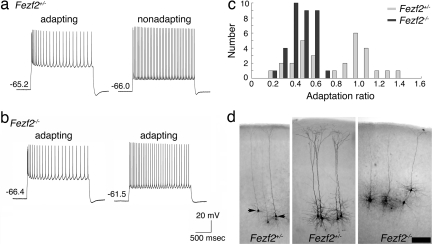
Projection neurons in layer 5 can be categorized into several electrophysiological classes that correlate with their projection patterns. Callosal projection neurons exhibit strong spike frequency adaptation in response to intracellular current injections, whereas many neurons that extend axons to the spinal cord, thalamus, or trigeminal nucleus fire trains of single action potentials without adaptation, and corticotectal neurons fire in bursts (15). To ascertain whether Fezf2 regulates the physiological fates of layer 5 neurons, we blinded each animal's genotype and recorded from individual layer 5 neurons in brain slices from Fezf2+/− and Fezf2−/− mice. In Fezf2+/− slices, neurons responded to depolarizing current injections in one of two manners: some showed spike frequency adaptation, whereas others were nonadapting (Fig. 2a). In slices from Fezf2−/− mice, all recorded layer 5 neurons demonstrated spike frequency adaptation in response to depolarizing current injections (Fig. 2b). To quantify these data, we plotted the number of neurons that exhibited a given adaptation ratio, where 1 is no adaptation and 0 is complete adaptation. This plot (Fig. 2c) revealed a bimodal distribution of layer 5 neurons in Fezf2+/− slices, consisting of neurons with either high (≥0.75) or low (<0.75) adaptation ratios. Fezf2+/− neurons that showed little or no spike frequency adaptation had an average adaptation ratio of 1.05 ± 0.04 (n = 13), whereas the average ratio for adapting neurons was 0.48 ± 0.02 (n = 18). The distribution of adaptation ratios of Fezf2+/− cells was significantly different from a unimodal distribution (P = 0.04; Hartigan's statistic for unimodality). In contrast, all of the layer 5 neurons in slices from Fezf2−/− animals showed an adapting phenotype (average adaptation ratio 0.44 ± 0.02, n = 36), and the ratios showed a unimodal distribution (P = 0.45) (Fig. 2c). The adaptation ratios of mutant neurons were significantly different from those of nonadapting neurons in heterozygous controls (P = 1.61 × 10−13; Student's t test) but were not significantly different from those of adapting neurons in control slices (P = 0.92; Student's t test). Because we recorded at random from layer 5 neurons in the slices, it is unlikely that our sample of cells in Fezf2 mutants included only normal callosal projection neurons. These data indicate that many subcortical projection neurons in layer 5 of Fezf2−/− mice have undergone a physiological fate switch and have adopted a callosal phenotype.
Fig. 2.
Fezf2 mutant neurons exhibit the electrophysiological characteristics of callosal projection neurons. (a) Responses to depolarizing current injection of layer 5 neurons in brain slices from Fezf2+/− mice at P17–P24. (b) Responses to depolarizing current injection of layer 5 neurons in slices from Fezf2−/− mice at P17–P24. (c) Histogram plotting the number of layer 5 neurons exhibiting a given adaptation ratio. Layer 5 neurons in slices from Fezf2+/− mice exhibit a bimodal distribution of neurons with high adaptation ratios (≥0.75) and those with low adaptation ratios (<0.75). Layer 5 neurons recorded in Fezf2−/− mice showed a unimodal distribution, all exhibiting low adaptation ratios. (d) Representative biocytin-filled layer 5 neurons in cortical slices. Arrows point to neurons in Fezf2+/− mice that showed spike frequency adaptation. The unmarked neurons in the Fezf2+/− mice showed no adaptation. (Scale bar, 200 μm.)
Altered Dendritic Morphologies in Fezf2−/− Layer 5 Neurons.
In rat, subcortical and callosal projection neurons exhibit distinct dendritic morphologies, with the apical dendrites of subcortical projection neurons giving rise to many branches and extending into layer 1, whereas those of callosal neurons contain fewer branches and terminate in or below layer 2/3 (2, 10, 13, 14, 16). Although it has been assumed that such differences also exist in mouse layer 5 neurons, the literature contains no definitive comparisons of the dendritic morphologies of layer 5 neurons with known long-distance projections. We examined the dendritic morphologies of biocytin-labeled layer 5 cells in slices from control Fezf2+/− mice (Fig. 2d), but we saw no significant differences in the heights or branching patterns of apical dendrites between adapting and nonadapting neurons (n = 10; data not shown). In the absence of clear differences between these two populations in Fezf2+/− slices, it was not surprising that the heights of the apical dendrites of Fezf2−/− layer 5 neurons in Fezf2 mutants (Fig. 2d) did not differ significantly from those in controls (n = 28, P = 0.38). However, the apical dendrites of mutant neurons showed significantly fewer terminal branches (average, 2.0 ± 1.7) than did those of adapting (9.5 ± 3.8) or nonadapting (11.0 ± 3.9) neurons in control slices (ANOVA, f = 44.899, P < 0.0001). Thus, although dendritic morphologies may not differ clearly between adapting and nonadapting neurons in mouse, our data suggest that Fezf2 is required for their full elaboration in layer 5 neurons, consistent with results obtained by using an RNAi construct to knock down Fezf2 function (21).
Satb2 Is Up-Regulated in the Deep Layers of Fezf2−/− Mice.
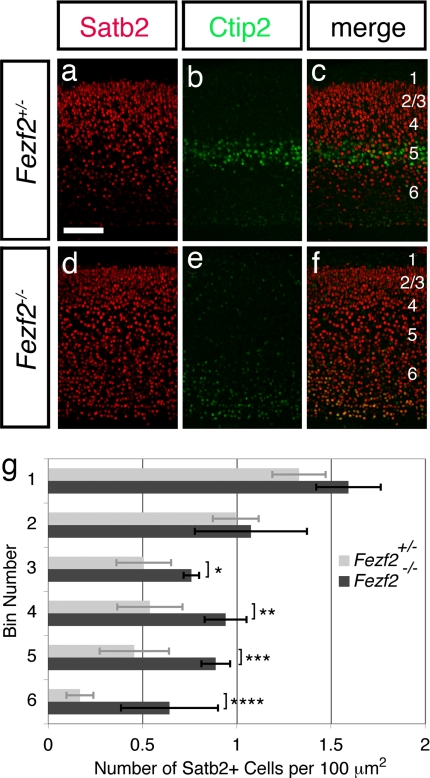
The above results are consistent with the hypothesis that Fezf2 specifies the fates of subcortical projection neurons and that at least some of these cells in Fezf2−/− mice adopt a callosal projection neuron identity. To explore this notion at a molecular level, we assessed the expression of Satb2, a DNA-binding protein required for the differentiation of callosal projection neurons (17, 18). Sections of Fezf2 mutant brains stained for Satb2 and Ctip2 showed the expected decrease in Ctip2 expression in layer 5, although some immunostaining was still detected in layer 6 (Fig. 3 e and f). Satb2 staining, in contrast, revealed similar numbers of Satb2+ neurons in the upper layers of cortex, whereas increased staining was apparent in layers 5 and 6 (Fig. 3 a, c, d, and f). To quantify this result, we delineated 200-μm-wide regions of the cortical plate in posterior and anterior–medial regions of cortex, divided each region into 67-μm-thick bins extending from layer 2/3 to subplate, then assessed the density of Satb2+ neurons at each depth. Our results revealed significant increases in the density of Satb2-expressing cells in the deep layers of Fezf2−/− mice compared with controls, whereas the density of Satb2+ neurons in the upper layers was comparable [Fig. 3g and supporting information (SI) Fig. S1]. These results suggest that many subcortical projection neurons become Satb2+ callosal projection neurons in the absence of Fezf2 function. These data further suggest that the cell fate transformations include neurons in layer 6 and those in layer 5 because Satb2 expression is altered in both layers.
Fig. 3.
The deep layers of Fezf2−/− mice show increased expression of Satb2. Anatomically matched sections from posterior regions of control (a–c) and Fezf2−/− (d–f) mice at P4 were immunostained for Ctip2 (green) to mark deep-layer neurons and for Satb2 (red). Satb2 expression was increased in the deep layers of Fezf2 mutants. (Scale bar, 100 μm.) (g) Histogram comparing the densities of Satb2+ neurons in mutants (n = 4 brains, 3 posterior sections per brain) vs. controls (n = 4 brains, 3 posterior sections per brain). Error bars represent SD. Statistically significant changes were observed in deeper but not more superficial positions. *, P = 0.00073; **, P = 0.00017; ***, P = 8.2E-05; ****, P = 0.00014 (α = 0.01, one-tailed Student's t test).
Ctip2 Is a Major Downstream Effector of Fezf2 in Regulating CST Formation.
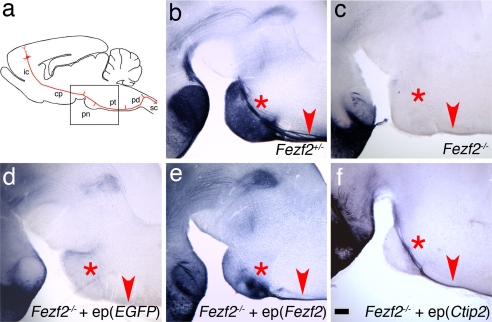
To explore the mechanisms by which Fezf2 regulates the development of subcortical projection neurons, we focused on the zinc finger transcription factor Ctip2. Ctip2 expression is perturbed in Fezf2 mutant mice (19), and Ctip2−/− mice show profound defects in the formation of the CST (22). To test the hypothesis that Ctip2 is an essential effector of Fezf2, we asked whether restoring Ctip2 expression in Fezf2−/− layer 5 neurons is sufficient to rescue their axonal phenotype. To this end, expression plasmids encoding Ctip2 were electroporated into the cerebral hemispheres of Fezf2−/− embryos at embryo day (E) 13.5, when layer 5 neurons are generated. As a positive control, we found that electroporation of a plasmid encoding Fezf2 rescued the Fezf2 mutant phenotype: PLAP-labeled axons failed to extend into the CST after electroporation of control vector (Fig. 4d), but PLAP+ axons populated the CST after electroporation of Fezf2 (Fig. 4e). Electroporation of Ctip2 also rescued the Fezf2−/− phenotype: expression of Ctip2 in Fezf2−/− neurons resulted in the extension of PLAP-labeled axons into the CST (Fig. 4f and Fig. S2). These data indicate that Ctip2 is a major downstream effector of Fezf2 in regulating CST development.
Fig. 4.
Expression of Ctip2 can rescue axon misguidance defects in Fezf2 mutant mice. (a) Representative scheme of corticospinal axon projections, modeled after Fig. 8 from ref. 34. The box represents the area shown in b–e. (b) In Fezf2+/− mice at P5, PLAP-labeled axons are visible in the CST above the pons (asterisk) and in the pyramidal tract (arrowhead). (c) In Fezf2−/− mice, no PLAP+ axons are visible near the pons or in the pyramidal tract. (d) When pCA-EGFP was electroporated into deep-layer neurons of Fezf2−/− brains, PLAP+ axons were absent from the pons and pyramidal tract at P5. (e) Electroporation of a pCA-Fezf2 plasmid into deep-layer neurons in the Fezf2−/− cortex results in a restoration of normal CST axon trajectories by P5. (f) When pCA-Ctip2 was electroporated into deep-layer neurons of the Fezf2−/− cortex, PLAP-labeled axons are visible in the CST above the pons and in the pyramidal tract at P1. Because of the young age, extensive PLAP-labeled axon collaterals in the pons are not visible. ep, electroporation (expression construct); ic, internal capsule; cp, cerebral peduncle; pd, pyramidal decussation; pn, pons; pt, pyramidal tract; sc, spinal cord. (Scale bar, 200 μm.)
To explore further the relationship between Fezf2 and Ctip2, we asked whether electroporating of Fezf2 into Fezf2−/− progenitors at E13.5 could restore Ctip2 expression. Intriguingly, even though electroporating Fezf2 rescued axon extension along the CST, Ctip2 protein was not detected in electroporated neurons at any time examined before postnatal day (P) 5 (E15.5, E16.5, E17.5, E18.5, or P0) (Fig. S3e). These data suggest that Fezf2 is required in progenitor cells before E13.5 to enable normal Ctip2 expression in postmitotic neurons. Collectively, the results of rescue experiments indicate that Ctip2 is sufficient to replace Fezf2 function but that Fezf2 can promote CST formation by affecting downstream components that are independent of Ctip2. Although the identity of these effectors is unknown, numerous genes show altered expression in Fezf2 mutant brains (19), and the functions of most remain to be tested.
Ectopic Expression of Fezf2 or Ctip2 in Layer 2/3 Neurons Alters Axon Targeting.
Previous work and the present experiments suggest that the Fezf2–Ctip2 genetic pathway is necessary for specification and differentiation of subcortical projection neurons. To ascertain whether this pathway is sufficient for specifying this fate, we ectopically expressed either Fezf2 or Ctip2 in layer 2/3 neurons of wild-type mice because these neurons normally form connections with ipsilateral and contralateral cortical areas but do not extend axons to the thalamus or CST. Expression plasmids encoding either Fezf2 or Ctip2 were electroporated into wild-type embryos at E15.5, when upper-layer neurons are generated. To label the axons of electroporated layer 2/3 cells, we coelectroporated a plasmid encoding green fluorescence protein (EGFP). Brains that contained labeled cells in layers 4, 5, or 6 were excluded from further analysis.
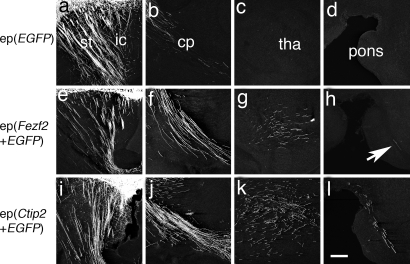
Electroporating EGFP alone into upper-layer neurons revealed their normal cortical projections (data not shown) and relatively small numbers of axons in the internal capsule and striatum (Fig. 5a and b). No labeled axons were visible in the thalamus, pons, or CST in any animal examined (Fig. 5 c and d). However, electroporating expression constructs encoding either Fezf2 or Ctip2 into upper-layer neurons produced marked changes in the behavior of EGFP-labeled axons. Both constructs resulted in increased numbers of labeled axons in the internal capsule (Fig. 5 e, f, i, and j) and in regions normally targeted by deep layer neurons, including the thalamus (Fig. 5 g and k) and the CST (Fig. 5 h and l). These striking alterations in axon trajectories suggest that the Fezf2–Ctip2 genetic pathway is sufficient to specify the subcortical projection neuron fate. Interestingly, even though the axonal trajectories of electroporated neurons were markedly altered, EGFP+ neurons electroporated with Fezf2 migrated into normal positions in layer 2/3 (Fig. S4 d and g), although some neurons electroporated with Ctip2 failed to migrate into the cortical plate (Fig. S4 a and c).
Fig. 5.
Ectopic expression of Fezf2 or Ctip2 in wild-type mice is sufficient to alter the axon trajectories of upper-layer neurons, which normally form corticocortical connections, at P5. (a–d) Axonal projections of layer 2/3 neurons electroporated with pCA-EGFP reveal that some labeled axons occupy the striatum (a) and a few descend through the internal capsule and cerebral peduncle (a and b). No EGFP-labeled axons were observed in the thalamus (c) or the vicinity of the pons (d). (e–h) Layer 2/3 neurons coelectroporated with pCA-Fezf2 and pCA-EGFP extend EGFP-labeled axons through the internal capsule (e) and cerebral peduncle (f). Labeled axons were abundant in the thalamus (g), and a few were present in the CST above the pons (h, arrow). (i–l) Upper-layer neurons coelectroporated with pCA-Ctip2 and pCA-EGFP showed a pattern similar to that after electroporation with Fezf2, but an even greater number of labeled axons were present in the thalamus (k) and CST (l). ep, electroporation (expression construct); cp, cerebral peduncle; ic, internal capsule; st, striatum; tha, thalamus. (Scale bar, 200 μm.)
Although ectopic expression of either Fezf2 or Ctip2 altered the axonal targeting of upper-layer neurons, the neurons transfected with Fezf2 did not express Ctip2 (Figs. S4 d–f and S5). As a positive control, Ctip2-transfected neurons did express Ctip2 protein (Fig. S4 a–c). Thus, even though Fezf2 function is necessary for Ctip2 expression in the deep layers, Fezf2 expression in upper-layer neurons is not sufficient to induce Ctip2. This may contribute to the apparently lower efficacy of Fezf2 in altering axon targeting of layer 2/3 neurons after electroporation (Fig. 5 g, h, k, and l).
Many upper-layer neurons normally express Satb2, which inhibits Ctip2 expression by altering chromatin structure at the Ctip2 locus (17, 18). The lack of Ctip2 expression in layer 2/3 neurons electroporated with Fezf2 led us to ascertain whether Fezf2 alters Satb2 expression in these neurons. Interestingly, neurons electroporated with Fezf2 showed no obvious loss of Satb2 (Figs. S4 g–i and S5), suggesting that Satb2 continues to repress Ctip2 expression in these cells. These data further emphasize the finding that Fezf2 can promote CST formation in a manner independent of Ctip2.
Discussion
Fezf2 is required for the differentiation and axon targeting of layer 5 subcortical projection neurons (19–21). Here, we show that Fezf2 regulates a choice between subcortical projection neuron and callosal projection neuron fates. In the absence of Fezf2, mutant neurons not only adopt the axonal targeting and the physiological properties of callosal projection neurons, but they also acquire expression of the callosal marker Satb2. Our data indicate that Ctip2 is a major downstream effector of Fezf2 and can rescue the axonal phenotype resulting from mutation of Fezf2. Finally, we show that ectopic expression of either Fezf2 or Ctip2 in upper-layer neurons is sufficient to redirect their axons subcortically, and Fezf2 can promote CST formation without inducing Ctip2 expression in these cells.
Fezf2 Regulates the Choice Between Subcortical and Callosal Projection Neuron Fates.
Projection neurons in layer 5 of the rodent cerebral cortex fall into two major classes that can be distinguished on the basis of their axonal projections, morphologies, and physiological properties in the adult (2, 10, 12). Interestingly, in mouse, electrophysiological differences between subcortical and callosal projection neurons are similar to those in rat; however, we did not observe clear morphological differences between these two classes of neurons. Thus, we focused our study on the long-distance axonal projections and electrophysiological characteristics that clearly distinguish these neurons in mouse.
The fact that layer 5 subcortical and callosal projection neurons are produced by progenitors at the same time and develop side-by-side in the same cortical layer has raised the question of how their distinct fates are determined. Defects in the layer 5 subcortical projection neurons in Fezf2−/− mice, including the absence of the CST and other subcortical projections, along with misregulation of gene expression in layers 5 and 6, have suggested that Fezf2 plays an essential role in deep-layer neuronal development (19, 20). However, it was unclear whether mutation of Fezf2 simply blocked the differentiation of subcortical projection neurons or caused them to adopt distinct fates.
The present experiments suggest that in the absence of Fezf2, many deep layer subcortical projection neurons adopt a callosal projection neuron fate. First, electrophysiological recordings of layer 5 neurons in mutant mice revealed that all recorded neurons exhibited strong spike frequency adaptation to current injection, a characteristic of callosal projection neurons, linking the electrophysiological identity of a cortical pyramidal neuron to the expression of a particular transcription factor. Second, instead of projecting in the CST or to midbrain targets, many PLAP+ axons in Fezf2−/− mice crossed the midline through the anterior commissure, as if trying to reach the contralateral hemisphere through the closest available commissure. Aggregation chimeric mice containing Fezf2−/− and wild-type cells restored callosal development, and many PLAP+ axons crossed the corpus callosum. Finally, deep layer neurons in Fezf2−/− mice exhibited dramatic increases in the expression of Satb2, a DNA-binding protein that represses Ctip2 expression and specifies callosal neuron identity (17, 18). The density of Satb2+ cells increased in both layers 5 and 6 of Fezf2 mutants, suggesting that some layer 6 neurons also contributed PLAP+ callosal axons in Fezf2−/− ↔ +/+ chimeras. Our data are consistent with the possibility that Fezf2 normally inhibits Satb2 expression in subcortical projection neurons, thus enabling Ctip2 expression in these cells. Collectively, these studies suggest the hypothesis that callosal (corticocortical) and subcortical projection neuron identities involve mutually repressive pathways, each of which confers the specification of distinct fates onto cortical neurons.
Fezf2 and Ctip2 Can Convert the Axon Targeting of Upper-Layer Cortical Projection Neurons.
Not only are Fezf2 and Ctip2 required for subcortical projection neuron development, but these molecules appear also to be sufficient for forming subcortical axon projections in other cortical cell types. Whereas normal layer 2/3 neurons form corticocortical projections and do not extend axons into the thalamus or CST, ectopic expression of Fezf2 or Ctip2 in layer 2/3 cells caused their axons to project subcortically. We note that not all GFP+ axons altered their projections in these experiments; some axons still projected across the corpus callosum (data not shown). We do not know whether these axons originated from a distinct subset of layer 2/3 neurons (such as those with lower Fezf2 or Ctip2 expression) or from cells that also formed subcortical projections. Interestingly, the migration of electroporated neurons was largely normal, with neurons still populating their normal superficial positions despite altered connectivity. These results suggest that the Fezf2–Ctip2 pathway controls specific aspects of fate determination related more to axon targeting than to cell body positioning or layer formation.
Previous transplantation experiments (6) and lineage analysis of cortical progenitors in vitro (25) have shown that late progenitor cells are restricted to generating upper-layer neurons. Neither Fezf2 nor Ctip2 is normally expressed by layer 2/3 neurons or their progenitors (19, 21, 23). We speculate that transplanting these cells into a younger brain environment is not sufficient to induce the expression of either gene. However, although misexpressing Fezf2 or Ctip2 in layer 2/3 neurons caused many axons to project subcortically, GFP+ axons were still visible in the corpus callosum, and expression of the callosal determinant Satb2 was not inhibited in electroporated neurons. This incomplete fate switch may be the result of the restricted developmental potential of late cortical progenitors.
In addition to their expression in layer 5, Fezf2 and Ctip2 are also expressed in layer 6 neurons, the majority of which normally project to the thalamus. Interestingly, electroporation of either Fezf2 or Ctip2 into layer 2/3 neurons resulted in the extension of EGFP+ axons into the thalamus, suggesting that Fezf2 and Ctip2 normally regulate axon targeting in both layer 5 and 6 neurons. In postnatal Fezf2−/− mice, cortical projections to the thalamus appear grossly normal (19), but at earlier stages the axons exhibit a transient delay in reaching this target (26). These data raise the question of how Fezf2 and Ctip2 regulate the development of multiple fates in the deep layers. Recent studies suggest that the precise levels of these proteins, in conjunction with the expression of other factors such as Sox5, can determine the identities of distinct subtypes of subcortical projection neurons in layers 5 and 6 (27).
Mechanisms of Fezf2 Function.
Because Fezf2 encodes a putative DNA-binding protein, it likely controls cell fates by regulating the expression of its target genes. Fezf2−/− mice exhibit defects in the cortical expression patterns of many transcription factors, including ER81, Grg4, Foxo1, and Foxp2 (19). Several of these genes are involved in fate determination and axon targeting in the spinal cord (28–31); however, their roles in cortical development are largely unknown. Here, we focused on the zinc finger transcription factor Ctip2 as an effector of Fezf2. The phenotype of Ctip2 mutants is reminiscent of that of Fezf2-deficient mice in that CST axons fail to reach the spinal cord (22). Fezf2 is expressed at a developmentally earlier stage than Ctip2 and is expressed in both cortical progenitors and subcortical projection neurons, whereas Ctip2 expression occurs later, in postmitotic neurons (19). In conjunction with the observation that Ctip2 expression is abrogated in the Fezf2−/− cortex (19, 20), these data suggest that Fezf2 acts upstream of Ctip2 in deep layer neurons. Indeed, restoration of Ctip2 expression in Fezf2−/− subcortical projection neurons was sufficient to rescue the targeting of PLAP-labeled axons to the spinal cord. Collectively, these observations suggest that Ctip2 is a major downstream effector of Fezf2 in regulating axon targeting.
Two lines of evidence, however, suggest that Ctip2 is unlikely to be the sole effector of Fezf2 (Fig. S6). First, although electroporating Fezf2 into E13.5 Fezf2−/− brains restored the extension of PLAP+ axons into the CST, Ctip2 protein expression was not detected in electroporated neurons. This suggests that the timing or level of Fezf2 expression may be critical in regulating Ctip2 expression. Second, ectopic expression of Fezf2 in wild-type layer 2/3 neurons was sufficient to promote axon extension to the CST and thalamus, but did not induce Ctip2 expression in these cells. In the latter case, it is possible that cofactors required for Ctip2 expression are absent in layer 2/3 neurons or that these cells actively repress Ctip2. Indeed, upper-layer neurons expressed Satb2 even after electroporation with Fezf2, suggesting that Fezf2 is not sufficient to repress Satb2 expression and that Satb2 continued to repress the Ctip2 locus in these cells.
How might Fezf2 promote the formation of subcortical projections, apart from (and in addition to) using Ctip2? Recent studies have identified several transcription factors that regulate the development of cortical projection neuron subtypes. For example, Tbr1 is required for subplate formation (32), whereas Sox5 controls the temporal sequence of differentiation and the identity of subplate, corticothalamic, and layer 5 subcortical projection neurons (27). We speculate that Fezf2 regulates the expression of other genes, in addition to Ctip2, that regulate the development of CSMNs (Fig. S6). Parallel pathways are commonplace in genetics, and the fact that Ctip2 is not an obligate target of Fezf2 in specifying subcortical connectivity is both important and interesting. We also note that the elaboration of subcortically directed axons in Ctip2 knockout mice appears more extensive than that observed in Fezf2 knockouts because CST axons extend at least to the level of the pons in Ctip2−/− brains (20). The identification of additional genes that show altered expression in Fezf2 mutants may provide further insight into the molecular mechanisms by which Fezf2 regulates the acquisition of subcortical projection neuron fates.
Methods
Animals.
Generation of Fezf2−/− mutant mice was described in ref. 19. Chimeric mice were generated by using established procedures (33). Experiments were carried out in accordance with protocols approved by the Administrative Panel for Laboratory Animal Care (APLAC) at Stanford University and at University of California at Santa Cruz.
Electrophysiology and Dendritic Analysis.
Recording from individual layer 5 neurons and dendritic analysis in Fezf2+/− or Fezf2−/− mice were performed as described in ref. 15. Details are available in SI Methods.
Electroporation.
In utero electroporation experiments (E13.5 for rescue experiments, E15.5 for misexpression experiments) were performed according to a published protocol (21). After electroporation, the embryos were allowed to survive for 2, 3, 4, 5, or 6 days after electroporation, or until P5, at which time axonal projections were visualized with EGFP or PLAP, and Ctip2 or Satb2 expression was analyzed by immunostaining.
PLAP Staining, Immunohistochemistry, and Cell Counts.
PLAP staining was performed as described in ref. 19. Immunohistochemistry staining was carried out by using standard protocol.
Quantitation of Satb2-expressing cells used three anatomically matched brain sections from each of four control (wild-type or Fezf2+/−) or Fezf2−/− mice at P0 and at P4. Sections were stained with antibodies against Ctip2 and Satb2 and visualized by using confocal fluorescence microscopy. Satb2+ cells were counted in a 200-μm-wide column through the cortical plate. The cortical plate was divided into bins of 67-μm-thickness from layer 2/3 at the top to the subplate at the bottom. The density of Satb2+ cells in each bin was calculated by dividing the number of Satb2+ cells in that bin by its area (200 μm × 67 μm). Cell densities in mutant and control brains were compared statistically by using the Student's t test.
Supplementary Material
Acknowledgments.
We thank Drs. Nenad Sestan and Mladen-Roko Rasin for providing the pEF1αZfp312iresGFP construct; Dr. Laura Schaevitz for performing ANOVA; and Will Mckenna, Sahar Taghvaei, and Laura Gaydos for technical assistance. This work was supported by National Institutes of Health Grants EY08411 (to S.K.M.) and MH066338 (to S.B.N.) and by California Institute of Regenerative Medicine RN1-00530-1 (to B.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804918105/DCSupplemental.
References
- 1.McConnell SK. Constructing the cerebral cortex: Neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- 3.Miller MW. In: Cerebral Cortex: Development and Maturation of Cerebral Cortex. Peter A, Jones EG, editors. Vol 7. New York: Plenum; 1998. pp. 133–175. [Google Scholar]
- 4.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: A review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 5.Luskin MB, Shatz CJ. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985;242:611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- 6.Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 7.McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 8.Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- 9.Larkman A, Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci. 1990;10:1407–1414. doi: 10.1523/JNEUROSCI.10-05-01407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasper EM, Lubke J, Larkman AU, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. III. Differential maturation of axon targeting, dendritic morphology, and electrophysiological properties. J Comp Neurol. 1994;339:495–518. doi: 10.1002/cne.903390404. [DOI] [PubMed] [Google Scholar]
- 11.Koester SE, O'Leary DD. Connectional distinction between callosal and subcortically projecting cortical neurons is determined prior to axon extension. Dev Biol. 1993;160:1–14. doi: 10.1006/dbio.1993.1281. [DOI] [PubMed] [Google Scholar]
- 12.Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Koester SE, O'Leary DD. Axons of early generated neurons in cingulate cortex pioneer the corpus callosum. J Neurosci. 1994;14:6608–6620. doi: 10.1523/JNEUROSCI.14-11-06608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards LJ, Koester SE, Tuttle R, O'Leary DD. Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J Neurosci. 1997;17:2445–2458. doi: 10.1523/JNEUROSCI.17-07-02445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- 16.Koester SE, O'Leary DD. Functional classes of cortical projection neurons develop dendritic distinctions by class-specific sculpting of an early common pattern. J Neurosci. 1992;12:1382–1393. doi: 10.1523/JNEUROSCI.12-04-01382.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alcamo EA, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Britanova O, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arlotta P, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Hirata T, et al. Zinc finger gene fez-like functions in the formation of subplate neurons and thalamocortical axons. Dev Dyn. 2004;230:546–556. doi: 10.1002/dvdy.20068. [DOI] [PubMed] [Google Scholar]
- 24.Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J Comp Neurol. 1987;263:567–580. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
- 25.Shen Q, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 26.Komuta Y, Hibi M, Arai T, Nakamura S, Kawano H. Defects in reciprocal projections between the thalamus and cerebral cortex in the early development of Fezl-deficient mice. J Comp Neurol. 2007;503:454–465. doi: 10.1002/cne.21401. [DOI] [PubMed] [Google Scholar]
- 27.Lai T, et al. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 29.Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- 30.Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 31.Muhr J, Andersson E, Persson M, Jessell TM, Ericson J. Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell. 2001;104:861–873. doi: 10.1016/s0092-8674(01)00283-5. [DOI] [PubMed] [Google Scholar]
- 32.Hevner RF, et al. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 33.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1994. pp. 190–193. [Google Scholar]
- 34.Weimann JM, et al. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–831. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.