Abstract
The success of the World Health Organization smallpox eradication program three decades ago resulted in termination of routine vaccination and consequent decline in population immunity. Despite concerns regarding the reintroduction of smallpox, there is little enthusiasm for large-scale redeployment of licensed live vaccinia virus vaccines because of medical contraindications and anticipated serious side effects. Therefore, highly attenuated strains such as modified vaccinia virus Ankara (MVA) are under evaluation in humans and animal models. Previous studies showed that priming and boosting with MVA provided protection for >2 years in a monkeypox virus challenge model. If variola virus were used as a biological weapon, however, the ability of a vaccine to quickly induce immunity would be essential. Here, we demonstrate more rapid immune responses after a single vaccination with MVA compared to the licensed Dryvax vaccine. To determine the kinetics of protection of the two vaccines, macaques were challenged intravenously with monkeypox virus at 4, 6, 10, and 30 days after immunization. At 6 or more days after vaccination with MVA or Dryvax, the monkeys were clinically protected (except for 1 of 16 animals vaccinated with MVA), although viral loads and number of skin lesions were generally higher in the MVA vaccinated group. With only 4 days between immunization and intravenous challenge, however, MVA still protected whereas Dryvax failed. Protection correlated with the more rapid immune response to MVA compared to Dryvax, which may be related to the higher dose of MVA that can be tolerated safely.
Keywords: biodefense, modified vaccinia virus Ankara, poxvirus, smallpox vaccine, neutralizing antibody
Smallpox, one of the most devastating diseases of man, was eradicated three decades ago through an intensive vaccination program spearheaded by the World Health Organization (1). A byproduct of this success was the cessation of routine vaccination and the consequent decline in population immunity. Although no animal reservoir exists, contingency vaccination plans have been made because of a concern that terrorists might release variola virus (VARV) (2, 3). The licensed smallpox vaccines consisting of live vaccinia virus (VACV) are highly efficacious, although serious side effects can occur particularly in individuals who are immunocompromised or have a history of eczema (4). During the recent immunization of potential medical first responders in the United States, the incidence of adverse reactions was lower than in historical records because of careful prescreening, although myocarditis was recognized as a new complication (5). However, such prescreening of the general population would be difficult in an emergency situation and further would eliminate many individuals and their families with high risk of severe side reactions to the vaccine. Therefore, the development and testing of safer live vaccines such as modified VACV Ankara (MVA) has been mandated. MVA was derived by >500 passages of the parent virus in chicken embryo fibroblast cells (6), leading to deletions and mutations that severely restrict the replication and virulence of the vaccine even in immunocompromised hosts (7–12). MVA essentially provides a single round “pseudo live” immunization because viral assembly and subsequent spread are blocked without impairing gene expression (13). To compensate for the inability of MVA to spread to other cells, relatively high doses have been used for vaccination (14). The immune responses to MVA and Dryvax are generally similar, although some differences have been found (15, 16).
MVA was safely administered to ≈120,000 individuals in Germany in the 1970s (6, 17) and to smaller groups in recent clinical trials as a potential smallpox vaccine and in recombinant form as a vaccine for other diseases including HIV and cancer (18–21). The efficacy of a MVA smallpox vaccine, however, is uncertain because it was not tested under field conditions before smallpox was eradicated. Consequently, there is a reliance on animal models to evaluate the efficacy of candidate vaccines. Thus far, there has been only limited success in developing a lethal animal model for VARV in monkeys (22). MVA has been shown to induce rapid, short-term protection in a mouse model using a homologous pathogenic VACV challenge (14, 23, 24). Monkeypox virus (MPXV), which belongs to the same Orthopoxvirus genus as VARV, causes a disease in humans that is clinically similar to smallpox, although the fatality rate and the spread from person to person are lower (25). Recent studies have shown that immunization with either MVA or a conventional licensed smallpox vaccine induces humoral and cellular immune responses that protect macaques against lethal infection with MPXV (26, 27). In those studies, the monkeys received two immunizations with MVA 4- 8 weeks apart and were challenged with MPXV after an additional 8–11 weeks. Furthermore, macaques immunized twice with recombinant MVA resisted a MPXV challenge >2 years after vaccination, demonstrating the durability of protective immunity (28, 29). However, the ability of a vaccine to rapidly elicit immunity is essential because widespread vaccination is unlikely before verification of smallpox. Historical and anecdotal evidence from the smallpox eradication campaign suggests that the conventional vaccine can confer protection if administered a few days after exposure to variola virus during the 1–2 week incubation prodrome (1, 30). The present study was designed to determine whether a single dose of MVA could induce rapid protective immunity in the monkeypox model.
Results
Immune Responses to MVA and Dryvax.
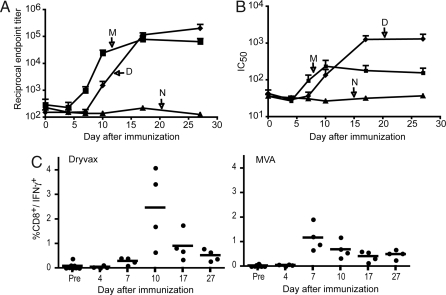
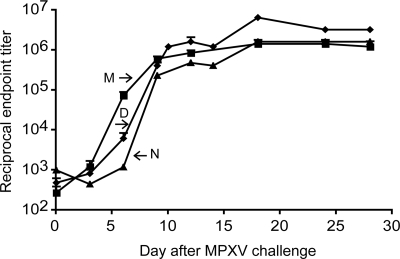
Macaques were immunized intramuscularly with 1 × 108 infectious units of MVA or 2.5 × 105 infectious units of Dryvax intradermally. These doses and routes of administration are comparable to those used in clinical smallpox vaccine trials (18, 31, 32). The temporal development of VACV-specific antibody and CD8+ T cell responses after immunizations are shown in Fig. 1. Antibodies were first detected by ELISA on days 7 and 10 for MVA and Dryvax, respectively (Fig. 1A). The difference between the two groups was statistically significant (P = 0.0286 on days 7 and 10, Mann–Whitney test) and was consistent with our previous data (26). By day 17, however, the titers plateaued to equivalent levels. Similarly, neutralizing antibody to VACV, measured by using a quantitative fluorescent reporter gene infectivity assay (33), was detected earlier in the MVA group (day 7) than in the Dryvax group (day 10), although by day 17 the antibody levels in the Dryvax group surpassed those in the MVA group (Fig. 1B). VACV-specific CD8+ T cell responses were measured by immunostaining fresh peripheral blood mononuclear cells. CD8+/INF-γ+ responses were detected in all animals with peaks on days 7 and 10 for the MVA and Dryvax groups, respectively (Fig. 1C). Although the average peak values were higher in the Dryvax than in the MVA group, the difference was not statistically significant.
Fig. 1.
Vaccine-induced immune responses. Monkeys received MVA (1 × 108 pfu) intramuscularly or Dryvax (2.5 × 105) by skin scratch. The animals were bled on the indicated days and serum and mononuclear cells were obtained for antibody and T cell analysis, respectively. (A) Serum ELISA titers were determined by using 96-well plates coated with purified VACV. The numbers of animals at each time point were 4 (M and D) and 2 (N). Groups determined by vaccination status: M, MVA; D, Dryvax; N, naïve unvaccinated. Averages with standard error are plotted. (B) Neutralizing antibodies were determined by using a flow cytometer to measure reduction in infectivity of VACV-expressing green fluorescent protein. (C) Fresh peripheral blood mononuclear cells were infected with VACV in the presence of brefeldin A overnight and then stained with antibodies to CD3, CD8, and IFN-γ conjugated to phycoerythrin, fluorescein isothyocyanate, and allophycocyanin, respectively, for analysis on a cytometer.
We also investigated systemic changes in the levels of INF-γ, ΤΝF-α, IL-2, IL-4, IL-5 and IL-10 from 2 to 27 days after Dryvax and MVA. The IFN-γ response was dominant, similar to the findings in healthy human adults that had received Dryvax (34). The timing and magnitude of the IFN-γ response was variable, with one monkey in each vaccine group showing an increase over base-line on days 4–10, two in each group showing an increase between days 7 and 10 and one in each group having no increase. There was no significant elevation of IFN-γ in the two unvaccinated animals.
In summary, a single injection of MVA induced more rapid antibody and CD8+/INF-γ+ responses than Dryvax, whereas the magnitudes of the responses were similar or slightly lower. The more rapid immune responses to MVA compared with Dryvax could be explained by the higher inoculum dose of the former. Whereas, the nonreplicating MVA was given as a bolus of 1 × 108 infectious units, only 2.5 × 105 infectious units of Dryvax were administered. The delay for Dryvax probably reflects the time needed to increase the immunogen mass by replication. Although theoretically the dose of Dryvax could be increased, that would be unlikely in view of the troublesome side effects accompanying the existing protocol.
Protection Against MPXV Challenge.
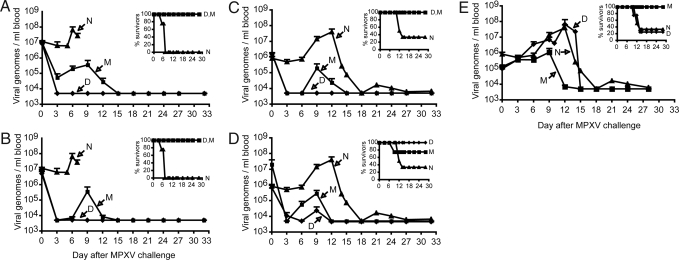
For protection studies, 50 cynomolgous macaques were divided into five groups of 10 animals. Each group was subdivided as follows: Four animals were immunized with Dryvax, four received MVA, one received saline intradermally, and one received saline intramuscularly. Groups were challenged intravenously with 5 × 107 plaque forming units (pfu) (≈10 LD50) of MPXV at 30 or 10 days after vaccination or with 5 × 106 pfu (≈1 LD50) at 10, 6, or 4 days after vaccination. The i.v. route of challenge, which mimics the systemic phase of smallpox (35), has been used previously and demonstrates reproducible disease endpoints (26, 36). After challenge with 5 × 107 pfu, all controls experienced severe disease requiring euthanasia between days 5 and 7. After challenge, the viral genome load in the blood of control animals (henceforth referred to as viremia) increased between days 5 and 7 reaching titers of 2 × 107 to 1.4 × 108 (mean 5.3 × 107) (Fig. 2A); total body skin lesion counts were in excess of 2,500 (Table 1). The first group of vaccinated animals was challenged with 5 × 107 pfu of MPXV on day 30, a time at which the antibody response had reached a plateau and CD8+ T cells had declined. These animals all survived the 32-day follow-up period (Fig. 2A). Viremia in the Dryvax-immunized animals dropped to base line levels in 3 days and none developed skin lesions (Fig. 2A and Table 1). Three of the four MVA-immunized animals displayed a transient viremia, reaching an average level 2 logs lower than in the controls before declining to baseline (Fig. 2A). In the fourth MVA-immunized animal, viremia dropped to base line in 3 days. The MVA-immunized animals had no clinical symptoms except for skin lesions that resolved by day 9 (Table 1). Next, we determined the outcome when the challenge with 5 × 107 pfu of MPXV occurred only 10 days after vaccination. The result was similar to the 30-day challenge (Fig. 2B and Table 1) except that two of the Dryvax-immunized animals developed a small number of skin lesions and one exhibited a low viremia. For individual MVA- and Dryvax-immunized monkeys, there was a good correlation between the levels of viremia and numbers of skin lesions (Table 1). Thus, a single inoculation with MVA provided substantial protection to the severe MPXV challenge given 10 or 30 days later but was inferior to Dryvax based on virus load and skin lesions.
Fig. 2.
Viral load and survival after MPXV challenge. (A–E) The number of MPXV genomes per ml of blood was determined by quantitative TaqMan 3′-minor groove binder PCR. The lower limit of detection was 5000 genomes/ml. Average values with standard error are shown. Survival curves for each group are shown in the Insets. Groups determined by vaccination status: M, MVA; D, Dryvax; N, naïve unvaccinated. Challenge with 5 × 107 pfu of MPXV at 30 (A) and 10 (B) days after vaccination. Four naïve unvaccinated animals were challenged with this dose of MPXV and are included in the above panels. Challenge with 5 × 106 pfu of MPXV at 10 (C), 6 (D), and 4 (E) days after vaccination. Six naïve unvaccinated animals were challenged with this dose of MPXV and are included in panels C–E. The immune responses in Fig. 1 were determined from the same animals used in panel A of this figure.
Table 1.
Signs of monkeypox disease
| 5 × 107 pfu MPXV |
5 × 106 pfu MPXV |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 d* |
10 d* |
10 d* |
6 d* |
4 d* |
||||||||
| Naive | Dryvax | MVA | Dryvax | MVA | Naive | Dryvax | MVA | Dryvax | MVA | Dryvax | MVA | |
| Pox lesions† | TNTC | 0 | >702 | 29 | >996 | >1188 | 0 | 125 | 109 | 625 | >1,800 | 389 |
| Viral load† | 20,620,000 | <5,000 | 1,384,600 | 5,490 | 1,370,000 | 138,000,000 | <5,000 | 700,000 | <5,000 | 130,000 | 8,920,000 | 1,132,000 |
| >4,200 | 0 | 414 | 4 | 169 | >871 | 0 | 30 | 70 | 157 | >1,541 | 364 | |
| 139,820,000 | <5,000 | 380,000 | <5,000 | 33,800 | 44,000,000 | <5,000 | <5,000 | 60,000 | 620,000 | 4,420,000 | 3,540,000 | |
| >3,000 | 0 | 106 | 0 | 50 | >798 | 0 | 15 | 21 | 16 | >1,093 | 197 | |
| 29,840,000 | <5,000 | 26,000 | <5,000 | 8,426 | 28,400,000 | <5,000 | 77,000 | 29,000 | <5,000 | 196,400,000 | 666,000 | |
| >2,500 | 0 | 20 | 0 | 2 | 463 | 0 | 0 | 18 | 10 | 303 | 41 | |
| 21,120,000 | <5,000 | <5,000 | <5,000 | 7,768 | 2,140,000 | <5,000 | <5,000 | <5,000 | <5,000 | 1,190,000 | 700,000 | |
| 277 | ||||||||||||
| 15,000,000 | ||||||||||||
| 263 | ||||||||||||
| 18,000,000 | ||||||||||||
| Day of onset of pox lesions | 5–6 | NA | 6 | 6–9 | 6–9 | 6 | NA | 6–9 | 6 | 6–9 | 6 | 6 |
| Days to lesion resolution | NR | NA | 6–9 | 3–6 or NA | 3–9 | 9–15 or NR | NA | 3–6 or NA | 3–9 | 6–9 or NR | 9 or NR | 9 |
| Non-survivors | 4/4 | 0/4 | 0/4 | 0/4 | 0/4 | 4/6 | 0/4 | 0/4 | 0/4 | 1/4 | 3/4 | 0/4 |
| Day to euthanasia | 5, 7 | NA | NA | NA | NA | 12, 14 | NA | NA | NA | 9 | 10, 14 | NA |
| Temperature ″spike″ | Yes | Norm | Norm | Norm | Norm | Yes | Norm | Norm | Norm | Yes/no‡ | Yes/no‡ | Norm |
| Weight loss | 2–6% | Norm | Norm | Norm | Norm | 4–8% | Norm | Norm | Norm | 6%/no‡ | 15%/no§ | Norm |
| Activity | Leth | Norm | Norm | Norm | Norm | Leth | Norm | Norm | Norm | Leth/Norm‡ | Leth/Norm‡ | Norm |
| Loss of appetite | Yes | No | No | No | No | Yes | No | No | No | Yes/no‡ | Yes/no‡ | No |
TNTC, too numerous to count; NA, not applicable; NR, not resolved; Norm, normal; Leth, lethargic.
*Days between immunization and challenge.
†Pox lesions are on the upper lines; viral loads are on lower lines.
‡Temperature spike, weight loss, lethargic activity and loss of appetite only in nonsurvivors.
§Two nonsurvivors developed generalized subcutaneous edema and weight gain at time of euthanasia.
Because the challenge dose of 5 × 107 pfu of MPXV induced severe disease and death within only 5 to 7 days in unvaccinated animals, the dose was lowered to 5 × 106 pfu for further studies. Under these conditions, viremia in unimmunized animals increased between days 6 and 9, reaching peak values of 2.1 × 106 to 1.4 × 108 (mean of 4.1 × 107) and large numbers of skin lesions developed (Fig. 2 C and Table 1). Four of these animals were killed because of severe disease and two recovered after 18 days. A challenge at 10 days after vaccination was repeated under the new conditions. The Dryvax group was completely protected, with no viremia or skin lesions (Fig. 2 C and Table 1). Only two monkeys in the MVA group exhibited viremia (7.8 × 104 and 7 × 105); three had relatively low numbers of skin lesions and one had none (Fig. 2 C and Table 1). When the time between vaccination and challenge was decreased to 6 days, the protection achieved with Dryvax and MVA was comparable. In each group, a rise in MXPV genome loads was detected in two of four animals and all developed skin lesions (Fig. 2 D and Table 1). In addition, one monkey in the MVA group was killed on day 9 after challenge. Although that animal had a high lesion count of 625, the viral load was much lower than of unvaccinated animals. When the period between immunization and challenge was shortened to 4 days, a striking difference was observed between the Dryvax and MVA groups. The viremia was controlled by the MVA group but not by the Dryvax group. Indeed, viremia, lesion counts, and survival in the Dryvax group were indistinguishable from that of the unimmunized controls (Fig. 2 E and Table 1). Three of the four monkeys in the Dryvax group were killed because of severe disease by days 10–14. In contrast, in the MVA group, disease was transient (peak viral loads were 27- and 37-fold lower than unimmunized and Dryvax groups, respectively) and all of the animals survived.
The immune responses shown in Fig. 1 were from the monkeys that had been immunized 30 days before challenge. Based on those data, we considered that the immune response of animals vaccinated with MVA at 4 days before challenge would have occurred early enough to modulate the infection. To confirm this, we compared the kinetics of antibody induction with viremia and clinical signs. No antibody to VACV was detected on the day of challenge or 3 days later (4–6 days after immunization) (Fig. 3). However, by day 6 after challenge (day 10 after immunization), antibody titers were >10-fold higher in the MVA group compared to Dryvax (P = 0.0286, Mann–Whitney test). These values represented vaccine-induced responses because naïve controls were still negative on day 6 (Fig. 3). By 9 days after challenge (day 14 after immunization), the naïve animals had made a strong antibody response and the antibody levels of the Dryvax group had caught up to MVA (Fig. 3). In the MVA vaccinated animals, the viremia was cleared and the pox lesions healed by 9 days after challenge, 3 days after the antibody response was detected (Fig. 2 and Table 1). Although the naïve and Dryvax groups had made antibody by day 9 after challenge, they were unable to clear the virus and succumbed within a few days (Fig. 2 and Table 1). Because of the biological containment level, we were unable to remove live cells to determine T cell responses after MPXV challenge. However, based on the timing in Fig. 1B, we can predict that the MVA-induced CD8+ response could also have contributed to the clinical outcome.
Fig. 3.
Antibody responses after MPXV challenge. ELISA titers were performed as in Fig. 1 with samples from monkeys challenged 4 days after immunization. Groups determined by vaccination status: M, MVA; D, Dryvax; N, naïve unvaccinated.
Discussion
Conventional smallpox vaccines, such as Dryvax, induce immune responses that offer rapid and long-lasting protection after a single dose (1). Previous studies had shown that two immunizations with MVA could provide protection for >2 years in a non-human primate monkeypox model (28, 29). However, because the second immunization with MVA gave a substantial boost in antibody response in monkeys, the efficacy of a single MVA vaccination could not be predicted. The purpose of the present study was to determine whether a single MVA vaccination would protect and to assess the time required for development of immunity. Previous studies had shown that smallpox vaccine-induced antibodies are necessary and sufficient for protection in the monkeypox model (36).
The present studies were carried out by using two challenge doses of MPXV administered intravenously. With the high dose of 5 × 107 pfu (≈10 LD50), skin lesions on naïve animals developed in 5–6 days and all had to be killed. At the more moderate dose of 5 × 106 pfu (≈1 LD50), the skin lesions appeared on day 6 coincident with viremia but resolved in 4 of 6 control animals. To estimate the times required to develop protective immunity, monkeys were challenged 4, 6, 10 or 30 days after vaccination. The degree of protection was determined by counting skin lesions and measuring viremia, as well as by clinical observations. There was a good correlation between the lesion number and viremia (Table 1). Using Dryvax as the standard, we found that complete protection against the high dose occurred when the animals were challenged at thirty days after vaccination, as judged by absence of viremia or skin lesions. With a dose of 5 × 106 pfu, complete protection was achieved when the animals were challenged at 10 days after Dryvax. After a day 6 challenge, viremia was detected and all monkeys developed skin lesions although all survived. However, when the monkeys were challenged at 4 days after Dryvax, only one of four animals survived compared with four of six controls, and viremia and skin lesions were similar to the controls. The picture was somewhat different with MVA. On the one hand, the animals all survived when challenged only 4 days after vaccination and only a single animal in the entire study became seriously ill and had to be killed. Furthermore, the viremia and skin lesions were greatly reduced compared with controls regardless of the challenge dose or the time interval after MVA vaccination. Nevertheless, complete protection as judged by the absence of skin lesions was rare.
The MVA and Dryvax immunizations differed with regard to dose (1 × 108 pfu versus 2.5 × 105 pfu, respectively) and route of administration (intramuscular versus skin scarification, respectively). Previous studies with mice demonstrated that the 400-fold greater amount of MVA compared to Dryvax was necessary to achieve comparable antibody levels after a single injection (14). MVA resembles an inactivated vaccine with regard to the relatively large inoculum and replication deficiency but mimics a live virus with regard to intracellular protein synthesis and induction of a CD8+ T cell response. Because of the low inoculum, a week is necessary for Dryvax to replicate and produce a substantial immunogenic mass as judged by the formation of a skin lesion. Presumably for this reason, antibody and T cell responses occurred more rapidly after MVA than Dryvax, as previously observed (26). The timing of the antibody and T cell responses correlated with the rapid acquisition of protective immunity after MVA. Stimulation of innate immune responses may also contribute to protection at short times after vaccination. In this regard, MVA has been shown to induce higher NF-kB responses in cultured cells than a replicating strain of VACV (37, 38). However, we detected a similar systemic elevation of IFN-γ in the monkeys vaccinated with MVA and Dryvax.
The finding that a single inoculation of MVA induces rapid protection in the monkeypox model suggests that it might be used effectively to vaccinate unimmunized individuals in an emergency situation caused by a sudden outbreak of smallpox. Analysis of historical records suggests that primary vaccination within 4 days after exposure to smallpox is usually protective of serious illness (30). Because the incubation period preceding systemic smallpox is ≈2 weeks (35), it is understandable that Dryvax administered only 4 days before an i.v. challenge would not be protective. The more rapid immune responses of monkeys to MVA compared to Dryvax suggest that the window of opportunity for protective vaccination might be extended by several days. Further comparisons of the MPXV model with human smallpox are difficult in view of the differences between the viruses, host animals, routes of infection, and time to develop disease (35). The emphasis of this study was to determine whether a single vaccination could provide rapid protection but the duration beyond 30 days was not investigated. However, a second dose has been shown to boost antibody responses to levels equal to that of Dryvax (26, 27) and to provide protection against MPXV for >2 years (28, 39).
Materials and Methods
Virus Preparation.
MVA 1974/NIH clone 1 (14) was propagated in chicken embryo fibroblast cells and purified by sedimentation through a sucrose cushion. Dryvax (Wyeth Laboratories, Inc.) was obtained from the U.S. Centers for Disease Control and Prevention, diluted and stored as recommended. MPXV strain Zaire-79 was obtained from the National Institutes of Health Biodefense and Emerging Infections Research Resources Repository.
VACV ELISA.
Microtiter plates were coated with 106 pfu per well of sucrose gradient purified VACV strain WR and fixed with paraformaldehyde. Two-fold serial dilutions of heat inactivated sera were added, followed by horseradish peroxidase conjugated anti-monkey IgG-POD (Accurate Chemical and Scientific Corp.) and BM blue substrate (Roche Molecular Biochemicals).
VACV Neutralization Assay.
A VACV neutralization assay based on flow cytometric detection of green fluorescent protein was used (33). Two-fold serial dilutions of sera were incubated with VV-NP-S-EGFP for 1 h at 37°C. HeLa S3 cells were added and incubated overnight in the presence of cytosine arabinoside. Fluorescent cells were enumerated with a FACScalibur flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, Inc.). IC50 values were determined with PRISM software (Graph Pad).
Intracellular Cytokine Staining.
Fresh peripheral blood mononuclear cells were infected overnight with VACV (10 pfu per cell) in the presence of Brefeldin A then stained with antibodies to CD3, CD8, and IFN-γ conjugated to phycoerythrin, fluorescein isothyocyanate, and allophycocyanin (BD PharMingen), respectively. Cells were enumerated with a FACScaliber flow cytometer and analyzed with FlowJo software.
Viral Genome Load.
Quantitative TaqMan 3′-Minor Groove binder PCR was used to determine the number of MPXV genomes in the blood as previously described (40).
Serum Cytokine Measurements.
Cytokines INF-γ, TNF-α, IL-2, IL-4, IL-5, and IL-10 were measured in sera taken before vaccination and on days 2, 4, 7, 10, 17, and 27 after vaccination by using a Bio-Plex system (Bio-Rad Laboratories) according to the manufacturer's protocol.
Animals.
Cynomolgous monkeys (Macaca fascicularis) of Chinese origin were obtained from Three Springs Scientific, Inc. and housed at Southern Research Institute, Frederick, MD. Housing and care of animals was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Association for Assessment and Accreditation of Laboratory Animal Care). The experimental design was approved by the Animal Care and Use Committee at Southern Research Institute.
Acknowledgments.
We thank Mark Challberg and Robert Johnson for many helpful discussions and Ted Pierson, Mark Buller, and Hana Golding for comments on the manuscript. This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, through Contract N01 AI 30063 (to P.M.S.) and by Division of Intramural Research funding.
Footnotes
The authors declare no conflict of interest.
References
- 1.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and Its Eradication. Geneva: World Health Organization; 1988. [Google Scholar]
- 2.Henderson DA, et al. Smallpox as a biological weapon: Medical and public health management. J Am Med Assoc. 1999;281:2127–2137. doi: 10.1001/jama.281.22.2127. [DOI] [PubMed] [Google Scholar]
- 3.Lane JM, Goldstein J. Evaluation of 21st-century risks of smallpox vaccination and policy options. Ann Int Med. 2003;138:488–493. doi: 10.7326/0003-4819-138-6-200303180-00014. [DOI] [PubMed] [Google Scholar]
- 4.Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: A review, part II. Adverse events. Clin Inf Dis. 2003;37:251–271. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 5.Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: A review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23:2078–2081. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Mayr A, Hochstein-Mintzel V, Stickl H. Passage history, properties, and applicability of the attenuated vaccinia virus strain MVA (Translated from German) Infection. 1975;3:6–14. [Google Scholar]
- 7.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 8.Carroll M, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: Propagation and generation of recombinant viruses in a non-human mammalian cell line. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 9.Drexler I, Heller K, Wahren B, Erfle V, Sutter G. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol. 1998;79:347–352. doi: 10.1099/0022-1317-79-2-347. [DOI] [PubMed] [Google Scholar]
- 10.Antoine G, Scheiflinger F, Dorner F, Falkner FG. The complete genomic sequence of the modified vaccinia Ankara strain: Comparison with other orthopoxviruses. Virology. 1998;244:365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: Implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 12.Stittelaar KJ, et al. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine. 2001;19:3700–3709. doi: 10.1016/s0264-410x(01)00075-5. [DOI] [PubMed] [Google Scholar]
- 13.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci USA. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tscharke DC, et al. Identification of poxvirus CD8(+) T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies DH, et al. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus Ankara is comparable to that of Dryvax. J Virol. 2008;82:652–663. doi: 10.1128/JVI.01706-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochstein-Mintzel V, Huber HC, Stickl H. Virulence and immunogenicity of a modified vaccinia virus (strain MVA) (Translated from German) Z Immun Forsch. 1972;144:140–145. [PubMed] [Google Scholar]
- 18.Vollmar J, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24:2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Parrino J, et al. Safety, immunogenicity and efficacy of modified vaccinia Ankara (MVA) against Dryvax (R) challenge in vaccinia-naive and vaccinia-immune individuals. Vaccine. 2007;25:1513–1525. doi: 10.1016/j.vaccine.2006.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters BS, et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine. 2007;25:2120–2127. doi: 10.1016/j.vaccine.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Dorrell L, et al. Safety and tolerability of recombinant modified vaccinia virus Ankara expressing an HIV-1 gag/multiepitope immunogen (MVA.HIVA) in HIV-1-infected persons receiving combination antiretroviral therapy. Vaccine. 2007;25:3277–3283. doi: 10.1016/j.vaccine.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Jahrling PB, et al. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc Natl Acad Sci USA. 2004;101:15196–15200. doi: 10.1073/pnas.0405954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCurdy LH, Rutigliano JA, Johnson TR, Chen M, Graham BS. Modified vaccinia virus Ankara immunization protects against lethal challenge with recombinant vaccinia virus expressing murine interleukin-4. J Virol. 2004;78:12471–12479. doi: 10.1128/JVI.78.22.12471-12479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staib C, Suezer Y, Kisling S, Kalinke U, Sutter G. Short-term, but not post-exposure, protection against lethal orthopoxvirus challenge after immunization with modified vaccinia virus Ankara. J Gen Virol. 2006;87:2917–2921. doi: 10.1099/vir.0.82068-0. [DOI] [PubMed] [Google Scholar]
- 25.Jezek Z, Fenner F. Human monkeypox. Monographs Virol. 1988;17:1–140. [Google Scholar]
- 26.Earl PL, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 27.Stittelaar KJ, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Earl PL, et al. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology. 2007;366:84–97. doi: 10.1016/j.virol.2007.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nigam P, et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366:73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortimer PP. Can postexposure vaccination against smallpox succeed? Clin Infect Dis. 2003;36:622–629. doi: 10.1086/374054. [DOI] [PubMed] [Google Scholar]
- 31.Artenstein AW, et al. A novel, cell culture-derived smallpox vaccine in vaccinia-naive adults. Vaccine. 2005;23:3301–3309. doi: 10.1016/j.vaccine.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg RN, et al. Safety and immunogenicity of new cell-cultured smallpox vaccine compared with calf-lymph derived vaccine: A blind, single-centre, randomised controlled trial. Lancet. 2005;365:398–409. doi: 10.1016/S0140-6736(05)17827-1. [DOI] [PubMed] [Google Scholar]
- 33.Earl PL, Americo JL, Moss B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J Virol. 2003;77:10684–10688. doi: 10.1128/JVI.77.19.10684-10688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rock MT, Yoder SM, Talbot TR, Edwards KM, Crowe JE. Adverse events after smallpox immunizations are associated with alterations in systemic cytokine levels. J Inf Dis. 2004;189:1401–1410. doi: 10.1086/382510. [DOI] [PubMed] [Google Scholar]
- 35.Breman JG, Henderson DA. Current concepts—Diagnosis and management of smallpox. New Eng J Med. 2002;346:1300–1308. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]
- 36.Edghill-Smith Y, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nature Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 37.Oie KL, Pickup DJ. Cowpox virus and other members of the Orthopoxvirus genus interfere with the regulation of nf-kappa b activation. Virology. 2001;288:175–187. doi: 10.1006/viro.2001.1090. [DOI] [PubMed] [Google Scholar]
- 38.Shisler JL, Jin XL. The vaccinia virus K1L gene product inhibits host NF-kB activation by preventing IkBa degradation. J Virol. 2004;78:3553–3560. doi: 10.1128/JVI.78.7.3553-3560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arlen PM, Gulley JL, Madan RA, Hodge JW, Schlom J. Preclinical and clinical studies of recombinant poxvirus Vaccines for carcinoma therapy. Crit Rev Immunol. 2007;27:451–462. doi: 10.1615/critrevimmunol.v27.i5.40. [DOI] [PubMed] [Google Scholar]
- 40.Heraud JM, et al. Subunit recombinant vaccine protects against monkeypox. J Immunol. 2006;177:2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]