Abstract
The regulation of plasmin generation on cell surfaces is of critical importance in the control of vascular homeostasis. Cell-derived microparticles participate in the dissemination of biological activities. However their capacity to promote plasmin generation has not been documented. In this study, we show that endothelial microparticles (EMP) from TNFα-stimulated endothelial cells, served as a surface for the generation of plasmin. The generation of plasmin involved expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) at the surface of EMP and was further increased by their ability to bind exogenous uPA on uPAR. Plasminogen was activated at the surface of EMP in a dose-dependent, saturable and specific manner as indicated by the inhibition of plasmin formation by ε-amino-caproic acid (ε-ACA) and carboxypeptidase B. EMP-induced plasmin generation affects tube formation mediated by endothelial progenitor cells. However, low amounts of EMP increased tube formation whereas higher concentrations inhibited it. Prevention of these effects by inhibitors of either uPA or plasmin, underscore the key role of EMP-induced plasmin generation. In conclusion, we demonstrated that EMP act as vectors supporting efficient plasmin generation and dissemination, a new pathway in the regulation of endothelial proteolytic activities with potential involvement in inflammation, angiogenesis and atherosclerosis.
Keywords: Cell Line, Endothelial Cells, metabolism, Enzyme Activation, drug effects, Humans, Intracellular Membranes, metabolism, Neovascularization, Physiologic, Plasmin, metabolism, Plasminogen, metabolism, Protein Binding, Receptors, Cell Surface, metabolism, Stem Cells, metabolism, Urinary Plasminogen Activator, pharmacology
Keywords: Angiogenesis, endothelial microparticles, plasminogen, plasmin, urokinase, endothelial progenitors
Introduction
Microparticles are vesicles resulting from the blebbing of the cellular membrane of most activated or apoptotic cells.1 These microvesicles have been described in various cellular models and in different pathological conditions as reliable hallmarks of cell damage.2 Because they convey various bioactive effectors originating from the parent cells, microparticles (MP) may exhibit a spectrum of biological activities; they regulate endothelial or blood cells functions, participate in inflammatory responses or angiogenesis and propagate biological responses involved in haemostatic balance.3 We previously reported the capacity of endothelial cells to release microparticles after inflammatory stimulation, and the presence of increased levels of circulating endothelial microparticles (EMP) in patients with thrombotic disorders.4 Since this initial report, elevated levels of EMP have been documented in various pathological conditions including coronary syndromes5, renal failure6, diabetes7, antiphospholipid syndrome8, thrombotic thrombocytopenic purpura9 and sickle cell disease10, in which they reflect endothelial dysfunction and are associated with a poor clinical outcome. EMP provide procoagulant phospholipid surfaces for the assembly and activation of coagulation factors, mainly through phosphatidylserine translocation to the exoplasmic leaflet as a result of membrane remodeling. Their involvement in thrombin generation also results from their capacity to harbor, deliver or induce tissue factor activity.11–13 However, a more complex contribution to the haemostatic balance is suggested by their expression of thrombomodulin, tissue factor pathway inhibitor and endothelial protein C receptor thus providing a possible antithrombotic counterbalance.14, 15
Another key regulator of the vascular homeostasis is the plasminogen activation system. Plasminogen activation is mediated by two serine proteases: tissue-type plasminogen activator (t-PA), which is mainly implicated in fibrinolysis, and urokinase-type plasminogen activator (uPA), which is critically involved in pericellular proteolysis due to its high affinity cell surface receptor uPAR.16 Plasmin generation induced by uPA and subsequent activation of matrix metalloproteinases (MMP) promotes cell migration through interstitial matrix and participates in processes such as tissue remodeling, cancer invasion and angiogenesis.17–19 Importantly, we have shown that uncontrolled plasminogen activation can have deleterious consequences by inducing cell detachment and apoptosis.20, 21 The regulation of plasmin generation at the endothelial surface is therefore of critical importance in the control of vascular homeostasis.
Because MP convey protein and functional systems expressed by the parent cell, we hypothesized that EMP may serve as active surfaces for interaction with plasminogen and plasmin formation, a hitherto undescribed function. The objective of this study was therefore to analyze the capacity of EMP to bind and convert plasminogen into active plasmin and to define the impact of plasmin generation on EMP capacity to regulate angiogenic responses mediated by endothelial progenitor cells (EPC) in vitro.
Materials and Methods
Preparation of plasminogen and plasminogen-free fetal calf serum
Plasminogen was purified by lysine-affinity chromatography and molecular sieving as reported previously.22 Plasminogen-free fetal calf serum (FCS) was obtained by three cycles affinity adsorption on lysine-Sepharose (4 FCS vol/1 gel vol) and was subsequently checked and found to contain less than 0.1 nM plasmin(ogen) activity.
Cell culture
The human microvascular endothelial cell line (HMEC-1), obtained from Dr. Ades23 (Centers for Disease Control, Atlanta, GA, USA), was cultured in MCDB 131 medium (Invitrogen Life Technologies, Cergy Pontoise, France) supplemented with 10% MP-free FCS, 10 ng/ml human recombinant epithermal growth factor (Upstate Cell Signaling Solutions, Lake Placid, NY, USA) and 1 μg/ml hydrocortisone (Sigma, St Quentin Fallavier, France). Human vein endothelial cells (HUVEC) were obtained by collagenase digestion as previously described24, cultured into 0,2 % gelatin coated flasks in EGM2 medium and used at passage 2. Human saphenous vein endothelial cells were purchased from Clonetics (Grand Island, NY, USA) and cultured into 0,2 % gelatin coated flasks in EGM2-MV medium and used at passage 6.
Generation, harvesting and flow cytometry enumeration of EMP
For most experiments, EMP were from HMEC-1 origin as this cell line is well characterized and considered representative of the microcirculation, the core of the vascular tree.23,25 EMP were purified from culture medium conditioned by sub-confluent HMEC-1 stimulated for 48 hours with 100 ng/ml TNF-α (PeproTech Inc, Rocky Hill, NJ, USA) as previously described, with minor modifications.4 Culture supernatants from flasks were collected and cleared from detached cells or large cell fragments by centrifugation at 4300g for 5 minutes. The supernatants were then centrifuged at 20000g for 120 minutes at 4°C. Pelleted EMPs were washed 2 times and re-suspended in phosphate-buffered saline (PBS). The absence of residual TNF-α in this EMP samples was verified using an ELISA assay (R&D system, Minneapolis, MN, USA). Aliquots of 10 μl EMP suspension, 1/100 diluted, were labeled using fluorescein isothiocyanate (FITC)-conjugated annexin V (Abcys, Paris, France) and EMP were enumerated by flow cytometry as previously described.26 The same protocol was used to obtain EMP from quiescent HMEC-1, saphenous endothelial cells and endothelial progenitor-derived cells (EPDC).
Isolation and culture of endothelial progenitor cells from cord blood
Human umbilical cord blood samples (30–50 ml) were collected from donors, in compliance with French legislation, in a sterile tube containing heparin (200 UI/ml). Mononuclear cells (MNC) were isolated by density gradient centrifugation. Briefly, blood was diluted 1:1 in phosphate-buffered saline containing 2 mM ethylenediaminetetraacetic (PBS/EDTA) and layered over lymphocyte separation medium (Eurobio, Les Ulis, France). After a 30 min centrifugation at 400g, MNC were washed three times in PBS/EDTA. Cord blood MNC were pre-plated in RPMI/10% FCS for 24 hours in plastic flasks. Non-adherent cells were plated onto 0.2% gelatin-coated 24-well plates (5.106 cells per well) and maintained in endothelial basal medium-2 (EBM-2) supplemented with EGM-2 SingleQuots (EGM-2 medium, Clonetics, Walkersville, MD, USA). The medium was changed every 4 days. The appearance of well-circumscribed colonies with a cobblestone morphology was monitored daily. For expansion of EPDC, colonies were trypsinized and cells were replated on a 6 wells plate (passage 1). Subsequently, confluent cells were trypsinized and re-plated in T75 flasks for further passages. Cells were maintained under standard conditions (humidified atmosphere, 5% CO2, 37°C).
Progenitor endothelial cell tube formation in Matrigel
Flat-bottom 96-well plates were pre-coated with 1:1 mixture of cold Matrigel Basement Membrane (10 mg/ml, BD Biosciences, Bedford, MA, USA) and RMPI-10% FCS medium. After 45 minutes of polymerization at 37°C, EPDC were plated at 2.105 cells/well in RPMI/10% FCS with increasing concentrations of EMP from TNF-α-stimulated HMEC-1 or an identical volume of the supernatant from the last EMP washing. To test the role of FCS plasminogen in this system, native FCS was replaced by plasminogen-free FCS supplemented or not with 1 μM human plasminogen. For inhibition experiments, EMP were pre-incubated with either an anti-uPA antibody (394O, American Diagnostica, Greenwich, CT, USA, 25 μg/ml) or an irrelevant control IgG1 (25 μg/ml) or with serine-protease inhibitors (aprotinine, 100 k.u.i./ml or α2-antiplasmin, 1 μM). After 24 hours, capillary tube formation was evaluated by measuring the number of polygons formed in a well under an inverted light microscope (Olympus) at 400× magnification. This time point has been chosen following previous kinetics experiments indicating that it is optimum to analyze tube formation in the presence of EMP. All experiments were performed in triplicate.
EMP immobilization
A physico-chemical adsorption principle was used to immobilize negatively charged EMP on a polycation surface. For that purpose, round-bottom PVC plates were activated with 25 μg/ml poly-L-lysine and various concentrations of EMP in PBS were then incubated over night at 4°C with the activated surface. The plate was then washed and the immobilized EMP were tested as indicated.
Plasmin generation test
In round-bottom 96-well PVC plates, various concentrations of EMP, either in suspension in PBS supplemented with 0.8% bovine serum albumin (PBSA) or immobilised on poly-L-lysine coated plates, were incubated with 50 μl of 1 μM plasminogen and 0.75 mM of a chromogenic substrate selective for plasmin (methyl-malonyl)-hydroxypropylarginine-p-nitroanilide (CBS0065, Stago, Asnière, France). An identical volume of supernatant from the last EMP washing was used as control. In order to determine the Michaëlis constant of plasmin generation by EMP, a fixed number (2.105) of EMP and varying concentrations of plasminogen (0 to 5 μM) were used. Kinetics of plasmin generation were followed during 9h in a multiwell plate counter (MX5000, Dynex) at 37°C by measuring the change in absorbance at 405 nm produced by the release of p-nitroaniline. In the case of activation experiments performed on immobilized EMP, the plate was washed with PBSA to eliminate unbound reactants and the plasmin bound to the immobilized EMP was detected by adding 50 μl/well of 0.75 mM CBS0065 and measuring the change in absorbance at 405 nm. When indicated, the following inhibitors were pre-incubated with the EMP: 1 μg/ml goat anti-human tPA (Biopool, Uppsala, Sweden), 50 μg/ml mouse anti-human uPA (394O), 50 μg/ml rabbit anti-αvβ3 ( gift of J.C.Lissitzky ), 100 μg/ml anti-α-enolase (gift of R. Lopez Alemany)_and the respective irrelevant control IgGs (Biocytex, Marseille, France); the uPA inhibitor, amiloride, was used at 100 μM and carboxypeptidase B (CpB) at 50 μg/ml, final concentrations. In separate experiments, the inhibitors α2-antiplasmin, aprotinin and ε-amino-caproic acid (ε-ACA) were added to the plasminogen activation solutions at 100 nM, 100 k.i.u./ml and 0.1 M final concentrations, respectively.
Characterisation of uPA and uPAR in cell and EMP extracts
HMEC-1 and pelleted MP were lysed in 100 mmol/1 Tris-HCl buffer pH 8.1, containing 0.5% Triton X-100 and supplemented with complete protease inhibitor mixture (Roche Diagnostic GmbH, Mannheim, Germany) except for zymography samples. Lysates were clarified and protein concentrations were determined using the BCA kit (Pierce, Rockford, IL, USA). uPA and uPAR total antigen levels were assayed by ELISA (894 and 893 IMUBIND® ELISA kits, American Diagnostica) according to the manufacturer’s instruction. The results were expressed as ng of uPA or uPAR per mg of total proteins. For zymography, protein extracts (5 μg), molecular weight markers and purified scuPA were electrophoresed under non-reducing conditions in 10% SDS-polyacrylamide gel copolymerised with 1 mg/ml α-casein from bovine milk (Sigma) and 20 μg/ml human plasminogen. After electrophoresis, SDS was eluted from the gel by washing for 1 h in 2.5% Triton X-100 buffer. The gels were then incubated for 40h at 37°C in 50 mmol/1 Tris-HCl buffer, pH 8, containing 5 mmol/1 CaCl2, 138 mmol/1 NaCl and 0.03% Brij 35. Zymograms were developed by staining with Coomassie Brillant Blue Dye and destained to reveal clear bands of casein lysis, indicative of enzymatic activity. Samples run on casein gels without plasminogen or gels incubated in lysis buffer supplemented with 1 mM amiloride (Sigma) served as controls of uPA activity. For Western-blot analysis, protein extracts and purified human uPAR (American Diagnostica, Greenwich, CT, USA) used as positive control (20 μg/lane), were separated under non-reducing conditions by 8% SDS-polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. After blocking, the membranes were incubated overnight at 4°C with rabbit anti-human uPAR (399R, American Diagnostica, 0.5 μg/ml) antibodies, followed by the horseradish peroxidase-conjugated secondary antibodies. Immunocomplexes were visualized with the Supersignal West Pico chemiluminescence kit (Pierce).
Immunoelectron microscopy of EMPs
For uPA/uPAR immunogold labeling of EMP, droplets of MP isolated from TNF-α-stimulated HMEC-1 were applied to 300 mesh nickel Formvar-carbon-coated grids (Agar Scientific, Essex, England) for 10 min. Samples were immunolabeled with either anti-uPA (3689, American Diagnostica, 10 μg/ml) or anti-uPAR (3932, American Diagnostica, 10 μg/ml) antibodies for 1 hour, washed with PBS and then reacted with the 15 nm beads gold-labeled secondary antibody (BBInternational, Cardiff, UK) for 1 hour. Grids were rinsed and negatively stained with 0.3% phosphotungstic acid (pH 7) before observation with a JEOL 1220 electron microscope. Specificity of immunolabeling was determined in comparison to results obtained with an irrelevant control antibody or with gold-labeled secondary antibody alone.
Flow cytometry for uPA and uPAR on EMP
EMP (10 μl) were labeled with either anti-uPA (394O, 25 μg/ml) or anti-uPAR (3932, 25 μg/ml) antibodies for 1h, then FITC-labeled secondary antibody was added and incubated for 30 min before samples were analyzed on a Cytomics FC500* flow cytometer (Beckman Coulter, Fullerton, CA, USA). MP were analysed according to the FSC/SSC characteristic in a gate with the upper limit defined by beads of 0.9 μm and the lower limit defined by 0.3 μm. Specificity of labelling was determined in comparison to results obtained with an irrelevant control antibody or with the secondary antibody alone.
Binding of uPA to EMP
A fixed concentration of EMP was immobilised on poly-L-lysine coated 96-well plates. Increasing concentrations (0 to 18.5 nM) of sc-uPA (a kind gift of Dr HR Lijnen, University of Leuven) were incubated during 1h at 37°C with the immobilized microparticles in the presence of 4 μg/ml of poly-L-lysine in order to eliminate non specific binding of sc-uPA to the poly-L-lysine surface. To verify the specificity of the binding, a fixed concentration of native sc-uPA (0.1 nM) was mixed with a 10 molar excees (1 nM) of a recombinant inactive form “rsc-uPA Ile 159→Gly” (H.R. Lijnen, University of Leuven). The native sc-uPA, specifically bound to EMP, was detected by measuring plasmin generation as describe above.
Statistical analysis
Data are expressed as mean +/− standard deviation. Statistical analysis was performed with Prizm software (GraphPad Software Inc., San Diego, CA, USA) and with KaleidaGraph software (Synergy Software, Reading, PA, USA). Significant differences were determined using non parametric Mann-Whitney test. A p value less than 0.05 was considered significant.
Results
EMP are able to activate plasminogen into plasmin
To investigate the ability of EMP to generate plasmin, the microparticles were incubated with plasminogen and a plasmin-selective chromogenic substrate. As shown in Fig. 1A, plasmin generation occurred as a function of time and was proportional to the number of EMP. At 106 EMP/well the plasmin generation rate was 47.6 ±1.1 A405nm×10−3/min, 53-fold higher than at 103 EMP/well (0.9 ±0.3 A405nm×10−3/min) (Fig. 1B). At identical EMP concentrations (2. 105 50 μl) comparable results were obtained using EMP derived from quiescent or TNF-α-stimulated HMEC-1. In contrast, the level of plasmin generated by EMP was shown to vary according to their endothelial cell origin (Table 1). Thus, a more pronounced activity was produced by HMEC-1-derived MP as compared to MP of macrovascular origin (saphenous vein, HUVEC) whereas intermediate values were obtained for EPDC-derived MP. Plasminogen incubated with EMP was activated in a dose-dependent, saturable and specific manner (Fig. 1C, Km = 0.122μM, Vmax= 25.2 A405nm/min.l03). As expected, plasmin activity was completely blocked in the presence of α2-antiplasmin or aprotinin (Fig. 1D).
Figure 1. EMP are able to activate plasminogen into plasmin.
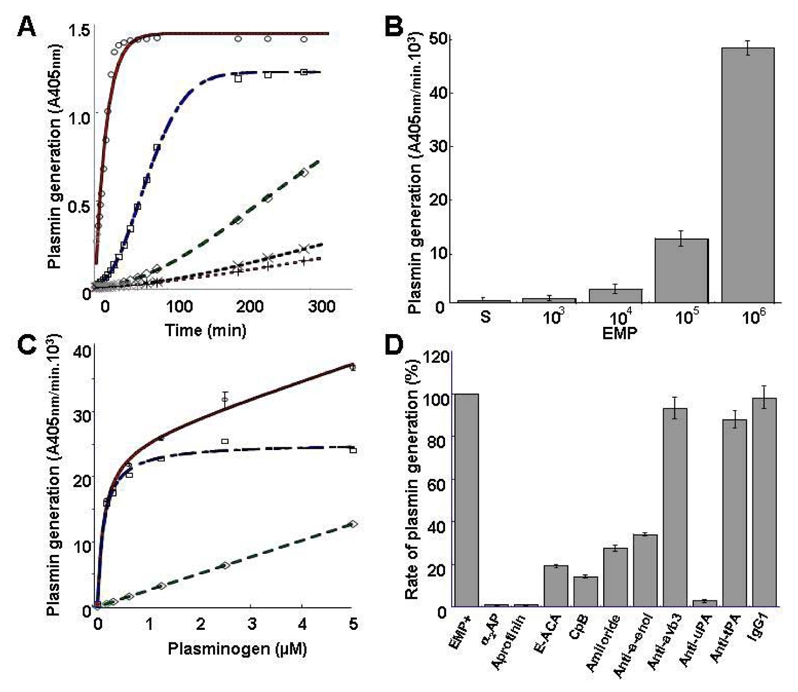
(A) Plot of plasmin generated versus time at varying EMP amounts per 50 μl/well (○ = 106; □ = 105; ◇ = 104; x = 103; + = control without EMP) and fixed final concentrations of plasminogen (1 μM) and a plasmin-selective chromogenic substrate (0.75 mM). Representative graph of four independent experiments. (B) Similar experiment as in A expressed as change in absorbance at 405 nm per minute versus EMP amount per well (S: EMP last washing supernatant used as control). (C) Plasmin generated at varying plasminogen concentrations (0 to 5 μM) and a fixed amount (2.105/50 ul) of EMP was detected with a chromogenic substrate as in A. Raw data (○) were fitted to the Michaelis-Menten equation allowing calculation of non-specific activity (◇) and a Km = 0.122) μM for specific plamin generation (□). (D) Effect of various inhibitors on the generation of plasmin by EMP (2.105/50 μl) at 0.5 μM plasminogen (α2AP = α2-antiplasmin; ε-ACA = ε-amino-caproïc acid; CPB = Carboxypeptidase B; antibodies to uPA and tPA. αvβ33 and α-enolase as compared to an isotype control IgGl) Results are the mean±SD of three independent experiments.
Table 1.
Plasmin generation by endothelial microparticules
| EMP (2.105/well) derived from | Plasmin generation (%, mean±SD) |
|---|---|
| TNFα-stimulated HMEC-1 | 100 |
| Quiescent HMEC-1 | 98.0± 6.3 |
| EPDC | 27.7 ± 5.5 |
| Saphenous vein endothelial cells | 13.0 ± 2.6 |
| HUVEC | 7.6 ± 3.3 |
HMEC-1: Human microvascular endothelial cell type 1. EPDC: Endothelial progenitor-derived cells. HUVEC: Human umbilical vein endothelial cells.
Plasminogen is activated by uPA at the surface of EMP
Supernatants from EMP washing failed to generate plasmin, ruling out the contribution of soluble factors and suggesting that the activation of plasminogen was dependent on factors associated with the EMP surface. In order to test this hypothesis, plasminogen activation experiments were performed on immobilized microparticles. In this system, plasmin generation was a function of the number of immobilized EMP (Fig. 2A). Plasmin was already detected on immobilized EMP at a concentration of 25000 EMP/well and increased progressively in a dose-dependent manner. At the end of the activation experiments the immobilized EMP were washed and bound plasmin was detected using a plasmin-selective chromogenic susbtrate. The amount of EMP-bound plasmin increased as a function of the number of immobilised EMP (Fig. 2B). Since some plasmin was released into the medium during its generation, the amount bound after washing was lower than the total amount of plasmin formed.
Figure 2. Plasminogen is activated at the surface of EMP by uPA.
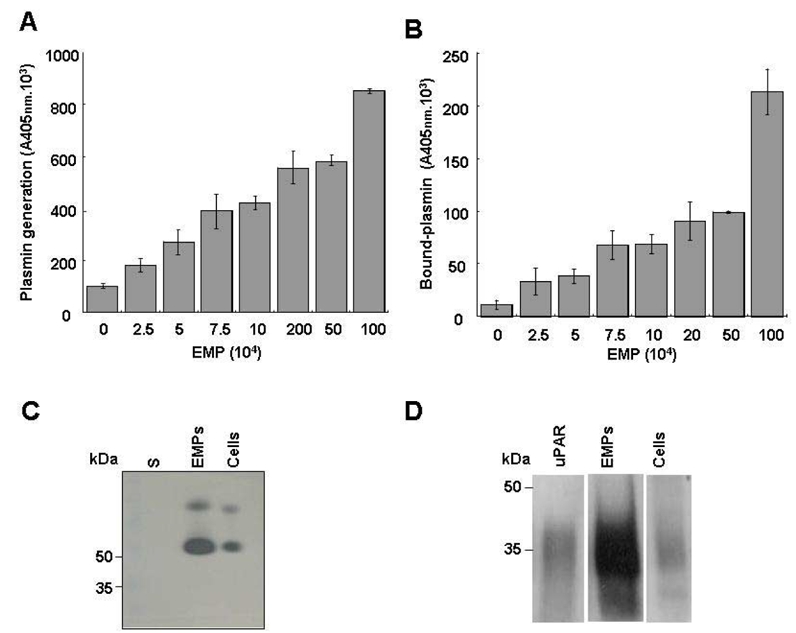
(A & B) A variable number of EMP immobilised on poly-L-lysine surfaces were incubated with fixed concentrations of plasminogen (0.5 μM) and the plasmin-selective chromogenic substrate (0.75 mM). The graph in (A) shows an increase in the formation of plasmin as a function of the number of immobilised microparticles. Unbound reagents were then washed off and the chromogenic substrate added to detect plasmin that remained bound to the immobilized EMP; the graph (B) shows an increase in bound plasmin as a function of the number of immobilised EMP. Representative graphs (Mean±SD) of three independent experiments. (C & D) Protein extracts from HMEC-1 and its derived EMPs were processed for zymography (C) to detect plasminogen activator activity (5 μg protein/lane; S: EMP last washing supernatant) and for immunoblot (D) using rabbit antibodies against uPAR and purified uPAR as reference (20 μg protein/lane). The ~50 kDa and upper bands in (C) were inhibited by antibodies to urokinase (not shown). A space has been inserted to indicate where a gel lane was cut. These gels came from different experiments as indicated by the space between the gels.
In parallel experiments using EMP in suspension, plasmin generation was inhibited by the lysine analogue ε-ACA suggesting a lysine-dependent mechanism for plasminogen binding and activation that was confirmed by abrogation of plasmin formation upon cleavage of C-terminal lysine residues on EMP by CpB (Fig 1D). Among proteins bearing C-terminal lysine residues, α-enolase was identified as a major binding protein as indicated by 65 % inhibition of plasmin formation with the specific antibody 11G1 (Fig 1D). The integrin αvβ3 that may influence plasminogen activation, mainly through interactions with uPA/uPAR system, was shown not to be involved in plasmin generation by EMP as indicated by the absence of effect of a specific neutralizing antibody (Fig 1D).
The uPA/uPAR complex is present on EMP
The value determined for the apparent Km of plasminogen activation on EMP was within the range determined for the uPA/uPAR system on endothelial cells and a variety of other cell types.27, 28 The plasminogen activator conveyed by EMP was identified as uPA as indicated by the inhibition of plasmin generation by (i) amiloride, a specific inhibitor of uPA, and by (ii) an antibody specific for uPA (>95% inhibition) (Fig. 1D). By contrast, an antibody directed against tPA had no significant effect on plasmin generation. The presence of uPA activity on EMP was further confirmed by a lytic band on casein zymography corresponding to the molecular mass of uPA (Fig. 2C) which was also absent in gels into which amiloride (1 mM) was incorporated (data not shown). Comparison of the lytic bands from the EMP and the parent cells, normalized for protein content, showed a 3–4 fold increased of uPA in the EMP (representative experiment in Fig. 2C). Similarly, when uPA antigen in EMP and cells were measured by ELISA, a 3-fold increase in uPA was found in the EMP (23.9 +/− 12.6 versus 8.3 +/− 3.17 ng uPA/mg protein respectively, p<0.05). UPAR was identified in EMP and cell lysates by Western-blot and was found to co-migrate with mammalian uPAR. As for uPA, the EMP had more uPAR than the cells when normalized for equivalent total protein concentrations, (Figure 2D). The uPAR antigen level measured by ELISA was 4-fold higher in the EMP than in the parent HMEC-1 (57.7 +/− 14.54 versus 4.4 +/− 1.17 ng/mg protein respectively, p<0.05). The presence of uPA or uPAR on the EMP surface was also analysed by flow cytometry (figure 3A and 3B). The shift of fluorescence histogram after specific labeling of EMP with uPA and uPAR antibody compared to the irrelevant staining confirmed the presence of uPA and uPAR at the EMP surface.
Figure 3. Identification of uPA, uPAR and uPA/uPAR complex on EMP.
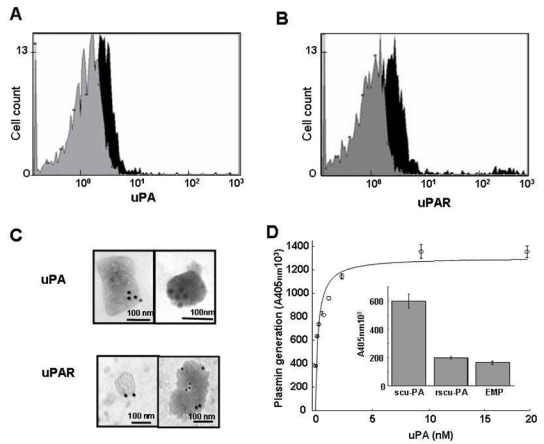
(A & B) Measured fluorescence intensity by flow cytometry analysis of EMP with anti-uPA (A) and anti-uPAR (B) antibodies (black) and the corresponding isotype controls (gray). (C) Representative images of immunogold labeling of uPA (top) and uPAR (bottom) analysis on EMP by transmission electron microscopy. The dimension of the bars indicate the relative small size of these EMP and the clusters of 15 nm gold particles (black dots) indicate the presence of uPA and uPAR at the surface of EMP. (D) Main graph: isotherm of the binding of varying amounts of scuPA incubated with EMP immobilized on a poly-L-lysine surface. The amount of bound scuPA was detected by its ability to activate plasminogen using a chromogenic substrate selective for plasmin. Data fitted to the Langmuir equation as indicated in Methods allowed calculation of a dissociation constant, Kd = 0.1 nM, for the interaction of scuPA with its receptor. Representative graph (Mean±SD) of three independent experiments. Inset: specificity of the binding of native scuPA is demonstrated by its inhibition with a modified recombinant form of scuPA (r-scuPA, ILel59→Gly) that bind to its receptor but cannot be activated. The bars represent the mean±SD of three independent experiments.
EMP were also analyzed by electron microscopy. Negative staining of the pellet revealed intact irregular shaped EMP ranging from 100 to 500 nm in size. Molecules of uPA and uPAR were detected on the outer surface after immunogold labeling with specific antibodies (Figure 3C). No labeling was observed in experiments using control antibodies.
Binding of sc-uPA to EMP-uPAR
Since the amount of uPAR measured by ELISA was higher on EMP than on cells, we tested the capacity of immobilized EMP to bind exogenous scuPA specifically. A range of concentrations of scuPA was incubated with the immobilized EMP, washed and then tested in a plasminogen activation assay. Figure 3D shows that scuPA binding was dose-dependent, saturable and specific, as indicated by the inhibition of the binding by a modified form of scuPA that resists activation (Fig 3D, insert). Analysis of raw data with the Langmuir equation for single-site binding allowed calculation of a very low dissociation constant (Kd = 0.1 nM), indicating a very high affinity of scuPA for its EMP anchored receptor, in agreement with previous published data for the uPA/uPAR system.28 Since elution of intrinsic uPA could not be performed before the binding experiments these results indicate that the amount of uPA originally bound is relatively small compared with the high uPAR binding capacity of EMP.
EMP affect tube formation by endothelial progenitor cells in vitro
EPC were supplemented with increasing amounts of EMP (2.103 to 8.105) and tube formation in Matrigel was evaluated using the supernatant from the last EMP washing as control (100% tube formation). As shown on figure 4A, a biphasic effect was observed: at low EMP concentrations (≤ 2.103/well), polygon number and tube formation increased by 20% over control (p= 0.012) whereas higher amounts of EMP (≥ 2.105 EMP/well) had the reverse effect. Thus, tube formation decreased progressively with the increase in the number of EMP reaching 55% decrease below control (p<0.001) at 8.105 EMP/well. Concomitant with the decrease in tube formation, morphologic cell changes were observed (rounded cells and retracted cell clusters) (fig 4B). The moderate pro-angiogenic effect of MP seems to be less potent as compared to VEGF (see Supplementary data and figure available on the Blood website)..
Figure 4. EMP affect the angiogenic properties of endothelial progenitors in vitro: effects on tube formation in Matrigel.
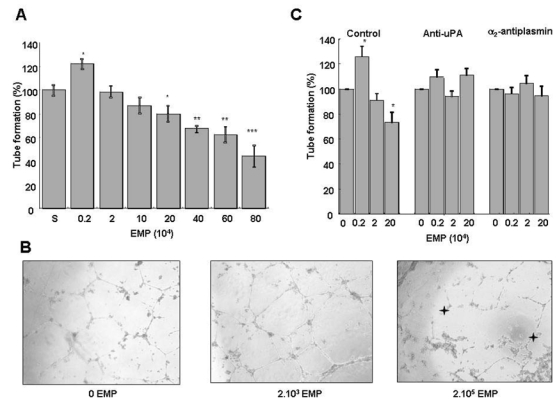
EPDC in RPMI/10% FCS and varying amounts of EMP were plated on Matrigel and tube formation was evaluated after 24 h of incubation. (A) Biphasic effect of EMP on tube formation: at low EMP concentrations (≤ 2.103/well), polygon number and tube formation increased by 20% (p= 0.012, n=12) over control (S: EMP last washing supernatant). However, higher amounts of EMP (≥ 2.105 BMP/well) had the reverse effect and progressively decreased the number of tubes to 50% at 8.105 EMP/well. Stars indicate significant changes (* p<0.05, ** p<0.01, *** p <0.001). Results are the mean±SD of twelve independent experiments. (B) Inverted light microscope (Olympus) representative images (400x magnification) showing EPDC tube formation in Matrigel in the absence and presence of 2.103 or 2.105 EMP per well. Stars indicate retracted cell clusters. (C) Experiments were performed in the presence of inhibitors of either plasmin formation (anti-uPA antibody) or activity (α2-antiplasmin, α2-AP). The biphasic effect of EMP on tube formation (left bar panel) was abolished by the anti-uPA antibody (middle bar panel) and by α2-AP (right bar panel). Stars indicate significant changes (p<0.05). Results are the mean±SD of three independent experiments.
Plasmin is involved in the EMP effects on tube formation
An anti-uPA antibody and α2-antiplasmin were used to determine whether the observed effects of EMP on EPC tube formation were related to plasmin generation. As illustrated in Fig. 4C, in the presence of these inhibitors, EMP had no more effect on tube formation. We also considered the presence of plasmin in the Matrigel medium because the gel itself and the culture medium (RPMI/10% FCS) contain plasminogen29 and the EMP are a source of uPA. The plasmin chromogenic substrate was added with the EMP in Matrigel allowing detection of increased plasmin generation from 105 EMP (p<0.001) at 2h of incubation (Fig. 5A). The contribution of FCS plasminogen to plasmin formation was estimated using plasminogen-free FCS (Fig 5B). Under these conditions plasmin generation was moderately reduced (−11.8%, p=0.0001) compared to native FCS and was not influenced by the amount of EMP added, suggesting the presence of a basal plasmin generation in the Matrigel. The addition of plasminogen to plasminogen-free FCS restored plasmin formation to the level observed with native FCS in the absence of EMP and was again importantly increased as a function of the number of added EMP (more than 75% increase at 2.105 EMP/well, Fig 5B). These data confirmed the role of Matrigel and plasminogen from FCS in plasmin generation in the absence of EMP, and the strong potential capacity of EMP to enhance plasmin generation.
Figure 5. EMP are involved in plasmin generation in Matrigel.
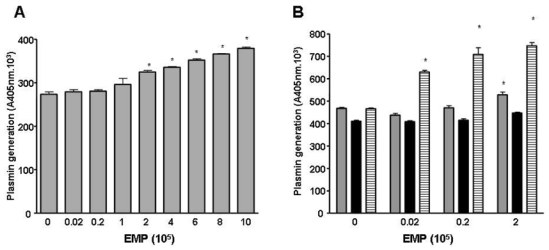
(A) Plasmin generation in Matrigel is dependent on the amount of EMP. The chromogenic substrate CBS0065 was added to the Matrigel with the EMP and under these conditions an increase in plasmin formation was detected from 105 EMP (p<0.001) after 2 h of incubation. Stars indicate significant changes (p<0.05). Results are the mean±SD of three independent experiments. (B) Plasmin generation in Matrigel at different EMP amounts is dependant on the supply of plasminogen by FCS. The graph shows plasmin activity detected after 3 h of incubation (gray bar, medium with 10% FCS; black bar, medium with plasminogen-free FCS; hatched bar, medium with plasminogen-free FCS supplemented with 1 μM plasminogen). Activity in the absence of EMP may represent low levels of plasmin and/or limited amounts of activators in Matrigel. Stars indicate significant changes (p<0.05). Results are the mean±SD of three independent experiments.
Altogether, these data indicate that the uPA/uPAR system of EMP is responsible for plasmin generation and that plasmin proteolytic activity is involved in the EMP effects on tube formation by EPC.
Discussion
The plasminogen activation system plays a pivotal role in maintaining vascular patency and facilitating cell migration and angiogenesis. Binding of plasminogen to fibrin or the cell surface is of critical importance to regulate and target the proteolytic activity. Although the presence of uPAR on endothelial microparticles has previously been described30, its functional consequences, to our knowledge, have not been investigated as yet._The present study is the first demonstration that EMP provide a catalytic surface for the conversion of plasminogen into plasmin by expressing uPA and uPAR. Plasmin generation was EMP surface-dependent and could be augmented by binding of exogenous uPA to EMP-uPAR. As a result, EMP were shown to modulate the angiogenic responses of endothelial progenitors in vitro.
The generation of plasmin at the EMP surface was supported by inhibition experiments using the lysine analog ε-ACA and CpB. The inhibitory effect of ε-ACA, which occupies the lysine-binding site of plasminogen kringles 1 and 4 indicated lysine-dependent binding of plasminogen to the activation surface. The inhibition of plasmin formation by CpB confirmed that plasminogen activation was dependent on its cell surface binding to C-terminal lysine residues. This mechanism was further confirmed by inhibition of plasmin generation with a monoclonal antibody directed against α-enolase, a major plasminogen binding protein on cell surfaces.31 Taken together, these data demonstrated that EMP bind plasminogen and provide a catalytic surface for plasmin generation. The kinetics of plasminogen activation by the EMP were similar to those described for cells expressing uPA and uPAR.27, 28 32 Accordingly, ELISA and zymography experiments revealed that EMP express uPA and uPAR. Part of this uPA and uPAR expression was located on the EMP surface as evidenced by electron microscopy and flow cytometry.
The major contribution of EMP-bound uPA to the plasmin generation was further demonstrated by the following observations (i) plasmin generation was blocked by the uPA inhibitor amiloride and by a uPA-neutralizing monoclonal antibody, (ii) tPA-dependent lytic activity was absent on zymograms and neutralizing tPA antibodies did not affect plasmin generation.
EMP were also shown to bind exogenous sc-uPA. Both, the affinity of sc-uPA binding and the competitive effect of a recombinant form of uPA (rsc-uPA Ilel59→Gly), consistent with uPA/uPAR interaction, demonstrate that the EMP surface bears uPAR molecules devoid of uPA, allowing the specific binding of exogeneous uPA. These data, together with the expression of uPAR in excess of uPA, suggest that plasmin generation by EMP can be amplified by uPA transferred from the local environment to the EMP surface. This mechanism may have implications in tumor angiogenesis where uPA secretion is promoted. Thus MP from tumor cells and EMP could participate in amplification of proteolytic processes.33
Several reports have demonstrated that cell surface-bound plasmin is responsible for pericellular proteolysis and matrix degradation as compared to the soluble form.27, 34 This efficient activity results from protection against inactivation by physiological inhibitors, a direct contact with its matrix substrates, and the existence of amplification loops for plasminogen activation.16 Interestingly, plasmin once formed, remains partly bound to the EMP surface. Collectively, these data show that EMP act as vectors of efficient plasmin generation.
This proteolytic activity is not the hallmark of cell activation by TNF-α since it was similarly observed on EMP derived from quiescent endothelial cells. The substantial differences in plasmin generation observed among various endothelial cell types is consistent with the heterogeneous expression level of uPA/uPAR system along the vascular tree.35
Vascular homeostasis is the result of an equilibrium between injury and capacity for repair.3 Regeneration of damaged endothelium has been recently shown to involve not only angiogenesis but also vasculogenesis mediated by endothelial progenitors, both processes being largely dependent on proteolytic activities.35 Thus, the capacity of EMP to behave as vector of proteolytic activities raises the possibility that EMP may modulate EPC mediated repair processes. Consistently, apoptotic bodies from HUVEC have been shown to stimulate differentiation of endothelial progenitor, thereby representing a potential signaling pathway linking damaged cells to progenitors in the facilitation of the repair process.36 In our study, co-culture experiments showed that EMP affect EPC angiogenesis in Matrigel in a concentration dependent manner. While low amounts of EMP increased tube formation, higher concentrations inhibited it. This dual effect involved EMP associated plasmin since (i) in our co-culture model, EMP retained the capacity to increase plasmin concentration by activating the plasminogen of the culture medium and (ii) EMP effect was abrogated in the presence of α2-antiplasmin and a uPA-blocking antibody. The proangiogenic effect of EMP is consistent with plasmin associated proteolytic activity that favours cell migration via extracellular matrix processing and direct interaction with αvβ3 integrin19. The latter is, however, not involved in the process of plasmin generation (Fig 1D). Although this proangiogenic effect was observed with EMP present in a low number, plasmin was readily detected indicating that the local concentration of plasminogen was sufficient to ensure efficient plasmin generation. Other mechanisms may also be involved. MP from HUVEC stimulated with growth factors have been shown to stimulate angiogenic properties of mature endothelial cells and this effect was related to their content in matrix metalloproteinases.37 Consistently, we observed an increase in MMP-2 and MMP-9 activities in condition media of Matrigel experiments in the presence of low dose of EMP (data not shown). Plasmin may also affect angiogenesis indirectly through activation of these MMPs.18
In our study, high concentrations of EMP produced high amounts of plasmin and a dose-dependent decrease in tube formation by EPC. This inhibitory effect is in line with the role of an excessive plasmin generation leading to extracellular matrix degradation, alteration of cell anchorage and apoptosis.20, 38 Morphological changes in EPC co-cultured with high EMP amounts were indeed observed: accumulation of round and retracted cells evoking cell detachment, an effect that precedes apoptotic cell death.21 Protective and noxious effects of plasmin formation have also been observed in a cellular model of amyloid β degradation, suggesting that a similar effect may occur in vivo.39 Our data add to the observations of Mezentsev et al (2005) that reported a major contribution of oxidative stress in the impairment of angiogenic behaviour of mature endothelial cells.40 Although, in this study no proangiogenic effect at low doses of EMP was observed, this discrepancy may be related to the specific properties of endothelial progenitors. EPC have been reported to display increased sensitivity to proangiogenic stimulation and reduced sensitivity to oxidative stress compared to mature endothelial cells.41 Hence, the magnitude of an MP angiogenic signal may be influenced not only by MP cellular origin and concentration but also by the nature of the target cells.
In conclusion, we have identified cell-derived MP as new actors in the plasminogen activation system, an undescribed facet of the multiple biological processes involving EMP in the control of vascular homeostasis. The role of EMP-bound plasmin in pathological settings involving inflammation, atherosclerosis, angiogenesis and tumor growth, remains to be investigated. The high concentration of MP reported in atherosclerotic plaques suggests that EMP induced plasmin generation could participate in the modulation of cell apoptosis angiogenesis balance influencing plaque vulnerability. In stroke, released EMP could be actors linking inflammation, cell damage and dysregulated repair processes.42
Acknowledgments
We gratefully acknowledge the contribution of Dr. H.R. Lijnen (Catholic University of Leuven, Belgium) for providing scu-PA and rsc-uPA Ile 159→Gly, Dr. R. Lopez Alemany (Institu Recerca Oncologica, Barcelona, Spain) for providing the monoclonal antibody 11G1 directed against α-enolase, and Dr. J.C. Lissitzky (UFR Pharmacie, Marseille, France) for providing the anti- αvβ3 antibody We also thank Pr Marc Gamerre and his staff (department of gynecology and obstetrics, CHU Conception, Marseille, France) for collection of cord blood samples. E. Angles-Cano and his team are funded by an Inserm-Avenir/Lower-Normandy Council grant.
Footnotes
Françoise Dignat-George (dignat@pharmacie.univ-mrs.fr) and *Eduardo Angles-Cano (angles @ cyceron.fr) are corresponding authors and share senior authorship for this study.
Françoise Dignat-George, Inserm UMR 608, UFR de Pharmacie, 27 Bd Jean Moulin, 13385-cdx Marseille 5, France, Tel: 33 4 91835600 Fax: 33 4 91835602
Eduardo ANGLES-CANO, Inserm/Cyceron/Université de Caen, Bd Henri Becquerel, 14074, Caen, France. Tel 33231470164 Fax 33 2 31 47 02 22
The authors declare no competing financial interests
Authors contributions: Romaric Lacroix performed the research (plasmin generation by microparticles, angiogenesis assays), collected data, analysed and interpreted data and participated in manuscript drafting.
Florence Sabatier contributed to isolation and culture of endothelial progenitors and participated in data interpretation and in manuscript drafting.
Agnès Mialhe performed the research (characterisation of uPA-uPAR expression by microparticles).
Agnès Basire performed the research (flow cytometry experiments, statistical analysis)
Ralph Pannell performed the research (zymography)
Hélène Borghi contributed to electronical microscopy experiments
Stephane Robert performed the research. (flow cytometry experiments)
Edouard Lamy performed the research. (zymography)
Laurence Camoin-Jau contributed to research design and microparticle production.
Laurent Plawinski performed the research (plasminogen preparations and fibrin-agar zymography)
Victor Gurewich contributed to study design and editing of the manuscript.
Eduardo Angles-Cano designed the research, contributed analytical tools, analyzed data and participated in manuscript drafting.
Françoise Dignat-George designed the research, analysed the data and participated in manuscript drafting.
References
- 1.Freyssinet JM. Cellular microparticles: what are they bad or good for? J Thromb Haemost. 2003;1:1655–1662. doi: 10.1046/j.1538-7836.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 2.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Morel O, Toti F, Hugel B, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26:2594–2604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 4.Combes V, Simon AC, Grau GE, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernal-Mizrachi L, Jy W, Jimenez JJ, et al. High levels of circulating endothelial microparticles in patients with acute coronary sydromes. Am Heart J. 2003;145:962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 6.Faure V, Dou L, Sabatier F, et al. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566–573. doi: 10.1111/j.1538-7836.2005.01780.x. [DOI] [PubMed] [Google Scholar]
- 7.Sabatier F, Darmon P, Hugel B, et al. Type 1 and 2 diabetic patients display différent patterns of cellular microparticles. Diabetes. 2002;51:2840–2845. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 8.Dignat-George F, Camoin-Jau L, Sabatier F, et al. Endothelial microparticles : a potential contribution to the thrombotic complications of antiphospholipid syndrome. Thromb Haemost. 2004;91:667–673. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez JJ, Jy W, Mauro LM, Horstmann LL, Soderland C, Ahn YS. Endothelial microparticles released in Thrombotic thromcytopenic purpura express von Willebrand factor and markers of endothelial activation. Br J Haematol. 2003;123:896–902. doi: 10.1046/j.1365-2141.2003.04716.x. [DOI] [PubMed] [Google Scholar]
- 10.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 11.Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller I, Klocke A, Alex M, et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17:476–478. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 13.Biro E, Sturk-Maquelin KN, Vogel GMT, et al. Human-cell derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;197:1585–1598. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 14.Steppich B, Mattisek C, Sobczyk D, Kastrati A, Schomig A, Ott I. Tissue factor pathway inhibitor on circulating microparticles in acute myocardial infarction. Thromb Haemost. 2005;93:35–39. doi: 10.1160/TH04-06-0393. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Casal M, Downey C, Fukudome K, Marx G, Toh CH. Activated protein C induces the release of microparticle-associated endothelial protein C receptor. Blood. 2005;105:1515–1522. doi: 10.1182/blood-2004-05-1896. [DOI] [PubMed] [Google Scholar]
- 16.Lijnen HR. Elements of the fibrinolytic system. Ann N Y Acad Sci. 2001;936:226–236. doi: 10.1111/j.1749-6632.2001.tb03511.x. [DOI] [PubMed] [Google Scholar]
- 17.Tkachuk V, Stepanova V, Little PJ, Bobik A. Regulation and role of urokinase plasminogène activator in vascular remodelling. Clin Exp Pharmacol Physiol. 1996;23:759–765. doi: 10.1111/j.1440-1681.1996.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 18.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86:324–33. [PubMed] [Google Scholar]
- 19.Tarui T, Majumdar M, Miles LA, Ruf W, Takada Y. Plasmin-induced migration of endothelial cells. A potential target for the anti-angiogenic action of angiostatin. J Biol Chem. 2002;277:33564–33570. doi: 10.1074/jbc.M205514200. [DOI] [PubMed] [Google Scholar]
- 20.Rossignol P, Ho-Tin-Noe B, Vranckx R, et al. Protease nexin-1 inhibits plasminogen activation-induced apoptosis of adherent cells. J Biol Chem. 2004;279:10346–10356. doi: 10.1074/jbc.M310964200. [DOI] [PubMed] [Google Scholar]
- 21.Rossignol P, Luttun A, Martin-Ventura JL, et al. Plasminogen activation: a mediator of vascular smooth muscle cell apoptosis in atherosclerotic plaques. J Thromb Haemost. 2006;4:664–670. doi: 10.1111/j.1538-7836.2005.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleury V, Angles-Cano E. Characterization of the binding of plasminogen to fibrin surfaces: the role of carboxy-terminal lysines. Biochemistry. 1991;30:7630–7638. doi: 10.1021/bi00244a035. [DOI] [PubMed] [Google Scholar]
- 23.Ades EW, Candal FJ, Swerlick RA, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouis D, Hospers GA, Meijer C, Molema G, Mulder NH. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis. 2001;4:91–102. doi: 10.1023/a:1012259529167. [DOI] [PubMed] [Google Scholar]
- 26.Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;991:3962–70. doi: 10.1182/blood.v99.11.3962. [DOI] [PubMed] [Google Scholar]
- 27.Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- 28.Ellis V. Plasminogen activation at the cell surface. Curr Top Dev Biol. 2003;54:263–312. doi: 10.1016/s0070-2153(03)54012-1. [DOI] [PubMed] [Google Scholar]
- 29.Farina AR, Tiberio A, Tacconelli A, Cappabianca L, Gulino A, Mackay AR. Identification of plasminogen in Matrigel and its activation by reconstitution of this basement membrane extract. Biotechniques. 1996;21:904–909. doi: 10.2144/96215rr03. [DOI] [PubMed] [Google Scholar]
- 30.Brodsky SV, Malinowski K, Golightly M, et al. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation. 2002;106:2372–2378. doi: 10.1161/01.cir.0000033972.90653.af. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Alemany R, Longstaff C, Hawley S, et al. Inhibition of cell surface mediated plasminogen activation by a monoclonal antibody against alpha-Enolase. Am J Hematol. 2003;72:234–242. doi: 10.1002/ajh.10299. [DOI] [PubMed] [Google Scholar]
- 32.Lamanuzzi LB, Mtairag el M, Pepe G, Angles-Cano E. Neutrophils stimulated by apolipoprotein(a) generate fragments that are stronger inhibitors of plasmin formation than apo(a) Thromb Haemost. 2004;92:1066–1075. doi: 10.1160/TH04-04-0241. [DOI] [PubMed] [Google Scholar]
- 33.Angelucci A, D’Ascenzo S, Festuccia C, et al. Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin Exp Metastasis. 2000;18:163–170. doi: 10.1023/a:1006778000173. [DOI] [PubMed] [Google Scholar]
- 34.Ellis V, Murphy G. Cellular strategies for proteolytic targeting during migration and invasion. FEBS Lett. 2001;506:1–5. doi: 10.1016/s0014-5793(01)02845-9. [DOI] [PubMed] [Google Scholar]
- 35.Basire A, Sabatier F, Ravet S, et al. High urokinase expression contributes to the angiogenic properties of endothelial cells derived from circulating progenitors. Thromb Haemost. 2006;95:678–688. [PubMed] [Google Scholar]
- 36.Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taraboletti G, D’Ascenzo S, Borsotti P, et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meilhac O, Ho-Tin-Noé B, Houard X, Philippe M, Michel JB, Angles-Cano E. Pericellular plasmin induces smooth muscle cell anoïkis. FASEB J. 2003;17:1301–1303. doi: 10.1096/fj.02-0687fje. [DOI] [PubMed] [Google Scholar]
- 39.Davis J, Wagner MR, Zhang W, Xu F, Van Nostrand WE. Amyloid beta-protein stimulates the expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) in human cerebrovascular smooth muscle cells. J Biol Chem. 2003;278:19054–19061. doi: 10.1074/jbc.M301398200. [DOI] [PubMed] [Google Scholar]
- 40.Mezentsev A, Merks RM, O’Riordan E, et al. Endothelial microparticles affect angiogenesis in vitro : role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:HI 106–1114. doi: 10.1152/ajpheart.00265.2005. [DOI] [PubMed] [Google Scholar]
- 41.Bompais H, Chagraoui J, Canron X, et al. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;103:2577–2584. doi: 10.1182/blood-2003-08-2770. [DOI] [PubMed] [Google Scholar]
- 42.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4:1296–1302. doi: 10.1111/j.1538-7836.2006.01911.x. [DOI] [PubMed] [Google Scholar]


