Abstract
Humans have relatively low plasma ascorbate levels and high serum uric acid levels compared to most mammals due to the presence of genetic mutations in L-gulonolactone oxidase and uricase, respectively. We review the major hypotheses for why these mutations may have occurred. In particular, we suggest that both mutations may have provided a survival advantage to early primates by helping maintain blood pressure during periods of dietary change and environmental stress. We further propose that these mutations have the inadvertent disadvantage of increasing our risk for hypertension and cardiovascular disease in today’s society characterized by Western diet and increasing physical inactivity. Finally, we suggest that a “planetary biology” approach in which genetic changes are analyzed in relation to their biologic action and historical context may provide the ideal approach towards understanding the biology of the past, present and future.
The reductionist paradigm for human biology has, over the past century, been remarkably successful. It has, in one sense, reached its apotheosis through the complete sequencing of the human genome[1]. This, together with analyses of the transcriptome, the metabolome, and their higher organization has provided a "parts list" for a living organism.
As these parts lists have become more complete, it has become increasingly clear that they do not provide anything approaching an understanding of human biology[2]. Additional approaches are needed if that understanding is to emerge and, with it, the opportunity to manipulate the biological parts to manage, treat, and cure human disease.
One approach is to exploit the axiom that a system can be fully understood only if we understand both its structure and its history. This is certainly true for the terrestrial living systems, which are the products of four billion years of random variation and natural selection, all constrained by physical and chemical law. Without understanding this history, we are no more likely to understand biology than we are to understand the QWERTY computer keyboard without knowing of the typewriter or the Federal Reserve banking system without the Panic of 1896.
This has led us and others to suggest that systems analyses involving a wide field disciplines (including paleontology, paleoecology, evolutionary molecular biology), a process that has been termed planetary biology[3], may provide a better approach to understand how we evolved to our current state. This approach may also provide hypotheses into why humans have certain diseases that appear rare among other species, including obesity, essential hypertension, preeclampsia, cardiovascular disease, alcoholism, and other conditions. Perhaps most importantly, a planetary analysis may provide means for testing evolutionary hypotheses, by reconstructing the ancestral genes and testing how the various mutations may have affected survival given the ecological conditions at that time [4, 5]. This approach may also be useful in understanding current genetic polymorphism in human populations that generates differential disease incidence and differential responses to therapies. Furthermore, by providing insight into active evolutionary processes, these analyses may also help predict the needs of our species in response to future changes in climate and ecology.
This paper concerns the differences between humans and other mammal species, with a focus on two key genetic differences. The first is the absence of the final enzyme (L-gulononolactone oxidase) in the pathway of vitamin C (ascorbate) biosynthesis. This enzyme was present in primitive primates, but was lost in the primate lineage leading to monkeys and apes in the Eocene (55-35 MYA). This generated a need for humans, monkeys, and other "higher" primates to obtain ascorbate from the diet.
The second is the absence of an active enzyme (urate oxidase, or uricase) involved in the degradation of purines. This arose through mutation of the gene encoding uricase in lineages leading to higher primates in the Miocene (5–23 MYA). This caused urate to be the final enzymatic endproduct in purine metabolism.
As a consequence of these mutations, the plasma concentrations of ascorbate and urate are quite different in humans and most other mammals. Interestingly, this means that two of the three most important water-soluble antioxidants in mammals (the other being glutathione) are different in humans and (for example), rats, an organism widely used in biomedical research to model human biology. We will discuss current hypotheses for why these mutations occurred, and how planetary biologic studies might be able to provide a better understanding for the mechanisms leading to these evolutionary changes.
Ascorbate Synthesis and its Role as an Antioxidant
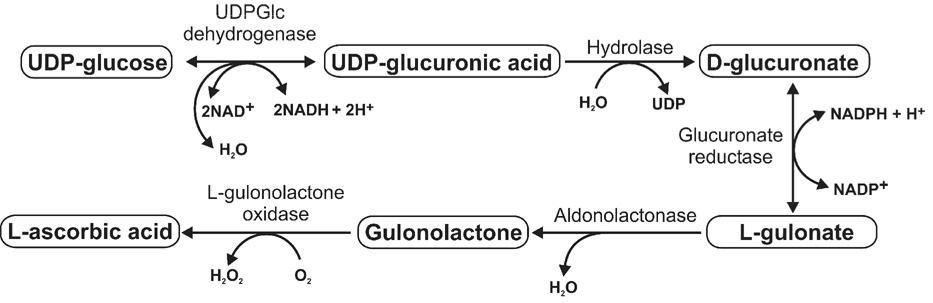
Ascorbate (vitamin C) is synthesized in most mammals by an active five-enzyme process that begins with an activated form of glucose, UDP-glucose. UDP-glucose is present primarily in the liver or kidney, depending on species (Figure 1). As such, its concentration is highly dependent on intracellular glucose reserves and glycogenolyis. Further, factors that reduce glucose reserves (starvation, low carbohydrate diets) or inhibit glycogenolysis (such as fructose) inhibit ascorbate synthesis[6].
Figure 1.
Ascorbate Metabolism
Ascorbate has numerous biologic functions, including important roles in the synthesis of collagen, creatine and catecholamine. However, one of its most important roles is to function as an electron donor, or anti-oxidant. Antioxidants are thought to play a key role in the protection of species by blocking lipid peroxidation, DNA damage and alkylation, and cell membrane injury[7,8].
An interesting feature of ascorbate is that it is recyclable[9]. Specifically, when ascorbate reacts with an oxidant, it is oxidized to the semidehydroascorbate radical and then dehydroascorbate. While some dehydroascorbate may be catabolized to various end products, dehydroascorbate can also be reduced back to ascorbate by reaction with glutathione or via an enzymatic pathway[6].
Ascorbate and dehydroascorbate enter cells via different transporters, with the ascorbate transporter being specific Na+ dependent co-transporters (SVCT1 and SCVT2)[10] and the dehydroascorbate transporter using members of the Glut family. Once transported, dehydroascorbate is reduced immediately into ascorbate [11]. The ascorbate transport system allows intracellular ascorbate levels to be in the range of 2–4 mM. despite plasma levels that are significantly lower (40–120 µM)[10]. Interestingly, when some cells, such as human neutrophils are activated by a variety of stimuli, intracellular concentration of ascorbate increases even more and can reach 14 mM, while its extracellular level remains unchanged [12]
Ascorbate is produced by many fish species, lobe-finned fish (lungfish and the coelacanth), amphibians, and other terrestrial vertebrates. Biosynthesis of ascorbate occurs primarily in the liver (most mammals and some birds) or kidney (amphibians and reptiles, some fish, and some birds)[13]. However, most primates lack the ability to make ascorbate due to a mutation in L-gulonolactone oxidase, which is the final enzyme involved in ascorbate synthesis[14]. The inability to synthesize ascorbate is observed among all primates except the prosimians (with the possible exception of Tarsius[15]) suggesting that the mutation occurred during the Eocene between 55 and 35 million years ago[14, 16]. A variety of other species also lack the ability to make ascorbate; these include the Indian fruit-eating bat, the guinea pig, the Indian red vented bul-bul bird, and some species of fish [17, 18].
One of the consequences of the loss of ascorbate synthesis is the disease scurvy, which was first recognized to be due to a dietary deficiency of fruits by James Lind in 1753[19]. It was nearly two centuries later when vitamin C (ascorbate) was identified as the critical nutrient for which a deficiency was responsible for the disease[20]. Today, scurvy is rare, and plasma ascorbate levels are generally in the range of 40–120 uM, which are well above levels associated with this disease (typically < 10 uM). These blood levels are two to four times lower than that observed in mammals that synthesize ascorbate[21]. Although the missing L-gulononolactone oxidase in humans might be a reason for somewhat lower concentration of ascorbate in the blood, its concentration in other biological fluids [22] and especially intracellular levels are much higher and in most cases comparable with the mammals that synthesize ascorbic acid [12, 13, 23, 24]
Uric acid and the Mutation of Uricase
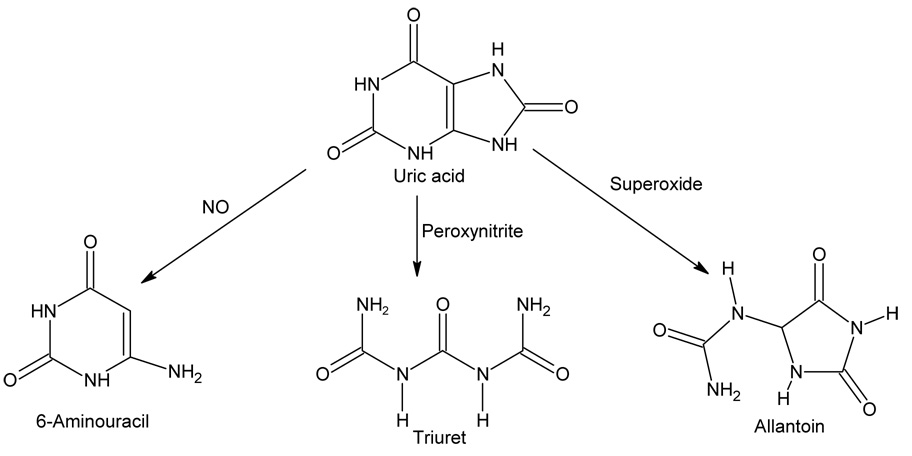
Uric acid is a metabolic product of purine metabolism generated from the breakdown of DNA, RNA and ATP (Figure 2). The immediate precursor enzyme is xanthine oxidoreductase which converts xanthine to uric acid with the generation of oxidants (superoxide anion or hydrogen peroxide) or NADH. In some species uric acid is then metabolized to allantoin by the enzyme uricase (urate oxidase). Depending on the species, allantoin may be further degraded by allantoinase and allantoincase to generate ammonia.
Figure 2.
Uric acid Metabolism
Uric acid is also recognized as a water soluble antioxidant and is considered to be one of the most important antioxidants in the plasma[25]. Uric acid can donate an electron to form the urate radical; but unlike ascorbate this radical is not recycled but rather is degraded via several different pathways. Uric acid can react with a variety of substances including hydrogen peroxide, hydroxyl radical, peroxynitrite, and nitric oxide[26–28]. For example, uric acid reacts with hydroxyl radical to form allantoin, with peroxynitrite to form triuret, and with nitric oxide to form 6-aminouracil (Figure 3). While these reactions might be beneficial under certain conditions as a means for reducing oxidative stress, some of these reactions, such as the uric acid reaction with peroxynitrite, also produces radicals and alkylating species that may be damaging27, [29].
Figure 3. Non enzymatic Pathways of Urate Degradation.
Nonenzymatic Pathways of Uric acid Degradation
Uric acid has also been proposed to have neurostimulant properties based on its similarity in chemical structure with caffeine[30] and due to epidemiological and experimental studies suggesting it may have a role in increasing reaction time, locomotor activity, and mental performance[31–35] .
Uric acid is also important in innate immune function. Specifically, uric acid may aid in the immune recognition of dying cells[36], help activate the inflammasome critical for interleukin-1 beta release[37], and in the immune rejection of tumor cells[38].
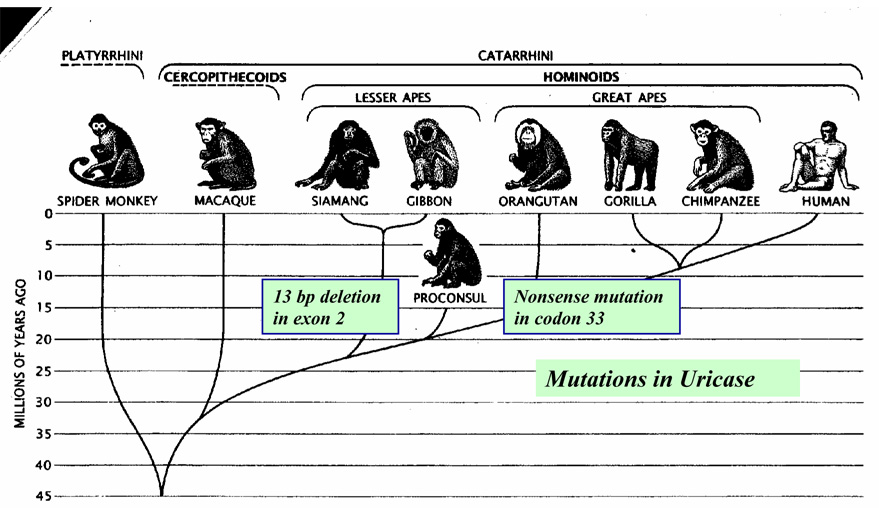
Most mammals have functional uricase, and have uric acid levels in the 1–2 mg/dl range (0.06 to 0.12 mM). In contrast, serum uric acid levels are higher in man and the Great and Lesser Apes due to parallel mutations of the uricase gene that occurred during the mid Miocene[39] (Figure 3). Uricase activity is also functionally absent or immunologically undetectable in certain New World monkeys (such as the woolly monkey (Lagothrix) and the macaque (Cynomolgus), consistent with a mutation of the uricase gene[40,41]. Uricase activity is also lower in both Old World and New World monkeys compared to other mammals[42, 43]. Recent studies strongly suggest that this may be due to mutations in the promoter regions[44]. Indeed, evidence now suggests that the loss of uricase in humans may have been stepwise, with a progressive loss in activity (due to mutations in the promoter region) followed by complete silencing of the gene[44].
Why did Early Primates lose their Ability to Synthesize Ascorbate?
Several hypotheses have been proposed to account for the loss of ascorbate synthesis by early primates. Pauling suggested that the loss of ascorbate synthesis resulted as a consequence of the lack of need, as early primates may have had ample access to dietary ascorbate from root tips, seed sprouts, fruits and green leaves[45]. However, Darwinian theory suggests that there was likely a positive selection mechanism that made it advantageous to lack ascorbate synthesis; otherwise, both polymorphisms would have been predicted to survive. In this regard, the enzyme L-gulonolactone oxidase generates not only ascorbate but also hydrogen peroxide (Figure 1), and therefore is redox neutral, whereas the dietary intake of ascorbate would increase antioxidant activity. Thus, Banhegyi et al have proposed there was a selection advantage to supplying ascorbate stores primarily by diet[6].
An alternative hypothesis has been proposed by Challem [46] and Challem and Taylor[47]. These authors have reviewed evidence that a primate retrovirus may have inserted Alu elements in the gene for L-gulonolactone oxidase that silenced the gene. While the loss of ascorbate may have been beneficial for the survival of the retrovirus, it may have also led to increased levels of free radicals that could increase the frequency of free radical-induced mutations that could help accelerate the evolution of these early primates[46–48].
What was the Advantage to having Uricase mutated?
The observation that parallel mutations involving uricase occurred in early hominoid evolution strongly suggests that there must have also been a selection advantage during the Miocene to having higher serum uric acid levels.
The most quoted hypothesis is that originally proposed by Proctor[49] and later Ames[25] who suggested that the uricase mutation may have occurred as a means to replace serum antioxidant activity after the loss of ascorbate synthesis. Indeed, uric acid can help maintain ascorbate levels[50]. Furthermore, the activity of other antioxidant systems, such as superoxide dismutase, are also higher in species lacking ascorbate[51]. Ames has suggested that the reason humans have longer longevity compared to other mammals may relate to the antioxidant benefits provided by the higher uric acid levels[25]. Challenging this hypothesis, however, is the observation that neither ascorbate or urate levels correlate with maximum life span in vertebrates[52].
Another hypothesis is that the increase in uric acid resulted in better reaction time and higher mental performance that helped accelerate the evolution of man[30]. While some epidemiological studies have supported this, the evidence has been weak at best. Alternative hypotheses could include the possibility that an increase in uric acid might improve innate immune function and the ability to ward off infections or tumors[36–38].
An Alternative Hypothesis: The Loss of Ascorbate and Rise in Uric acid had a Role in Maintaining Blood Pressure during Periods of Environmental Stress
Ascorbate and blood pressure
The inability to synthesize ascorbate would have forced early primates to ingest a vitamin C-rich diet to maintain their ascorbate stores. The gorilla, who lacks the ability to synthesize Vitamin C, eats about 4.5 g of ascorbate per day[53]. Pauling has calculated, based on synthetic rates in other mammals, that the average 70 kg human would have to ingest 1.8–4.1 g/d ascorbate to achieve similar blood levels[45].
What would happen if suddenly a change in climate occurred that resulted in less available dietary ascorbate? Serum ascorbate levels would fall, resulting in less protection from oxidative stress. Furthermore, low concentrations of ascorbate, particularly in the presence of catalytic metals such as copper or iron, can become pro-oxidative[54], and examples have been found where ascorbate increases rather than decreases intracellular oxidative stress[55].
In turn, oxidative stress has been shown to raise blood pressure[56]. For example, the depletion of glutathione in rats rapidly results in a rise in blood pressure[57]. Numerous animal models have shown a key role for oxidative stress (present in the circulation, blood vessels, and kidney) in mediating hypertension[56]. Ascorbate administration also lowers blood pressure in many hypertensive animal models[58–60].
Epidemiologic studies have confirmed a strong inverse relationship between serum ascorbate levels and blood pressure[61–63]. Plasma levels of ascorbate are lower in hypertensive subjects (mean 40 umol/L) compared to normotensive controls (mean 57 umol/L)[64]. Plasma ascorbate levels are also lower in African-Americans who have higher rates of hypertension compared to whites[65] and in other hypertensive conditions, such as preeclampsia[66] .
Vitamin C supplementation in patients with hypertension have also reported a reduction in blood pressure in some studies[67–69]. but was either negative or less effective in other studies [70, 71]. One potential explanation is that the effect of vitamin C on blood pressure appears to be more effective in the studies using younger subjects as opposed to studies that enrolled elderly subjects with longstanding hypertension, possibly because in later stages of hypertension the kidney is the primary driving force [72]. In one recent study of young subjects (age 30 to 59), a strong effect was observed with ascorbate levels and blood pressure after either taking a vitamin C deplete diet or following supplementation[68].
Given these considerations, it is tempting to speculate that the mutation in ascorbate synthesis occurred during a time of high vitamin C intake and was partially advantageous since dietary intake did not require the generation of oxidants that occurs with its synthesis. However, if a climatologic change resulted in famine or starvation, those primates that could still make vitamin C would decrease their synthesis (see above) with potential advantage of stimulating oxidative stress and raising blood pressure; but those who could not synthesize ascorbate at all might develop more severe oxidative stress and higher blood pressures that might have provided a superior survival advantage for that time.
Uric acid and blood pressure
We have recently proposed a hypothesis for how a mutation in uricase might provide a survival advantage by raising blood pressure[73]. Specifically, there is evidence that during the early Miocene there was a marked increase in ape species[74]. However, by the mid Miocene there was global cooling (‘the Miocene Disruption’) associated with the extinction of numerous species, likely including many species of apes. During this period large areas of rain forests dried out, leaving savannahs and grasslands, and forcing early hominoids to develop knuckle walking and change their diet. The Paleolithic diet was low in sodium[75], and hence survival would be optimized by those species that could maintain blood pressure and salt sensitivity.
Interestingly, while uric acid can function as an antioxidant, it can also function as a pro-oxidant on a variety of cell types and in vivo[27, 29]. Indeed, inhibiting uricase in rats results in a rise in blood pressure associated with systemic reduction in endothelial nitric oxide and a stimulation of the renin angiotensin system[76, 77]. Over time uric acid causes microvascular disease in the kidneys due to direct effects of uric acid on vascular smooth muscle cells and endothelial cells[78–81]. Once these changes occur the kidney preferentially holds onto salt (development of salt-sensitivity)[73].
Evidence that uric acid is involved in blood pressure in humans is equally compelling. There are now extensive studies showing that a high uric acid independently predicts the development of hypertension[82–84]; likewise, an elevated uric acid is common in early hypertension and was present in almost 90% of hypertensive adolescents in one study[85]. Furthermore, recent clinical trials have found that lowering uric acid lowers blood pressure in both adolescents and adults with hypertension (and D Feig and R Johnson, unpublished)[86, 87].
In addition to raising blood pressure, recent studies support uric acid as having a role in insulin resistance and obesity[88, 89]. Indeed, fructose, which rapidly raises uric acid, induces metabolic syndrome in animals and this can be ameliorated by lowering uric acid[88]. The mechanism by which uric acid mediates features of the metabolic syndrome is likely due to the ability of uric acid to block some of insulin’s actions by reducing endothelial nitric oxide as well as due to direct effects of uric acid on the adipocyte[76, 90].
Thus, the uricase mutation may have conferred a survival advantage by helping to raise blood pressure, stimulate salt-sensitivity, and induce insulin resistance and mild obesity, and thereby help promote survival during a period of famine or stress.
How could a mutation that was a survival advantage in the Miocene now be playing a role in the cardiovascular epidemic? The consequence of the uricase mutation is that humans not only have higher uric acid levels than most other mammals but they also can not regulate levels as effectively[91, 92]. Interestingly, because the current Western diet is high in meats and fructose, both which generate uric acid, humans today have higher uric acid levels (range 4–10 mg/dl) compared to primates that lack uricase (where uric acid levels are typically in the 3–4 mg/dl range)[92]. Our preliminary studies in Yanomamo Indians living in their original habitat and with their primitive diet found serum uric acid levels in the 2–4 mg/dl range, suggesting that primitive humans had lower uric acid levels than today (W. Oliver and RJ Johnson, preliminary data). Thus, in today’s society we are ingesting significantly more sweeteners (containing fructose) and meats (containing purines) such that those who obtain the highest uric acid levels develop high blood pressure (hypertension), insulin resistance and obesity, and possibly diabetes and cardiovascular disease.
Planetary Biology and the Approach to the Uricase/Ascorbate Hypotheses
How can planetary biology help determine which hypothesis is correct? By pinpointing the date of the mutations and cross checking with the paleoecological and anthropological record, we can evaluate the effects of environment with the genetic changes.
Emerging tools in paleogenetics provide an experimental tool to test hypotheses that emerge from this correlative science [93]. In a paleogenetics experiment, the structures of ancestral genes are inferred from the sequences of their descendents; the ancestral genes from extinct organisms are then resurrected in the laboratory, where their behavior is studied. This allows us to evaluate their function under cell culture and in animal models using inferred ancestral environmental conditions.
Finally, paleogenetic evaluation of changes in up- and downstream genes may allow additional testing of the hypothesis. For example, mice in which uricase has been knocked out do not survive due to a rapid rise in uric acid that leads to massive uricosuria, intrarenal crystal deposition, and acute renal failure[94]. Thus, there were likely changes in other genes that regulate uric acid production or excretion to prevent this complication in the primates that lost uricase. One possible change could have been a contemporaneous reduction in the rate of synthesis of uric acid.
Consistent with this possibility, humans have a 100-fold lower activity of xanthine oxidase than rodents[95]. This appears to be the result of a loss of transcription and core promoter activity for the gene encoding that enzyme[96]. In some New World primates, the level of serum uric acid is also not elevated due to enhanced mechanisms of urinary excretion[40]. Thus, identifying the temporal sequence for changes in xanthine oxidase and in renal urate transport may provide clues to the sequence by which the changes in gene expression occurred as it relates to the paleoecological and paleontological record.
Likewise, the mutation of ascorbate synthesis did not change dramatically the intracellular levels of ascorbate, even as it evidently lowered the serum levels of ascorbate. This suggests that changes in the management of ascorbate also occurred in more ancient primate lineages contemporary with the loss of ascorbate biosynthesis. An obvious system where compensatory changes might have been effective is in the ascorbate transporters.
These considerations suggest a historical paradigm for future research that should complement in this century the reductionist paradigm of the last. It is partly correlative science; interconnecting the evolutionary histories of related genes should provide hypotheses to interpret of the evolutionary biology of the biomolecular system. Paleogenetic resurrections will provide experimental tests of those hypothese. Animal model studies will provide orthogonal tests, completing the connection between the biomolecules, natural history, and physiology.
Figure 4. Uricase Mutation in Early Hominoid Evolution.
Cladogram of uricase mutations in hominoid evolution. Figure taken from Scientific American 1989;260:76–82.
Acknowledgments
SAB is indebted to the National Science Foundation for its support of this work via an OPUS grant (DEB 0717335).
Supported by NIH Grants HL-68607, DK-52121, and research funds from Gatorade.
Footnotes
Disclaimers: Dr Johnson is listed as an inventor on several patent applications related to lowering of uric acid in cardiovascular disease and also is author on a book on fructose that will be published in 2008 by Rodale press. Dr. Benner and Dr. Gaucher are listed as inventors on several patent applications to apply evolutionary analysis to understand protein evolution.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Collins FS, McKusick VA. Implications of the Human Genome Project for medical science. Jama. 2001;285:540–544. doi: 10.1001/jama.285.5.540. [DOI] [PubMed] [Google Scholar]
- 2.Woese CR. A new biology for a new century. Microbiol Mol Biol Rev. 2004;68:173–186. doi: 10.1128/MMBR.68.2.173-186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benner SA, Caraco MD, Thomson JM, Gaucher EA. Planetary biology-- paleontological, geological, and molecular histories of life. Science. 2002;296:864–868. doi: 10.1126/science.1069863. [DOI] [PubMed] [Google Scholar]
- 4.Liberles DA. Experimental Paleogenetics. New York: Academic Press; 2007. [Google Scholar]
- 5.Benner SA, Sassi SO, Gaucher EA. Molecular paleoscience: systems biology from the past. Adv Enzymol Relat Areas Mol Biol. 2007;75:1–132. doi: 10.1002/9780471224464.ch1. xi. [DOI] [PubMed] [Google Scholar]
- 6.Banhegyi G, Braun L, Csala M, Puskas F, Mandl J. Ascorbate metabolism and its regulation in animals. Free Radic Biol Med. 1997;23:793–803. doi: 10.1016/s0891-5849(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell B. The role of oxygen radicals in human disease, with particular reference to the vascular system. Haemostasis. 1993;23 Suppl 1:118–126. doi: 10.1159/000216921. [DOI] [PubMed] [Google Scholar]
- 8.Darley-Usmar V, Halliwell B. Blood radicals: reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm Res. 1996;13:649–662. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- 9.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. Febs J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 11.Welch RW, Wang Y, Crossman A, Jr, Park JB, Kirk KL, Levine M. Accumulation of vitamin C (ascorbate) and its oxidized metabolite dehydroascorbic acid occurs by separate mechanisms. J Biol Chem. 1995;270:12584–12592. doi: 10.1074/jbc.270.21.12584. [DOI] [PubMed] [Google Scholar]
- 12.Washko PW, Wang Y, Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem. 1993;268:15531–15535. [PubMed] [Google Scholar]
- 13.Chinoy NJ. Ascorbic acid levels in mammalian tissues and its metabolic significance. Comp Biochem Physiol A. 1972;42:945–952. doi: 10.1016/0300-9629(72)90400-8. [DOI] [PubMed] [Google Scholar]
- 14.Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem. 1994;269:13685–13688. [PubMed] [Google Scholar]
- 15.Pollock JI, Mullin RJ. Vitamin C biosynthesis in prosimians: evidence for the anthropoid affinity of Tarsius. Am J Phys Anthropol. 1987;73:65–70. doi: 10.1002/ajpa.1330730106. [DOI] [PubMed] [Google Scholar]
- 16.Ohta Y, Nishikimi M. Random nucleotide substitutions in primate nonfunctional gene for L-gulono-gamma-lactone oxidase, the missing enzyme in L-ascorbic acid biosynthesis. Biochim Biophys Acta. 1999;1472:408–411. doi: 10.1016/s0304-4165(99)00123-3. [DOI] [PubMed] [Google Scholar]
- 17.Nishikimi M, Kawai T, Yagi K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J Biol Chem. 1992;267:21967–21972. [PubMed] [Google Scholar]
- 18.King CG. The biological synthesis of ascorbic acid. World Rev Nutr Diet. 1973;18:47–59. doi: 10.1159/000394228. [DOI] [PubMed] [Google Scholar]
- 19.Lind J. In: A treatise of the scurvy. Stewart CP, Guthrie D, editors. Edinburgh: University of Edinburgh; 1953. originally published in 1753. [Google Scholar]
- 20.Waugh WA, King CG. The isolation and identification of Vitamin C. J Biol Chem. 1932;97:325–331. [Google Scholar]
- 21.Qiu S, Li L, Weeber EJ, May JM. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res. 2007;85:1046–1056. doi: 10.1002/jnr.21204. [DOI] [PubMed] [Google Scholar]
- 22.Luck MR, Jeyaseelan I, Scholes RA. Ascorbic acid and fertility. Biol Reprod. 1995;52:262–266. doi: 10.1095/biolreprod52.2.262. [DOI] [PubMed] [Google Scholar]
- 23.Wang YH, Dhariwal KR, Levine M. Ascorbic acid bioavailability in humans. Ascorbic acid in plasma, serum, and urine. Ann N Y Acad Sci. 1992;669:383–386. doi: 10.1111/j.1749-6632.1992.tb17130.x. [DOI] [PubMed] [Google Scholar]
- 24.Siman CM, Eriksson UJ. Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia. 1997;40:1416–1424. doi: 10.1007/s001250050844. [DOI] [PubMed] [Google Scholar]
- 25.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gersch C, Palii S, Angerhofer A, Imaram W, Johnson RJ, Henderson GN. Inactivation of Nitric Oxide by Uric acid:. Nucleosides Nucleotides Nucleic Acids. 2007 doi: 10.1080/15257770802257952. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gersch C, Palii S, Angerhofer A, Imaram W, Johnson RJ, Henderson GN. Uric acid's reaction with Peroxynitrite: Formation of Reactive Intermediates, Alklyation Products, and Triuret. Biochim Biophys Acta. 2007 submitted. [Google Scholar]
- 28.Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci (Lond) 2003;105:425–430. doi: 10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]
- 29.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372:285–294. doi: 10.1006/abbi.1999.1491. [DOI] [PubMed] [Google Scholar]
- 30.Orowan E. The origin of man. Nature. 1955;175:683–684. doi: 10.1038/175683a0. [DOI] [PubMed] [Google Scholar]
- 31.Stetten D, Jr, Hearon JZ. Intellectual level measured by army classification battery and serum uric acid concentration. Science. 1959;129:1737. doi: 10.1126/science.129.3365.1737. [DOI] [PubMed] [Google Scholar]
- 32.Montoye HJ, Mikkelsen WM. Serum uric acid and achievement in high school. Arthritis Rheum. 1973;16:359–362. doi: 10.1002/art.1780160310. [DOI] [PubMed] [Google Scholar]
- 33.Inouye E, Park KS, Asaka A. Blood uric acid level and IQ: a study in twin families. Acta Genet Med Gemellol (Roma) 1984;33:237–242. doi: 10.1017/s0001566000007273. [DOI] [PubMed] [Google Scholar]
- 34.Barrera CM, Ruiz ZR, Dunlap WP. Uric acid: a participating factor in the symptoms of hyperactivity. Biol Psychiatry. 1988;24:344–347. doi: 10.1016/0006-3223(88)90205-3. [DOI] [PubMed] [Google Scholar]
- 35.Hunter RE, Barrera CM, Dohanich GP, Dunlap WP. Effects of uric acid and caffeine on A1 adenosine receptor binding in developing rat brain. Pharmacol Biochem Behav. 1990;35:791–795. doi: 10.1016/0091-3057(90)90360-t. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 37.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 38.Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res. 2004;64:5059–5062. doi: 10.1158/0008-5472.CAN-04-1586. [DOI] [PubMed] [Google Scholar]
- 39.Wu XW, Muzny DM, Lee CC, Caskey CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- 40.Logan DC, Wilson DE, Flowers CM, Sparks PJ, Tyler FH. Uric acid catabolism in the woolly monkey. Metabolism. 1976;25:517–522. doi: 10.1016/0026-0495(76)90005-6. [DOI] [PubMed] [Google Scholar]
- 41.Usuda N, Reddy MK, Hashimoto T, Rao MS, Reddy JK. Tissue specificity and species differences in the distribution of urate oxidase in peroxisomes. Lab Invest. 1988;58:100–111. [PubMed] [Google Scholar]
- 42.Friedman TB, Polanco GE, Appold JC, Mayle JE. On the loss of uricolytic activity during primate evolution--I. Silencing of urate oxidase in a hominoid ancestor. Comp Biochem Physiol B. 1985;81:653–659. doi: 10.1016/0305-0491(85)90381-5. [DOI] [PubMed] [Google Scholar]
- 43.Christen P, Peacock WC, Christen AE, Wacker WE. Urate oxidase in primate phylogenesis. Eur J Biochem. 1970;12:3–5. doi: 10.1111/j.1432-1033.1970.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 44.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 45.Pauling L. Evolution and the need for ascorbic acid. Proc Natl Acad Sci U S A. 1970;67:1643–1648. doi: 10.1073/pnas.67.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Challem JJ. Did the loss of endogenous ascorbate propel the evolution of Anthropoidea and Homo sapiens? Med Hypotheses. 1997;48:387–392. doi: 10.1016/s0306-9877(97)90033-5. [DOI] [PubMed] [Google Scholar]
- 47.Challem JJ, Taylor EW. Retroviruses, ascorbate, and mutations, in the evolution of Homo sapiens. Free Radic Biol Med. 1998;25:130–132. doi: 10.1016/s0891-5849(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 48.Benzie IF. Evolution of dietary antioxidants. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:113–126. doi: 10.1016/s1095-6433(02)00368-9. [DOI] [PubMed] [Google Scholar]
- 49.Proctor P. Similar functions of uric acid and ascorbate in man? Nature. 1970;228:868. doi: 10.1038/228868a0. [DOI] [PubMed] [Google Scholar]
- 50.Sevanian A, Davies KJ, Hochstein P. Conservation of vitamin C by uric acid in blood. J Free Radic Biol Med. 1985;1:117–124. doi: 10.1016/0748-5514(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 51.Nandi A, Mukhopadhyay CK, Ghosh MK, Chattopadhyay DJ, Chatterjee IB. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radic Biol Med. 1997;22:1047–1054. doi: 10.1016/s0891-5849(96)00491-1. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Torres M, Perez-Campo R, Rojas C, Cadenas S, Barja G. Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech Ageing Dev. 1993;70:177–199. doi: 10.1016/0047-6374(93)90047-u. [DOI] [PubMed] [Google Scholar]
- 53.Bourne GH. Vitamin C and immunity. Brit J Nutr. 1949;2:346–347. [Google Scholar]
- 54.Buettner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996;145:532–541. [PubMed] [Google Scholar]
- 55.Inai Y, Bi W, Shiraishi N, Nishikimi M. Enhanced oxidative stress by L-ascorbic acid within cells challenged by hydrogen peroxide. J Nutr Sci Vitaminol (Tokyo) 2005;51:398–405. doi: 10.3177/jnsv.51.398. [DOI] [PubMed] [Google Scholar]
- 56.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 57.Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 58.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary vitamin C supplementation lowers blood pressure in spontaneously hypertensive rats. Mol Cell Biochem. 2001;218:97–103. doi: 10.1023/a:1007234027421. [DOI] [PubMed] [Google Scholar]
- 59.Vasdev S, Gill V, Parai S, Longerich L, Gadag V. Dietary vitamin E and C supplementation prevents fructose induced hypertension in rats. Mol Cell Biochem. 2002;241:107–114. doi: 10.1023/a:1020835229591. [DOI] [PubMed] [Google Scholar]
- 60.Elhaimeur F, Courderot-Masuyer C, Nicod L, Guyon C, Richert L, Berthelot A. Dietary vitamin C supplementation decreases blood pressure in DOCA-salt hypertensive male Sprague-Dawley rats and this is associated with increased liver oxidative stress. Mol Cell Biochem. 2002;237:77–83. doi: 10.1023/a:1016587201108. [DOI] [PubMed] [Google Scholar]
- 61.Ness AR, Chee D, Elliott P. Vitamin C and blood pressure--an overview. J Hum Hypertens. 1997;11:343–350. doi: 10.1038/sj.jhh.1000423. [DOI] [PubMed] [Google Scholar]
- 62.Kurl S, Tuomainen TP, Laukkanen JA, Nyyssonen K, Lakka T, Sivenius J, et al. Plasma vitamin C modifies the association between hypertension and risk of stroke. Stroke. 2002;33:1568–1573. doi: 10.1161/01.str.0000017220.78722.d7. [DOI] [PubMed] [Google Scholar]
- 63.Bates CJ, Walmsley CM, Prentice A, Finch S. Does vitamin C reduce blood pressure? Results of a large study of people aged 65 or older. J Hypertens. 1998;16:925–932. doi: 10.1097/00004872-199816070-00005. [DOI] [PubMed] [Google Scholar]
- 64.Pierdomenico SD, Costantini F, Bucci A, De Cesare D, Cuccurullo F, Mezzetti A. Low-density lipoprotein oxidation and vitamins E and C in sustained and white-coat hypertension. Hypertension. 1998;31:621–626. doi: 10.1161/01.hyp.31.2.621. [DOI] [PubMed] [Google Scholar]
- 65.Fulwood R, Johnson CL, Bryner JD. National Center for Health Statistics. vol. Vital and Health Statistics series 11. Washington DC: US Public Health Service; 1982. Hematological and Nutritional Biochemistry Reference Data for Persons 6 months-74 years of Age. United States, 1976–1980; p. 232. DHHS publication No (PHS) 283-1682. [PubMed] [Google Scholar]
- 66.Hubel CA, Kagan VE, Kisin ER, McLaughlin MK, Roberts JM. Increased ascorbate radical formation and ascorbate depletion in plasma from women with preeclampsia: implications for oxidative stress. Free Radic Biol Med. 1997;23:597–609. doi: 10.1016/s0891-5849(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 67.Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Jr, et al. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–2049. doi: 10.1016/s0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- 68.Block G, Mangels AR, Norkus EP, Patterson BH, Levander OA, Taylor PR. Ascorbic acid status and subsequent diastolic and systolic blood pressure. Hypertension. 2001;37:261–267. doi: 10.1161/01.hyp.37.2.261. [DOI] [PubMed] [Google Scholar]
- 69.Hajjar IM, George V, Sasse EA, Kochar MS. A randomized, double-blind, controlled trial of vitamin C in the management of hypertension and lipids. Am J Ther. 2002;9:289–293. doi: 10.1097/00045391-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Fotherby MD, Williams JC, Forster LA, Craner P, Ferns GA. Effect of vitamin C on ambulatory blood pressure and plasma lipids in older persons. J Hypertens. 2000;18:411–415. doi: 10.1097/00004872-200018040-00009. [DOI] [PubMed] [Google Scholar]
- 71.Ghosh SK, Ekpo EB, Shah IU, Girling AJ, Jenkins C, Sinclair AJ. A double-blind, placebo-controlled parallel trial of vitamin C treatment in elderly patients with hypertension. Gerontology. 1994;40:268–272. doi: 10.1159/000213595. [DOI] [PubMed] [Google Scholar]
- 72.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 74.Begun DR. Planet of the apes. Sci Am. 2003;289:74–83. doi: 10.1038/scientificamerican0803-74. [DOI] [PubMed] [Google Scholar]
- 75.Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 76.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 77.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 78.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 79.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 80.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 81.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266:8604–8608. [PubMed] [Google Scholar]
- 82.Johnson RJ, Feig DI, Herrera-Acosta J, Kang DH. Resurrection of uric acid as a causal risk factor in essential hypertension. Hypertension. 2005;45:18–20. doi: 10.1161/01.HYP.0000150785.39055.e8. [DOI] [PubMed] [Google Scholar]
- 83.Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, et al. Serum Uric Acid Predicts Incident Hypertension in a Biethnic Cohort. The Atherosclerosis Risk in Communities Study. Hypertension. 2006 doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 84.Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 85.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feig DI, Nakagawa T, Karumanchi SA, Oliver WJ, Kang DH, Finch J, et al. Hypothesis: Uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int. 2004;66:281–287. doi: 10.1111/j.1523-1755.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- 87.Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007 doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 88.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 89.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Fructose-induced hyperuricemia as a casual mechanism for the epidemic of the metabolic syndrome. Nature Clinical Practice Nephrology. 2006;1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 90.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classical antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 91.Johnson RJ, Rideout BA. Uric acid and diet--insights into the epidemic of cardiovascular disease. N Engl J Med. 2004;350:1071–1073. doi: 10.1056/NEJMp048015. [DOI] [PubMed] [Google Scholar]
- 92.Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25:3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 93.Liberles DA, editor. Liberles, Experimental Paleogenetics. NY: Academic Press; 2007. [Google Scholar]
- 94.Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery C, Jr, Jones P, et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A. 1994;91:742–746. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abadeh S, Killacky J, Benboubetra M, Harrison R. Purification and partial characterization of xanthine oxidase from human milk. Biochim Biophys Acta. 1992;1117:25–32. doi: 10.1016/0304-4165(92)90157-p. [DOI] [PubMed] [Google Scholar]
- 96.Xu P, LaVallee P, Hoidal JR. Repressed expression of the human xanthine oxidoreductase gene. E-box and TATA-like elements restrict ground state transcriptional activity. J Biol Chem. 2000;275:5918–5926. doi: 10.1074/jbc.275.8.5918. [DOI] [PubMed] [Google Scholar]