Abstract
Tumor necrosis factor receptor associated factors (TRAFs) play a variety of interesting and important roles in the regulation of B lymphocyte function. They act both as cytoplasmic regulatory molecules, and as signal transducers for receptors involved in both innate and adaptive humoral immune responses. In this brief review, we highlight the current state of knowledge of the diverse roles of TRAF molecules in the functions of B lymphocytes.
1. CD40
CD40 is the most well-studied member of the TNFR superfamily expressed by B lymphocytes, so its interactions with TRAF molecules have also been intensively investigated. B cell CD40 is important in the induction of B cell survival and expansion, immunoglobulin (Ig) production and isotype switching, upregulation of surface molecules involved in B cell antigen presentation, differentiation to B memory cells, and the production of various cytokines (reviewed in [1]. The TRAF molecules have been shown to contribute to each of these functions (reviewed in [2].
In 1994, TRAF3 became the first cytoplasmic molecule demonstrated to associate with CD40 (reviewed in [2]). However, for many years, its roles in CD40 signaling were largely unknown, and are still not completely understood. TRAF3-/- mice die shortly after birth, but adoptive transfer of fetal liver cells from these mice suggested that their B cells were capable of reconstituting the humoral response [3], implying that TRAF3 is not required to promote CD40-mediated antibody responses. This conclusion was supported by experiments in B cell lines demonstrating that inducible overexpression of TRAF3 inhibits both CD40 responses [4], and cooperation between CD40 and the BCR [5]. Subsequently, B cell lines made completely TRAF3-deficient via gene targeting by homologous recombination show no defects in CD40 signaling overall, and display enhanced CD40-mediated c-jun kinase (JNK) activation and IgM production [6]. Recently, mice made conditionally deficient in TRAF3 only in CD19+ B cells show no deficiencies in CD40 signaling in vitro and have enhanced antibody responses [7].
TRAF2 was initially demonstrated to bind CD40 by exogenous overexpression studies in adenocarcinoma cell lines, in which it promotes JNK activation and the activity of NF-κB reporter genes (reviewed in [2]. Because TRAF2-/- mice have an early lethal phenotype [8], they could not provide clear information on the roles played by TRAF2 in CD40 signaling to B cells. Mice transgenic for mutant CD40 molecules with reduced TRAF2 binding were studied by several groups, but they reached divergent conclusions regarding the importance of TRAF2-CD40 association in the humoral immune response, complicated by residual binding of TRAF2 to the mutants used, varying transgene dosage, and different CD40 external domains (reviewed in [9]. However, in contrast to TRAF3, TRAF2 overexpression in B cells promotes B cell activation [4], and B cell lines lacking TRAF2 show defects in JNK activation, Ig production, and CD40-BCR synergy (reviewed in [9]. Mice conditionally deficient in B cell TRAF2 show enhanced B cell survival and constitutive activation of the non-canonical NF-κB2 pathway, but this may be due to effects on signaling via other TNFR superfamily members (see below); the B cells do show reduced CD40-induced proliferation [10]. Another interesting function of TRAF2 revealed by TRAF2-/- B cell lines is its role as the “master regulator” of CD40-mediated degradation of both itself and TRAF3 [11, 12]. This degradation is an important feedback control mechanism for CD40 signaling [12].
TRAF1 associates with CD40 primarily indirectly by forming multimers with TRAF2, although a small amount binds CD40 directly if TRAF2 is absent [13]. Because TRAF1 is unique among the TRAFs in lacking a RING domain, its functions have been especially mysterious. TRAF1 is the only TRAF whose expression is substantially enhanced by CD40 signals (reviewed in [9]. TRAF1-/- mice have normal numbers and phenotype of B cells, and no obvious defects in humoral immunity or CD40-induced B cell proliferation [14]. However, few studies have specifically studied the role of TRAF1 in CD40-mediated signaling in B cells. Using B cell lines targeted to remove TRAF1, TRAF2, or both by homologous recombination, Xie et al. found that, while TRAF1 deficiency does not itself impair most CD40-mediated functions, a dual deficiency in TRAFs 1 and 2 impairs CD40 signaling to a considerably greater degree than a loss of TRAF2 alone. This suggests that TRAF1-TRAF2 interactions cooperate to enhance CD40 signals to B cells [13].
The role of TRAF5 in B cell CD40 functions is the least understood of all the TRAFs. To date, it has been very difficult to demonstrate direct association of TRAF5 with CD40 in B cells. It is possible that this association occurs indirectly via association with TRAF3 or TRAF2. CD40-induced B cell proliferation is decreased in TRAF5-/- mice [15], but a specific role for TRAF5 in in vivo CD40 functions has not yet been demonstrated. It may be that, similar to TRAF1-TRAF2 cooperation, TRAF5 interacts with additional TRAF molecules to regulate CD40 function, a hypothesis to be tested in future investigations.
Like TRAF1, TRAF6 appears to contribute to CD40 signaling to B cells in unique ways. TRAF6 directly binds CD40 via a membrane-proximal site distinct from the overlapping site used by other TRAFs [2], but recent evidence indicates that TRAF6 can also regulate CD40-induced JNK activation in B cells without binding to CD40 [16]. CD40 mutants that cannot bind TRAF6 show defects in B cell-mediated IL-6 production [17], and if expressed in TRAF2-/- B cell lines, cannot activate canonical NF-κB activation or CD80 upregulation [11]. In transgenic mice, such mutant molecules cannot mediate normal T-cell-dependent isotype switching or humoral memory (reviewed in [9]. However, CD40-mediated JNK activation in B cells apparently only requires cytoplasmic TRAF6 [16]. Thus, TRAF6 appears to contribute to CD40-mediated B cell regulation in multiple complex ways. It can function both bound to CD40 and unbound in the cytoplasm, and interacts with both TRAFs 2 and TRAF3.
2. LMP1
Latent membrane protein 1 (LMP1) is a multi-membrane spanning protein of 386 aa encoded by Epstein Barr virus (EBV), a gamma herpes virus that latently infects >90% of the human population. LMP1 is essential for EBV-mediated transformation of primary B cells and fibroblasts. (reviewed in [18]. LMP1 is a constitutively active mimic of CD40, and uses TRAFs for signaling (reviewed in [19, 20]). B cells are the major target of EBV, and due to the high rate of latent infection, nearly every human has some LMP1+ B cells. Additionally, most EBV associated B cell malignancies are LMP1+. However, initial work defining LMP1 signal transduction was performed mainly in transformed epithelial cell or mouse embryonic fibroblast (MEF) models. Recently, our laboratory has studied the TRAF requirements for LMP1 signaling in mouse B cell lines transfected with chimeric CD40-LMP1, using cell lines made deficient in TRAFs through gene targeting through somatic recombination [6, 11, 13, 21]. Use of these cell lines has helped to clarify the TRAF requirements for LMP1 signaling in B cells.
The cytoplasmic tail of LMP1 has two domains implicated in activation. The first, containing amino acids 194-232 (CTAR1), contains a TRAF binding site that has been shown to interact with TRAFs 1,2,3 and 5. The second activating domain, residues 351-386, (CTAR2) does not contain a TRAF binding site, though it does contain a PVQLSYYD site that when mutated may increase binding of TRAFs 1 and 2 [21]. The TRAF binding site in CTAR1 is PQQAT(DD). While PQQAT is a classical TRAF binding sequence, it has been shown that the second aspartic acid residue following the classical sequence also participates in stabilizing the interaction of LMP1-derived peptides and TRAF3 in crystallographic studies [22] Mutational analysis of the site has shown that single changes in any of the predicted contact residues indicated by underlining: PQQATDD decreased the ability of TRAF3 and LMP1 to co-immunoprecipitate [22]. It is of interest that EBV encoding LMP1 molecules with CTAR1 deleted are unable to transform primary B cells [23], implicating TRAF binding in this process.
TRAF1 is normally expressed at very low levels in lymphocytes, but can be induced by many B cell activating signals, including signals through LMP1 [24]. TRAF1 is expressed at high levels in LMP1+ lymphoma cells and in EBV-infected non-neoplastic lymphocytes, and LMP1 and TRAF1 co-localize in these cells [25]. Using B cell lines deficient in TRAF1, TRAF2, or both TRAF1 and 2, it was demonstrated that these TRAFs are not required for JNK phosphorylation, NFkB1 or NFkB2 activation in response to signals through LMP1, although these TRAFs do cooperate in CD40 signals to B cells [13]. This also contrasts with studies in 293 adenocarcinoma cells and TRAF2-/- MEFs implicating TRAF2 as a critical TRAF in LMP1 mediated NFkB activation [20, 26]. Thus, LMP1 signals to B cells may employ distinct TRAF usage, and as B cells are the principal target of EBV, it is important to understand how LMP1 signals specifically to B cells.
In addition to cell specificity, there is dramatic receptor specificity in how LMP1 uses TRAFs, in contrast to the CD40 receptor that LMP1 mimics. As mentioned above, unlike CD40, LMP1 signals to B cells are independent of TRAFs 1 and 2. TRAF3 negatively regulates CD40-activating signals to B cells [4, 6]. However, studies with TRAF3 deficient B cell lines made evident the central role TRAF3 plays in LMP1 signal transduction in B cells, as the presence of TRAF3 is required for JNK activation NFkB1 activation, IgM secretion and the upregulation of some surface molecules[6]. Not all LMP1 responses are dependent on TRAF3, as LMP1 in B cells deficient in TRAF3 can still activate ERK1,2, and Akt, and cytokine production is not affected [6]. TRAF5 has also been shown to associate with the PQAAT site in LMP1 [21]. To date, no specific investigation of the role of TRAF5 in LMP1 signal transduction in B cells has been reported.
TRAF6 does not appear to interact directly with LMP1 in B cells [21] or MEFs [27]. However, TRAF6 is required for signals through LMP1 in MEFs and epithelial cells [20, 27]. In a TRAF6 deficient B cell line, JNK activation through LMP1 is abrogated, though NFkB1 and NFkB2 activation is still observed (LLS & GAB, unpublished). Upregulation of CD80 induced by LMP1 signaling also requires the presence of full-length TRAF6 (LLS & GAB, unpublished). How does TRAF6 interact with LMP1? In adenocarcinoma overexpression models, TRAF6 has been hypothesized to interact with LMP1 indirectly through TRADD and/or RIP binding to CTAR2 (reviewed in [20]. More recently BS69 has been described as a potential bridging molecule between LMP1 CTAR2 and TRAF6 [28]. In mouse B cell lines, TRADD does not detectably interact with CTAR2 [21]. It is not yet known whether BS69 or RIP could serve to assist the binding of TRAF6 to LMP1 in B cells.
Signals through LMP1 are generally greater in magnitude and more prolonged than signals through CD40[29]. It is of interest that in comparison to CD40 signaling, which results in TRAF2 dependent degradation of TRAF2 and TRAF3[11] signaling through LMP1 does not result in TRAF degradation[29]. Thus, through both selective interaction with TRAFs, as well as subsequent TRAF modification, LMP1 makes unique use of this family of molecules to both mimic and dysregulate normal B cell signaling pathways.
3. TNF receptors
As the prototype members of the TNFR superfamily, TNF receptors 1 and 2 (CD120a and CD120b) are the best-studied of these receptors. However, the majority of studies have focused upon TNFR1/CD120a, while B lymphocytes express little to no detectable amounts of this receptor. B cells do express TNFR2/CD120b, and it has been known for many years that TNF has activating properties for B cells, but an understanding of how CD120b signals to B cells has been lacking.
Greater appreciation of the role of TRAF2 in CD120b signaling to B cells followed the puzzling finding that a truncated, “dominant negative” TRAF2 molecule could inhibit signaling not only through Wt CD40, but also via CD40 with a mutation in its TRAF2 binding site [4]. This suggested that CD40 could affect signaling via another TRAF2-utilizing receptor on B cells. It was known that B cells can both produce and respond to TNF [30]. It was subsequently discovered that CD40 can also induce B cell TNF secretion, which then signals to B cells via CD120b to enhance IgM production. This CD120b signal requires direct interaction with TRAF2 [11, 31].
The roles played by TRAF2 in CD120b signaling to B cells both overlap and contrast with its roles in CD40 signals. While TRAF2 is important to stimulating the CD40-mediated JNK pathway, CD120b-mediated JNK activation is not significantly affected by an absence of TRAF2 [32]. Both CD40 and CD120b can activate NF-κB pathways in B cells; CD40 can activate both the nuclear translocation of p50/p65 heterodimers (NF-κB1/canonical pathway), as well as the processing of p100 to p52 and the nuclear translocation of p52 and RelB (NF-κB2/alternative pathway). However, CD120b only stimulates NF-κB2 activation in B cells [32]. For both receptors, TRAF2 deficiency inhibits the induction of NF-κB activation [32], and antibody production [11].
TRAF1 also associates with TNFR2, and while its role in signaling to B cells by TNFR superfamily members appears to be primarily cooperative with TRAF2, TRAF1 deficiency also inhibits TNF-mediated IgM production [13]. Interestingly, while initial studies in other cell types did not demonstrate TRAF3 binding to TNF receptors, TRAF3 does directly associate with CD120b in B cells [32]. This is consistent with previous findings indicating that TNFR superfamily members that do not associate detectably with TRAF3 in other cell types can do so in B cells (reviewed in [9]. The specific roles played by TRAF3 in CD120b function in B cells remain to be investigated, but preliminary findings indicate that as for CD40, a deficiency in TRAF3 leads to enhanced CD120b signaling in B cells (GAB, unpublished data). Thus, TRAF3 may also negatively regulate these signals.
4. CD27
CD27 is a member of the TNF-R superfamily expressed by naïve and memory T lymphocytes in both mice and humans. In contrast to T cells, the expression pattern of CD27 by B lymphocytes is species-specific. In the human, CD27 is expressed on B cells following stimulation through the B cell antigen-receptor[33] and is expressed by memory B cells[34]. In the mouse, CD27 expression is extremely transient and exists only on centroblasts and on a very low frequency of memory B cells[35]. Data from the CD27 deficient mouse reveal that CD27 appears to have only a minor role in germinal center formation[35]. Other experiments in this mouse demonstrated that in the mouse, CD27 is more important in the generation of effector and memory T cells[36, 37]. In the human CD27 has been shown to be important for antibody production and plasma cell differentiation[38]. One possible mechanism is through the increased transcription of Blimp-1 and BSAP[39]. In vitro data suggests that CD27 signaling is important for isotype switching[39, 40] but it remains unclear whether this is due to CD27 signals directly driving plasma cell differentiation or providing survival signals to switched antibody secreting cells.
CD27 is capable of activating signal transduction pathways inducing activation of nuclear factor kappa-B (NF-κB)[15, 41] and JNK[15, 42] Like many other members of the TNF-R superfamily, CD27 activates both the classical (p65/RelA heterodimers) and alternative (p52/RelB heterodimers) NF-κB pathways[43, 44]. Regulation of the classical and alternative NF-κB activation is important because the activation of these two NF-κB pathways result in functionally distinct outcomes for the cell. The classical NF-κB pathway is involved in cellular activation[45], whereas the alternative pathway is linked to the initiation of pro-cell survival pathways[46] and in lymphatic tissue development[47].
TNF-receptor associated factors (TRAFs) are the most proximal signaling proteins in CD27 signaling and play an important role in the regulation of these two NF-κB pathways. The cytoplasmic tail of CD27 contains a single TRAF binding motif (PIQED) which is essential for the activation of NF-κB and JNK pathways[41, 48]. TRAF3 associates with CD27[41] and is a powerful regulator both the classical[6, 41] and alternative NF-κB pathways in B lymphocytes[7]. CD27 also associates with TRAF2[41, 48] and TRAF5[48] to activate the classical NF-κB pathway[48]. There are other proximal signaling proteins that have putative roles in CD27 function and may regulate or be regulated by TRAFs and in doing so could potentially regulate NF-κB signaling. One such protein is the cytoplasmic protein, NF-κB-inducing kinase (NIK). NIK is involved in the activation of these pathways but it is unclear how TRAFs interact with and influence NIK function[43]. Another protein often associated with CD27 function is the pro-apoptotic Siva. Siva exists in two splice variants and was first discovered in a yeast-two-hybrid screen using CD27 as bait[49]. A relationship between TRAFs and Siva has yet to be elucidated. More work is needed in order to determine whether TRAFs are involved in the regulation of NIK and Siva.
JNK activation by CD27 is facilitated by the recruitment of TRAF2 and TRAF5[42, 48]. As with activation of the classical NF-κB pathway, it appears that TRAF2 and TRAF5 share overlapping roles where one can compensate for the loss of the other. This is well demonstrated in the TRAF5 deficient mouse where NF-κB and JNK signaling remain intact when T cells are stimulated through CD27[15]. This redundancy may be species specific and more work is needed to determine whether TRAF5 and TRAF2 have distinct roles for CD27 signaling in human memory B cells.
5. CD30
CD30 is not expressed by resting B lymphocytes but is upregulated on the surface of B cells activated through CD40[50]. Work by Cerruti and colleagues demonstrated that engagement of CD30 on activated B cells negatively regulated isotype switching but had no effect on IgM secretion or cell survival[50]. An interesting structural aspect of CD30 is that the cytoplasmic tail of CD30 contains two TRAF binding motifs. One contained between residues 556-567 (PEQET) is similar to that of CD40 (PVQET) and binds TRAF3[51]. The other between residues 573-583 (VMLSVEE) resembles that of TNF-R2 (VPFSKEE) and binds TRAF1[51]. TRAF2 was shown to be able to bind to both sequences[51]. TRAF5 also has been reported to bind to CD30 and recognizes the PEQET site[52]. As with CD27, CD30 uses TRAF2 and TRAF5 to activate the NF-κB pathway and like many other TNF-R superfamily members, CD30 can activate both the classical and alternate NF-κB pathways[44, 52]. Another similarity with CD27 and other TNF-R superfamily members is that CD30 is capable of promoting both cell survival and apoptosis. Work by Duckett and colleagues showed that CD30 can make cells more sensitive to TNF-R1 mediated cell death[53]. This sensitivity to TNF-R1 was found to be due to CD30 mediated degradation of TRAF2[53]. While this is an interesting finding, the work was conducted entirely in epithelial cells and it remains to be seen if this occurs in B or T cells. While TRAF1 has been reported to bind to CD30, the role of this molecule remains mysterious. TRAF1 has been shown to be a positive regulator of NF-κB through the use of dominant-negative TRAF1 molecules expressed in non-lymphoid cell lines[54]. This study also showed that in addition to dominant-negative TRAF1 and TRAF2, full-length TRAF3 negatively effected NF-κB activation by CD30 suggesting that TRAF3 is a negative regulator of CD30 signaling[54].
There is one report linking increased TRAF1 expression to resistance to apoptosis in Hodgkin-Reed-Sternberg (HR-S) cells via CD30 signaling[55]. It will be interesting to see if this putative role for TRAF1 as an antiapoptotic factor in CD30 signaling holds up in B cells stimulated through CD30. The next step in this field is to elucidate the role of individual TRAFs in CD30 signaling in B lymphocytes. The introduction of various conditional TRAF knockout mice should help move these types of investigations forward.
6. Receptors for BAFF and APRIL
B cell activation factor (BAFF) is a fundamental survival factor during B cell maturation [56]. BAFF binds to three receptors of the TNF-R superfamily: B-cell maturation antigen (BCMA), transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), and BAFF receptor (BAFF-R) [56]. TACI and BCMA are also shared by a proliferation-inducing ligand (APRIL), another member of the TNF family that is closely related to BAFF [56]. APRIL also binds to heparin sulfate proteoglycan structures [56]. Dysregulation of BAFF and/or its receptors has been associated with a variety of human B cell disorders, including autoimmunity, malignancy, and immunodeficiency [57]. However, the signal transduction pathways of BAFF and its receptors in B cells are still being elucidated.
Interestingly, among the three receptors for BAFF, BAFF-R appears to be the sole mediator of the BAFF-mediated B cell survival signal, as only BAFF-R-/- mice resemble the phenotype of BAFF-/- mice, which display an almost complete loss of mature B lymphocytes and marginal zone B cells, and are deficient in mounting T-dependent humoral immune responses [56]. Available evidence indicates that BAFF-R interacts with TRAF3, but not other members of the TRAF family in B cells [56, 58]. A major outcome of BAFF-R stimulation is the activation of the alternative NF-κB (NF-κB2) pathway, which is known to be essential for the survival of primary B cells [56]. Recent evidence suggests that BAFF-R triggers this pathway by recruiting TRAF3, and thus inhibiting TRAF3 from binding to NF-κB-inducing kinase (NIK) [56, 59]. Interaction between TRAF3 and NIK induces proteasome-mediated degradation of NIK [56, 59]. Recruitment of TRAF3 by BAFF-R may thus allow NIK protein to accumulate and increase NF-κB processing. Consistent with this model, TRAF3-/- resting splenic B cells exhibit prolonged survival ex vivo and increased constitutive level of nuclear NF-κB2 [7]. Another prominent effect of BAFF-R signaling is the inhibition of the nuclear translocation of the proapoptotic protein kinase Cδ (PKCδ) [60]. Interestingly, deletion of TRAF3 from B cells leads to a decreased level of nuclear PKCδ [7]. However, how TRAF3 regulates PKCδ nuclear translocation awaits further investigation. Additionally, BAFF signals trigger Akt phosphorylation in primary B cells, which may also contribute to BAFF-mediated survival of B cells. Although it has been shown that PKCβ and PI3K are required for BAFF-mediated Akt activation [61], whether and how TRAF3 participates in this signaling pathway remain to be determined.
TACI appears to be a negative regulator of overall B cell activation and expansion, because TACI-/- mice display increased numbers of peripheral B cells, and eventually develop systemic lupus erythematosus (SLE)-like disorders and lymphoid cancers [56]. Paradoxically, T-independent type II antibody responses are impaired in these mice [56], and recent evidence indicates that this is due to requirement of TACI for efficient plasma cell differentiation in response to T-independent type II antigens [62]. In human, TACI mutations have been associated with common variable immunodeficiency [56]. Interaction between TACI and TRAF3 has been demonstrated in the B lymphoma cell line BJAB [58]. Overexpressed TACI co-immunoprecipitates with TRAF2, TRAF5 and TRAF6 in 293 epithelial cells [63]. Future study is needed to understand how TRAF molecules regulate TACI-mediated signaling events in B cells.
BCMA is preferentially expressed on plasma cells, plasmablasts, and tonsillar germinal center B cells. BCMA-/- mice are born with no major immune defect apart from impaired survival of some plasma cells in the bone marrow [56]. It has been shown that BCMA binds to TRAF1, 2, and 3 in COS7 fibroblasts [64]. Experiments using TRAF1-/-, TRAF2-/-, and TRAF3-/- plasma cells will be helpful in elucidating the functions of TRAFs in BAFF/BCMA-mediated plasma cell survival.
7. Toll-like receptors (TLRs)
TLRs are key sensors of microbial pathogen-derived molecules. B cells express TLRs 1-9 [65]. It has been shown that B cells can respond to a variety of TLR ligands/agonists, such as peptidoglycan (TLR2), poly I:C (TLR3), lipopolysaccharides (LPS, TLR4), flagellin (TLR5), R848 (TLR7/8) and CpG (TLR9) [65, 66]. B cell responses to stimulation by TLRs include proliferation, differentiation, cytokine secretion, up-regulation of adhesion and co-stimulatory molecules, antibody production, and Ig isotype switching [65, 66]. Furthermore, TLRs can synergize with BCR or CD40 to promote B cell activation, thus providing powerful protection against various microbial pathogens [65, 67, 68] However, in the case of co-ligation of TLRs and BCR by autoantigens (DNA or RNA containing complexes), autoantibody production and autoimmunity may develop [65].
The functions of TRAF6 in signaling by TLRs have been extensively studied in epithelial cells, macrophages, and dendritic cells [69, 70]. However, information regarding roles played by TRAF6 in B cell TLR-mediated signals is relatively limited. B cells from TRAF6-/- mice show impaired NF-κB activation and proliferation in response to LPS, a TLR4 agonist [71]. Using a TRAF6-/- B cell line, Rowland et al. recently reported that LPS-induced NF-κB and JNK activation is defective in TRAF6-/- B cells, and that the TRAF-C domain of TRAF6 is required for this function in LPS signaling [16]. Roles of TRAF6 in signaling by TLR2, 3, 5, 7/8, and 9 should be an important area for future research.
Involvement of TRAF3 in TLR signaling has just begun to be appreciated. By examining myeloid cells derived from chimeric mice reconstituted with TRAF3-/- fetal liver cells, two recent studies showed that TRAF3 is required in two distinct signaling pathways that lead to the production of type I interferons: (1) a TRIF-dependent pathway in macrophages via TLRs 3 and 4; (2) a MyD88-dependent pathway in plasmacytoid dendritic cells via TLRs 7/8 and 9 [72, 73]. In contrast, TRAF3 inhibits TLR signaling in B lymphocytes [66]. B cells from B cell-specific TRAF3-/- mice show enhanced responses to agonists for TLR3, 4, 7/8 and 9, including surface molecule up-regulation, cytokine production, and Ig isotype switching [66]. Detailed molecular mechanisms underlying TRAF3-mediated inhibition of TLR signaling in B cells are currently under investigation.
8. Constitutive functions of TRAF molecules in B cells
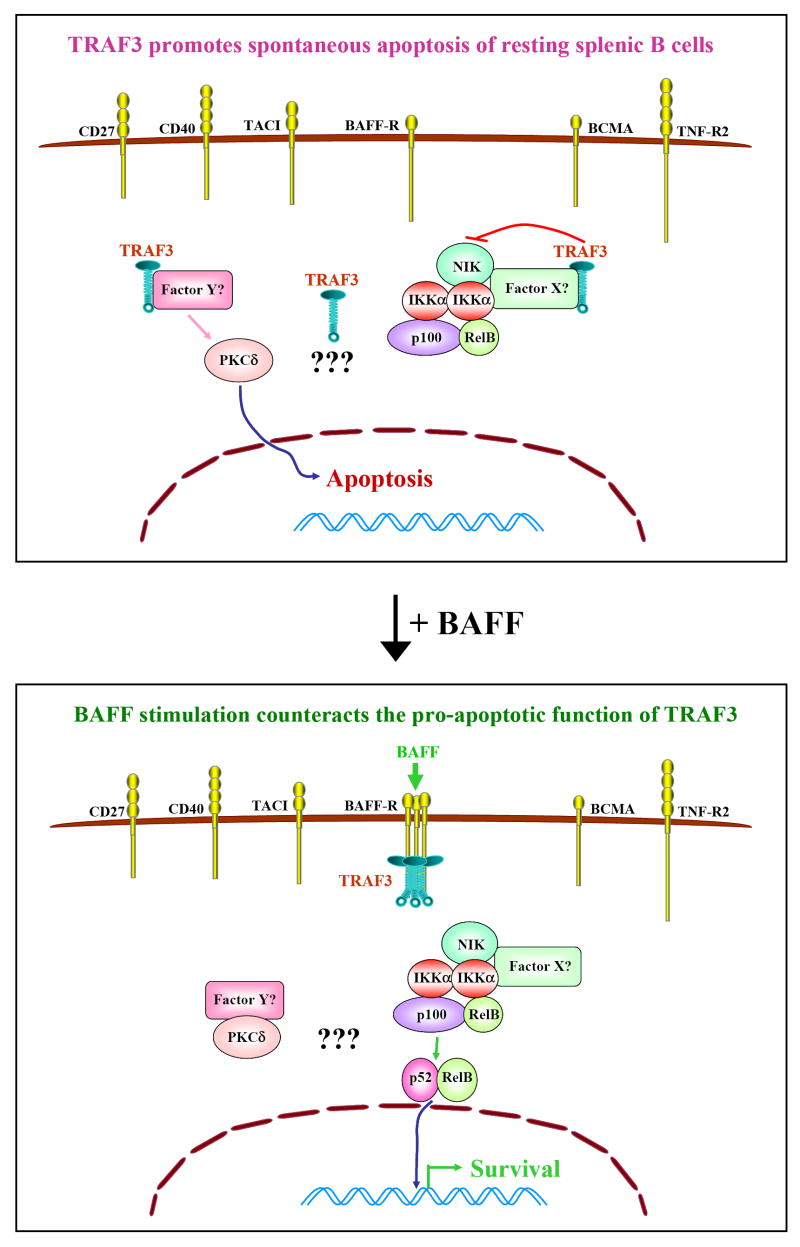
TRAF molecules were originally described as adaptor proteins that transduce signals from receptors of the TNF-R superfamily [9, 74]. Recent evidence suggests that TRAF molecules, at least TRAF3, may regulate B cell function in the absence of receptor engagement. Resting splenic B cells from B cell-specific TRAF3-/- mice exhibit remarkably prolonged survival ex vivo independent of BAFF, and show increased levels of active NF-κB2 but decreased levels of nuclear PKCδ in the absence of any stimulation. Co-culture of TRAF3-/- and littermate control B cells does not promote the survival of the latter, indicating that TRAF3-/- B cells do not spontaneously secret any survival factors such as BAFF or IL-6. Furthermore, in vivo administration of TACI-Ig, a soluble fusion protein that blocks both BAFF and APRIL from binding to their receptors, could not reverse peripheral B cell hyperplasia of B cell-specific TRAF3-/- mice [7]. Taken together, these results suggest that TRAF3 may constitutively inhibit NF-κB2 activation and promote PKCδ nuclear translocation to induce spontaneous apoptosis in peripheral B cells (depicted in Figure 1). Similarly, resting splenic B cells from a conditional TRAF2-/- mouse strain also show improved survival and higher levels of nuclear NF-κB2 ex vivo in the absence of stimuli [10]. Further experiments using TACI-Ig or BAFF-Ig, or alternatively, breeding conditional TRAF2-/- mice with BAFF-/- or BAFF-R-/- mice are required to determine whether TRAF2-mediated inhibition of B cell survival is downstream of BAFF/APRIL signaling or a constitutive TRAF2 function.
Figure 1. Constitutive function of TRAF3 in mature B cells.
Although six TRAF3-binding receptors are expressed on resting splenic B cells, the predominant function of TRAF3 not associated with receptors is to promote spontaneous apoptosis. In the absence of receptor engagement, TRAF3 is evenly distributed in the cytoplasm of B cells. However, TRAF3 may bind to the kinase NIK directly or indirectly through an unknown factor X, and thus inhib p100 processing. Meanwhile, TRAF3 may bind to another unknown Factor Y (or a multi-protein complex Y), and thus prevent factor Y from binding to PKCδ, allowing PKCδ to enter the nucleus. This explains why, in the absence of stimulation, mouse splenic B cells die by apoptosis. It is also possible that yet unknown pathways may be involved. Upon BAFF signaling, BAFF-R is trimerized or multimerized, and TRAF3 is recruited to the BAFF-R signaling complex in membrane rafts. Thus, BAFF signaling functions to remove TRAF3 from its cytoplasmic binding partners. Factor Y can then bind to PKCδ, and sequester it in the cytosol. Meanwhile, NIK can activate IKKα to process p100 into p52, allowing p52/RelB dimers to move into the nucleus and activate transcription of antiapoptotic proteins, leading to B cell survival.
Interestingly, no evidence of B cell hyperplasia was reported in chimeric mice reconstituted with TRAF3-/- fetal liver cells, although B cells from these mice do exhibit prolonged survival ex vivo and elevated constitutive NF-κB2 activation [3, 59]. Lack of peripheral B cell hyperplasia in such chimeric mice may be due to the following confounding issues: First, it is known that fetal liver cells most efficiently repopulate CD5+ B-1 cells, but have relatively poorer capacity to repopulate conventional B-2 (including follicular and marginal zone) B cells [75]. However, the predominantly expanded B cell populations in B cell-specific TRAF3-/- mice are conventional B-2 B cells. Second, TRAF3-/- mice display a reduced percentage of B220+IgM- B lineage precursor cells in the bone marrow. Similarly, chimeric mice reconstituted with TRAF3-/- fetal liver cells also show a partial reduction of B220+IgM- B lineage precursors in the bone marrow [3]. These data suggest that TRAF3 plays a role in early B lymphopoiesis, particularly from hematopoietic stem cell (HSC) to pro/pre-B (B220+IgM-) cell stages. Inasmuch as CD19Cre-mediated deletion of TRAF3 starts from the pro-B cell stage, but does not occur in earlier progenitor cells or HSC, the effect of TRAF3 on early B cell development could not overshadow its peripheral function in the B cell-specific TRAF3-/- mouse model, but may do so in the reconstituted TRAF3-/- chimeric mice. Third, in addition to B cells, all other hematopoietic cell lineages in reconstituted TRAF3-/- chimeric mice are also TRAF3 deficient, which may complicate the investigation of specific functions of TRAF3 in B cell homeostasis. Thus, TRAF3 may play distinct roles in progenitor B cells in the bone marrow versus in mature B cells in secondary lymphoid organs. How TRAF3 regulates early B cell development awaits further investigation.
Biographies

Gail A. Bishop earned the Ph.D. from the University of Michigan, followed by postdoctoral work at the University of North Carolina. She has been a member of the University of Iowa faculty since 1989, where she is currently Distinguished Professor of Microbiology, and Holden Chair of Cancer Biology. She also serves as Director of the Immunology Program and Associate Director for Basic Science Research of the Comprehensive Cancer Center. She is a current member of the NIH Tumors, Tolerance and Transplantation study section. She is also a member of the American Association of Immunologists Council, and a Council Delegate (Medical Sciences) to the AAAS.

Zach Kraus graduated from California State University at Chico with a B.S. in Microbiology. He joined Gail Bishop?s laboratory in the spring of 2004 where his primary interest is in the role of TNF-receptor superfamily members in T cell costimulation. He is currently funded by an American Heart Association predoctoral fellowship.

Ping Xie earned the Ph.D. from the Hong Kong University of Science & Technology, followed by postdoctoral work at the University of Illinois at Chicago and the University of Iowa. She is currently an Assistant Research Scientist at the University of Iowa. She was a Special Fellow of the Leukemia & Lymphoma Society from 2002-2005. Her research is currently supported by a National Scientist Development Grant from the American Heart Association.

Laura L. Stunz received her Ph.D. from the University of Iowa, Dept. of Microbiology in 1986. After postdoctoral work at the same institution in the Department of Internal Medicine, she became a research investigator with appointments at the DVA Medical Center in Iowa City and in the Department of Internal Medicine at the U of Iowa. Dr. Stunz joined Dr. Bishop’s laboratory in 2001 as an Associate Research Scientist. Dr. Stunz is a member of the AAI, and has served as an associate editor for the Journal of Immunology. She also has served as a Councilor for the Autumn Immunology Conference.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine & Growth Factor Reviews. 2003;14:297–309. doi: 10.1016/s1359-6101(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 2.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. In: Wu H, editor. TRAFs. New York: Landes Biosciences; 2007. in press. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–15. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 4.Hostager BS, Bishop GA. Cutting Edge: Contrasting roles of TRAF2 and TRAF3 in CD40-mediated B lymphocyte activation. J Immunol. 1999;162:6307–11. [PubMed] [Google Scholar]
- 5.Haxhinasto SA, Bishop GA. A novel interaction between PKD and TRAFs regulates BCR-CD40 synergy. J Immunol. 2003;171:4655–62. doi: 10.4049/jimmunol.171.9.4655. [DOI] [PubMed] [Google Scholar]
- 6.Xie P, Hostager BS, Bishop GA. Requirement for TRAF3 in signaling by LMP1, but not CD40, in B lymphocytes. J Exp Med. 2004;199:661–71. doi: 10.1084/jem.20031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. TRAF3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–67. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh W, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, et al. Early lethality, functional NF-kB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–25. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 9.Bishop GA. The multifaceted roles of TRAFs in the regulation of B cell function. Nat Rev Immunol. 2004;4:775–86. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 10.Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-κB activation in mature B cells. Immunity. 2004;21:629–42. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Hostager BS, Haxhinasto SA, Rowland SR, Bishop GA. TRAF2-deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling. J Biol Chem. 2003;278:45382–90. doi: 10.1074/jbc.M306708200. [DOI] [PubMed] [Google Scholar]
- 12.Moore CR, Bishop GA. Differential regulation of CD40-mediated TRAF degradation in B lymphocytes. J Immunol. 2005;175:3780–9. doi: 10.4049/jimmunol.175.6.3780. [DOI] [PubMed] [Google Scholar]
- 13.Xie P, Hostager BS, Munroe ME, Moore CR, Bishop GA. Cooperation between TRAFs 1 and 2 in CD40 signaling. J Immunol. 2006;176:5388–400. doi: 10.4049/jimmunol.176.9.5388. [DOI] [PubMed] [Google Scholar]
- 14.Tsitsikov E, Laouini D, Dunn IF, Sannikova TY, Davidson L, Alt FW, et al. TRAF1 is a negative regulator of TNF signaling: enhanced TNF signaling in TRAF1-deficient mice. Immunity. 2001;15:647–57. doi: 10.1016/s1074-7613(01)00207-2. [DOI] [PubMed] [Google Scholar]
- 15.Nakano H, Sakon S, Koseki H, Takemori T, Tada K, Matsumoto M, et al. Targeted disruption of Traf5 gene causes defects in CD40 and CD27-mediated lymphocyte activation. Proc Natl Acad Sci (USA) 1999;96:9803–8. doi: 10.1073/pnas.96.17.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowland SR, Tremblay ML, Ellison JM, Stunz LL, Bishop GA, Hostager BS. A novel mechanism for TRAF6-dependent CD40 signaling. J Immunol. 2007;179:4645–53. doi: 10.4049/jimmunol.179.7.4645. [DOI] [PubMed] [Google Scholar]
- 17.Jalukar SV, Hostager BS, Bishop GA. Characterization of the roles of TRAF6 in CD40-mediated B lymphocyte effector functions. J Immunol. 2000;164:623–30. doi: 10.4049/jimmunol.164.2.623. [DOI] [PubMed] [Google Scholar]
- 18.Bishop GA, Busch LK. Molecular mechanisms of B lymphocyte transformation by Epstein-Barr virus. Microbes Infec. 2002;4:853–7. doi: 10.1016/s1286-4579(02)01605-2. [DOI] [PubMed] [Google Scholar]
- 19.Bishop GA, Hostager BS. Signaling by CD40 and its mimics in B cell activation. Immunol Res. 2001;24:97–109. doi: 10.1385/IR:24:2:097. [DOI] [PubMed] [Google Scholar]
- 20.Soni V, Cahir-McFarland E, Kieff E. LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol. 2007;597:173–87. doi: 10.1007/978-0-387-70630-6_14. [DOI] [PubMed] [Google Scholar]
- 21.Xie P, Bishop GA. Roles of TRAF3 in signaling to B lymphocytes by CTAR regions 1 and 2 of the EBV-encoded oncoprotein LMP1. J Immunol. 2004;173:5546–55. doi: 10.4049/jimmunol.173.9.5546. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Xie P, Welsh K, Li C, Ni C, Zhu X, et al. LMP1 protein from EBV is a structural decoy in B lymphocytes for binding to TRAF3. J Biol Chem. 2005;280:33620–6. doi: 10.1074/jbc.M502511200. [DOI] [PubMed] [Google Scholar]
- 23.Izumi KM, Kaye KM, Kieff ED. The EBV LMP1 amino acid sequence that engages TRAFs is critical for primary B lymphocyte growth transformation. Proc Natl Acad Sci (USA) 1997;94:1447–52. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosialos G, Birkenback M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The EBV transforming protein LMP1 engages signaling proteins for the TNF-R family. Cell. 1995;80:389–99. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 25.Siegler G, Kremmer E, Gonnella R, Niedobitek G. Epstein-Barr virus encoded latent membrane protein 1 (LMP1) and TNF receptor associated factors (TRAF): colocalisation of LMP1 and TRAF1 in primary EBV infection and in EBV associated Hodgkin lymphoma. Mol Pathol. 2003;56:156–61. doi: 10.1136/mp.56.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaye KM, Devergne O, Harada JN, Izumi KM, Yalamanchili R, Kieff E, et al. TRAF2 is a mediator of NF-κB activation by LMP1, the EBV transforming protein. Proc Natl Acad Sci (USA) 1996;93:11085–90. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultheiss U, Püschner S, Kremmer E, Mak TW, Engelmann H, Hammerschmidt W, et al. TRAF6 is a critical mediator of signal transduction by the viral oncogene LMP1. EMBO J. 2001;20:5678–91. doi: 10.1093/emboj/20.20.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan J, Zhang W, Wu L, Bai T, Zhang M, Lo K, et al. BS69, a specificadaptor in the LMP1-mediated JNK pathway. Mol Cell Biol. 2006;26:448–56. doi: 10.1128/MCB.26.2.448-456.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown KD, Hostager BS, Bishop GA. Differential signaling and TRAF degradation by CD40 and the EBV oncoprotein LMP1. J Exp Med. 2001;193:943–54. doi: 10.1084/jem.193.8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieckmann P, D’Allessandro F, Nordan RP, Fauci AS, Kehrl JH. IL-6 and TNF-α. Autocrine and paracrine cytokines involved in B cell function. J Immunol. 1991;146:3462–8. [PubMed] [Google Scholar]
- 31.Hostager BS, Bishop GA. Role of TRAF2 in the activation of IgM secretion by CD40 and CD120b. J Immunol. 2002;168:3318–22. doi: 10.4049/jimmunol.168.7.3318. [DOI] [PubMed] [Google Scholar]
- 32.Munroe ME, Bishop GA. Role of TRAF2 in distinct and overlapping CD40 and TNFR2/CD120b-mediated B lymphocyte activation. J Biol Chem. 2004;279:53222–31. doi: 10.1074/jbc.M410539200. [DOI] [PubMed] [Google Scholar]
- 33.Maurer D, Holter W, Majdic O, Fischer GF, Knapp W. CD27 expression by a distinct subpopulation of human B lymphocytes. Eur J Immunol. 1990;20:2679–84. doi: 10.1002/eji.1830201223. [DOI] [PubMed] [Google Scholar]
- 34.Klein U, Rajewsky K, Küppers R. Human IgM+IgD+ peripheral blood B cells expressing the CD27 surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Y, Hendricks J, Langerak P, Jacobs H, Borst J. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J Immunol. 2004;172:7432–41. doi: 10.4049/jimmunol.172.12.7432. [DOI] [PubMed] [Google Scholar]
- 36.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–80. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–40. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 38.Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman SF. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses. J Immunol. 1997;159:2652–7. [PubMed] [Google Scholar]
- 39.Nagumo H, Agematsu K, Shinozaki K, Hokibara S, Ito S, Takamoto M, et al. CD27/CD70 interaction aguments IgE secretion by promoting the differentiation of memory B cells into plasma cells. J Immunol. 1998;161:6496–502. [PubMed] [Google Scholar]
- 40.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman SF, Morimoto C. CD27-CD70 interactions regulate B-cell activation by T cells. Proceedings of the National Academy of Sciences (USA) 1995;92:11249–53. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto H, Kishimoto T, Minamoto S. NF-kB activation in CD27 signaling: Involvement of TRAFs in its signaling and identification of functional region of CD27. J Immunol. 1998;161:4753–9. [PubMed] [Google Scholar]
- 42.Gravestein LA, Amsen D, Boes M, Calvo CR, Kuisbeek AM, Borst J. The TNFR family member CD27 signals to JNK via TRAF2. Eur J Immunol. 1998;28:2208–16. doi: 10.1002/(SICI)1521-4141(199807)28:07<2208::AID-IMMU2208>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 43.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and canonical NF-κB activation pathways by NIK. Immunity. 2004;21:477–89. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Hauer J, Püschner S, Ramakrishnan P, Simon U, Bongers M, Federle C, et al. TRAF3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci (USA) 2005;102:2874–9. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–30. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 46.Viatour P, Bentires-Alj M, Chariot A, Deregowski V, de Leval L, Merville MP, et al. NF- kappa B2/p100 induces Bcl-2 expression. Leukemia. 2003;17:1349–56. doi: 10.1038/sj.leu.2402982. [DOI] [PubMed] [Google Scholar]
- 47.Weih DS, Yilmaz ZB, Weih F. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J Immunol. 2001;167:1909–19. doi: 10.4049/jimmunol.167.4.1909. [DOI] [PubMed] [Google Scholar]
- 48.Akiba H, Nakano H, Nishinaka S, Sindo M, Kobata T, Tasuta C, et al. CD27, a member of the TNF-R superfamily, activates NF-κB and SAPK/JNK via TRAF2, TRAF5, and NIK. J Biol Chem. 1998;273:13353–8. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 49.Prasad KVS, Ao Z, Yoon Y, Wu MX, Rizk M, Jacquot S, et al. CD27, a member of the TNFR family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci (USA) 1997;94:6346–51. doi: 10.1073/pnas.94.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerutti A, Schaffer A, Shah S, Zan H, Liou H, Goodwin RG, et al. CD30 is a CD40-inducible molecule that negatively regulates CD40-mediated Ig class switching in non-antigen-selected human B cells. Immunity. 1998;9:247–56. doi: 10.1016/s1074-7613(00)80607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boucher LM, Marengere LE, Lu Y, Thukral S, Mak TW. Binding sites of cytoplasmic effectors TRAF1, 2, and 3 on CD30 and other members of the TNF receptor superfamily. Biochem Biophys Res Commun. 1997;233:592–600. doi: 10.1006/bbrc.1997.6509. [DOI] [PubMed] [Google Scholar]
- 52.Aizawa S, Nakano H, Ishida T, Horie R, Nagai M, Ito K, et al. TRAF5 and TRAF2 are involved in CD30-mediated NF-κB activation. J Biol Chem. 1997;272:2042–5. doi: 10.1074/jbc.272.4.2042. [DOI] [PubMed] [Google Scholar]
- 53.Duckett CS, Thompson CB. CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Devel. 1997;11:2810–21. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duckett CS, Gedrich RW, Gilfillan MC, Thompson CB. Induction of NF-kB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol. 1997;17:1535–42. doi: 10.1128/mcb.17.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durkop H, Hirsch B, Hahn C, Foss HD, Stein H. Differential expression and function of A20 and TRAF1 in Hodgkin lymphoma and anaplastic large cell lymphoma and their induction by CD30. Am J Pathol. 2003;200:229–39. doi: 10.1002/path.1351. [DOI] [PubMed] [Google Scholar]
- 56.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–36. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18:305–17. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Xu L, Shu H. TRAF3 is associated with BAFF-R and negatively regulates BAFF-R-mediated NF-κB activation and IL-10 production. J Immunol. 2002;169:6883–9. doi: 10.4049/jimmunol.169.12.6883. [DOI] [PubMed] [Google Scholar]
- 59.He JQ, Zarnegar B, Oganesyan G, Saha SK, Yamazaki S, Doyle SE, et al. Rescue of TRAF3-null mice by p100 NF-κB deficiency. J Exp Med. 2006;203:2413–8. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mecklenbräuker I, Kalled SL, Leitges M, Mackay CR, Tarakhovsky A. Regulation of B cell survival by BAFF-dependent PKCδ-mediated nuclear signaling. Nature. 2004;431:456–61. doi: 10.1038/nature02955. [DOI] [PubMed] [Google Scholar]
- 61.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203:2551–62. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. 2007;179:2282–8. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 63.Xia X, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M, et al. TACI is a TRAF-interacting receptor for TALL-1, a TNF family member involved in B cell regulation. J Exp Med. 2000;192:137–43. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hatzoglou A, Roussel J, Bourgeade MF, Rogier E, Madry C, Inoue J, et al. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J Immunol. 2000;165:1322–30. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- 65.Gray D, Gray M, Barr T. Innate responses of B cells. Eur J Immunol. 2007 doi: 10.1002/eji.200737728. [DOI] [PubMed] [Google Scholar]
- 66.Xie P, Brown L, Stunz L, Bishop G. TRAF3 inhibits signaling by Toll-like receptors in B lymphocytes. J Immunol. 2008 in press. [Google Scholar]
- 67.Bishop GA, Ramirez LM, Baccam M, Busch LK, Pederson LK, Tomai MA. The immune response modifier, Resiquimod, mimics CD40-induced B cell activation. Cell Immunol. 2001;208:9–17. doi: 10.1006/cimm.2001.1769. [DOI] [PubMed] [Google Scholar]
- 68.Vanden Bush T, Bishop GA. TLR7 and CD40 cooperate in IL-6 production via enhanced JNK and AP-1 activation. Eur J Immunol. doi: 10.1002/eji.200737602. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi Y. Role of TRAF6 in the immune system. Adv Exp Med Biol. 2005;560:77–82. doi: 10.1007/0-387-24180-9_11. [DOI] [PubMed] [Google Scholar]
- 70.Inoue J, Gohda J, Akiyama T. Characteristics and biological functions of TRAF6. Adv Exp Med Biol. 2007;597:72–9. doi: 10.1007/978-0-387-70630-6_6. [DOI] [PubMed] [Google Scholar]
- 71.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective IL-1, CD40, and LPS signaling. Genes Devel. 1999;13:1015–21. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Häcker H, Redecke V, Blagoev B, Kratchmarova I, Hsu L-C, Wang GG, et al. Specificity in TLR signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–7. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 73.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, et al. Critical role of TRAF3 in the TLR-dependent and independent antiviral response. Nature. 2006;439:208–11. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 74.Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, et al. Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res. 2000;254:14–24. doi: 10.1006/excr.1999.4733. [DOI] [PubMed] [Google Scholar]
- 75.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–9. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]