Abstract
Recent studies have demonstrated nicotinamide (NAM), a soluble B-group vitamin, to be an effective treatment in experimental models of TBI. However, research on this compound has been limited to administration regimens starting shortly after injury. This study was conducted to establish the window of opportunity for NAM administration following controlled cortical impact (CCI) injury to the frontal cortex. Groups of rats were assigned to NAM (50 mg/kg), saline (1 ml/kg), or sham conditions and received contusion injuries or sham procedures. Injections of NAM or saline were administered at 15 min, 4 hrs, or 8 hrs post-injury, followed by five boosters at 24 hr intervals. Following the last injection, blood was taken for serum NAM analysis. Animals were tested on a variety of tasks to assess somatosensory performance (bilateral tactile adhesive removal and vibrissae-forelimb placement) and cognitive performance (reference and working memory) in the Morris water maze. The results of the serum NAM analysis showed that NAM levels were significantly elevated in treated animals. Behavioral analysis on the tactile removal test showed that all NAM-treated groups facilitated recovery of function compared to saline treatment. On the vibrissae-forelimb placing test all NAM-treated groups also were significantly different from the saline-treated group. However, the acquisition of reference memory was only significantly improved in the 15-min and 4-hr groups. In the working memory task both the 15-min and 4-hr groups also improved working memory compared to saline treatment. The window of opportunity for NAM treatment is task-dependent and extends to 8 hrs for the sensorimotor tests but only extends to 4 hrs post-injury in the cognitive tests. These results suggest that a 50 mg/kg treatment regimen starting at the clinically relevant time point of 4 hrs may result in attenuated injury severity in the human TBI population.
Keywords: traumatic brain injury, therapy, window of opportunity, vitamin, recovery of function
Conservative estimates place the incidence rate of traumatic brain injuries (TBI) at 1.4 million a year in the United States; of these, 235,000 require prolonged hospitalization and 50,000 individuals will die (Langlois et al., 2006). Those sustaining severe, moderate, or repeated mild TBI's acquire enduring emotional, motor, sensory, and/or cognitive deficits. Exacerbating this public health crisis is the fact that no therapeutics are currently available to attenuate secondary damage following TBI. As research on potential treatments has progressed more and more emphasis has been placed on determining the widow of opportunity for administration of novel therapeutics (Narayan et al., 2002). Following TBI, patients may not receive medical intervention for several hours because of the remoteness of accident site, logistics of removing the patient from the accident site (e.g., vehicle accidents), and stabilization of patient (e.g., intubation, internal bleeding) (Gilligan et al., 1999). Thus, it is critical that a novel therapeutic has a wide window opportunity if it is to translate from animal to human models of TBI.
Nicotinamide (NAM; vitamin B3) has been shown to be effective in attenuating behavioral and cortical pathology following multiple models of TBI and stroke. It was first shown that administration of NAM following permanent middle cerebral artery occlusion was effective at reducing infarction volumes in male rats (Ayoub et al., 1999). This study began treatment 1 hr prior to ischemia onset and tested the effectiveness of three doses: 50 mg/kg, 500 mg/kg, and 1000 mg/kg. Histological data revealed a inverted U-shaped distribution with the 50 and 1000 mg/kg groups failing to show neuroprotection as assessed by infarction volume. The 500 mg/kg group showed a significant reduction in infarction volume relative to saline 24 hrs post-stroke. A single dose of 500 mg/kg of NAM was found to have a window of opportunity that extended to 2 hrs, but not 3 or 4 hrs post-stroke using a measure of infarction volume (Ayoub et al., 1999). In another study, a 500 mg/kg dose was the most effective at reducing infarction volume; the window of opportunity remained at 2 hrs (Sakakibara et al., 2000). It was later demonstrated that the window of opportunity extended to 4 hrs post-stroke following transient MCAo with the 500 mg/kg dose as measured by infarction and neuroscore at 7 days post-stroke (Ayoub and Maynard, 2002).
Recently, NAM has been demonstrated to be effective in preclinical models of TBI. Hoane, Akstulewicz, & Toppen (2003) showed that NAM was effective at improving behavioral and histological outcome following TBI. Animals received bilateral cortical contusion injury (CCI) to the medial prefrontal cortex and were administered NAM (500 mg/kg) at 15 min with a booster at 24 hrs post-injury. Behavioral evaluation revealed that NAM-treated animals were significantly less impaired than saline-treated animals in sensorimotor (bilateral tactile adhesive removal task) and cognitive measures (Morris water maze). NAM was also shown to decrease the size of the lesion cavity and down regulate the glial response (Hoane et al., 2003).
NAM has also been shown to be effective in attenuating behavioral and histological measures at two different doses in a diffuse model of TBI: fluid percussion injury (FPI) {Hoane, 2006 #65}. Animals received FPI injuries and were treated with either 500 mg/kg or 50 mg/kg NAM at 15 mins post-injury with a 24 hr booster. Behavioral evaluation over 35 testing days showed significant improvements in performance relative to saline-treated animals on measures of sensorimotor performance (bilateral tactile adhesive removal task), sensorimotor integration (vibrissae-forelimb placing), and motor functioning (beam task). The 500 mg/kg treatment condition was effective in attenuating cognitive dysfunction (MWM), but the 50 mg/kg was not. Histologically, both treatment conditions significantly reduced cavity size and glial cell proliferation relative to saline-treated animals (Hoane et al., 2006c). A similar dose-effect with the 50 and 500 mg/kg doses of NAM were seen in their ability to reduce astrogliosis and neurodegeneration acutely following FPI (Holland et al., 2008).
Despite promising results from multiple models of cerebral insult, it remains to be determined how long post-TBI NAM is able to elicit these beneficial effects. This study was conducted to determine the window of opportunity for NAM administration following bilateral controlled cortical impact of the frontal lobes. While the 500 mg/kg dose of NAM has been repeatedly shown to be the most effective following trauma, it is unlikely this dose will ever be introduced in the human condition. Thus, the more clinically relevant dose of NAM at 50 mg/kg with 5 days of 24 hr boosters was evaluated in its ability to promote recovery of function following delayed treatment onset.
Experimental Procedures
Subjects
Thirty four Sprague-Dawley rats (Harlan, Indianapolis, IN, USA), 3−4 months of age were included in this experiment. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee and the study was conducted in a facility certified by the American Association for the Accreditation of Laboratory Animal Care. Rats were maintained on a standard 12 hr light/dark cycle with food and water available ad lib.
Surgery
The surgical procedure was performed using aseptic techniques and conditions. The CCI model utilized in the present study was based on previous studies (Hoane et al., 2003). Animals were anesthetized using a mixture of Isoflurane (5 − 2.5 %) and oxygen (0.8 L/min). When the animal became unresponsive (no ocular or pedal reflexes) the head was shaved and scrubbed with 70% alcohol and placed into a stereotaxic device. A midline incision was made in the skin and underlying fascia reflected. A circular craniotomy (6.0 mm) was performed using a Dremel tool and a specially designed drill bit that prevented the bit from damaging the meninges and cortex. The craniotomy was bilateral and centered 2.5 mm anterior to bregma exposing the cortical region containing the medial prefrontal cortex. The contusion injury was created with a sterile, stainless steel impactor tip (5.0 mm in diameter) that was attached to a magnetically activated piston (www.myneurolab.com, St. Louis, MO, USA). The impact tip traveled at a velocity of 2.76 m/s compressing the cortex to a depth of 2.5 mm during 0.5 sec of contact. Following contusion any bleeding was controlled with sterile sponges soaked in cold saline and the incision was closed with nylon suture material. To maintain normal body temperature during surgery and recovery the rats were maintained on isothermic (EZ Anesthesia, Palmer, PA, USA) heating units (37°C). Rats receiving sham surgeries underwent identical surgical prep as injured animals, received craniotomies, and were then sutured.
Drug Administration
Following injury treated animals received NAM injections (50 mg/kg, i.p.) (Sigma # N3376, St. Louis, MO, USA) at 15 min (n = 7), 4 hr (n = 7), or 8 hr (n = 7) post-injury, followed by five boosters (50 mg/kg, i.p.) at 24 hr intervals (Hoane et al., 2006c). Sham (n = 6) and injured-control (n = 7) animals received saline injections (0.9%, 1.0 mL/kg, i.p.) 15 min post-injury followed by five saline (0.9%, 1.0 mL/kg, i.p.) boosters at 24 hr intervals. All behavioral testing and histological analysis were carried out by experimenters blinded to treatment conditions.
NAM Assay
Based on a previous sampling study, 1 hr following the last injection, blood was collected from the tail vein (Hoane et al., 2006c). Samples were separated with a microcentrifuge and stored frozen at −80°C. NAM serum concentrations were determined with a modification of a published protocol using high pressure liquid chromatography (HPLC) on a Varian Pro Star 210 HPLC system (Gillmor et al., 1999). Briefly, the internal standard, 6-methylNAM was added to 0.1 mL serum sample and to serum standard samples. The samples were extracted on a Varian Bond Elute C18 extraction column using three extractions of 0.3 mLs 35% methanol and 40 mM ammonium acetate (pH 5.8) and acetonitrile. The gradient was run as 7% acetonitrile for 12 mins, 75% acentanitrile for 7 mins and then 7% acetonitrile for 7 mins at the UV detection of 262 nm. The retention times of NAM and 6-methylNAM were 6.3 mins and 12.2 mins, respectively.
Bilateral Tactile Adhesive Removal Test
This test has been previously shown to be a sensitive assessment of somatosensory and attentional deficits following injury to the frontal lobes and has been described in detail (Schallert et al., 2000, Hoane et al., 2003, Schallert and Woodlee, 2005, Hoane et al., 2006c, Hoane et al., 2007). A slightly modified version of this task was used in this experiment. A small rectangular patch (105 mm2) (Avery, product #05412) was applied to the radial aspect of each forelimb. The rat was returned to the home cage and the latency and order of contact and removal (right vs. left) of the stimuli was recorded. A trial ended when the rat either removed both patches or 2 minutes elapsed. Baseline latencies were recorded prior to CCI after which animals were tested on two trials per testing day (ITI 5 min), on post-operative days 2, 4, 6, 8, 10, 14, and 21.
Vibrissae - Forelimb Placing
Sensorimotor function was evaluated by scoring the forelimb placing reaction (Schallert et al., 2000, Schallert and Woodlee, 2005, Hoane et al., 2006c, Hoane et al., 2007). Each rat was held by the trunk ensuring the forelimbs were free to move. One side of the rat was oriented parallel to a Plexiglas surface and was slowly moved until the vibrissae on one side touched the surface. In intact rats, a reliable lateralized placing response was elicited each time the vibrissae made contact with the surface. A successful forelimb placing response was recorded if the animal raised its forelimb and placed it on the surface in response to stimulation of the vibrissae ipsilateral to the forelimb. Each rat was given 10 trials for each forelimb. If a placing response was not elicited within 5 sec of vibrissae stimulation, the trial was recorded as unsuccessful. Baseline performance was recorded prior to injury. The animals were tested on days 2, 4, 6, 8, 10, 14, and 21.
Cognitive Assessment
The MWM has been widely utilized to assess cognitive performance following brain injury (Lindner et al., 1998, Hoane et al., 2003). A 1.5 m diameter blue fiberglass pool was filled with water (24°C) to a depth of 32 cm and a 10 cm2 clear Plexiglas platform was submerged 1 cm below the surface of the water. Path length and latency to escape measures were recorded by a computerized video tracking system with SMART tracking software (San Diego Instruments, CA, USA).
All the animals were assessed on the acquisition of a reference memory task beginning on day 11−14 post-injury. Each rat was given 4 trials a day, starting from one of four release points in random order. The trial was terminated when the rat reached the submerged platform located in the center of the north-east quadrant, or when 90 sec had elapsed. Animals not reaching the platform were guided to the platform after the trial had ended. Each rat was allowed to remain on the platform for 15 sec after which it was placed in a warm holding cage for 8 min before the next trial.
Working memory performance was tested on days 15−17 post-injury using established methods (Lindner et al., 1998, Hoane et al., 2003). The platform was submerged at the center of a new randomly chosen quadrant (south-west, north-west, and south-east) each day. Each animal was given four trials per day, starting from one of four randomly chosen release points (ITI 8 min). The first trial on each of these three days was considered an information trial and was not included in subsequent analyses. Each trial ended when the animal located the platform, or when 90 sec had elapsed. Animals not reaching the platform were guided to the platform after the trial had ended.
Histology
At 30 days post-injury, rats were anesthetized with urethane (3.0 g/kg, 0.5g/ml, i.p.) and transcardially perfused with 0.9% phosphate buffered saline (PBS) followed by 10% phosphate buffered formalin (PBF). Brains were post-fixed in PBF following removal from the cranium for 48 hr and stored at 4°C. Brains were then processed in a Tissue TEK VIP tissue processor using a standard protocol for rodent brain. Paraffin embedded brains were sectioned at 8 μm on a Leica RM2125 rotary microtome and serial sections were mounted on electrostatically charged microscope slides. Immediately prior to staining, slides were deparaffinized in two 7 min washes of xylene, then transferred through a series of EtOH baths (2 × 5 min in 100%; 3 min each at 95%, 70%, and 50%), followed by a 1 min rinse in distilled H2O (dH2O) (Holland et al., 2008).
Lesion Analysis
A series of deparaffinized slides were stained with cresyl violet, dehydrated and cover slipped. The extent of the lesion was analyzed with an Olympus microscope (BX-51) and an Olympus 13.5 megapixel camera (DP-70). Images of the sections throughout the extent of the injury coordinates were captured using the digital capturing system and area measures of the lesioned tissue were determined using ImageTool software. The Cavalieri method was used to calculate remaining cortical volume (Coggeshall, 1992). The number of sections and the section thickness (8 μm) were multiplied by the mean area of remaining cortex calculated at 3 coordinates through the injury site (+2.50, +1.00, and −0.40 relative to bregma) (Paxinos and Watson, 2005). The extent of cortical injury was assessed by comparing remaining tissue volumes from the site of injury across groups (Hoane et al., 2003, Hoane et al., 2005).
Statistical Analysis
Analysis of variance (ANOVA) tests were performed using procedures for general linear models (SPSS 15.0 for Windows) with options for repeated measures where appropriate for all behavioral and cognitive measures. Between group factors were group (15-min NAM, 4-hr NAM, 8-hr NAM, saline, and sham). The within group factor was day of testing. Huynh-Feldt probabilities (HFP) and Fischer's least significant difference test (LSD) were used to correct for Type-1 error associated with repeated measures and post hoc mean comparisons, respectively. For all HFP corrections the actual corrected degrees of freedom were used and reported. Anatomical and serum data were analyzed by one-way ANOVA procedures with appropriate post-hoc examinations. A significance level of p < 0.05 was used for all statistical analyses. One animal in the sham group died following surgery and was not replaced.
Results
NAM Analysis
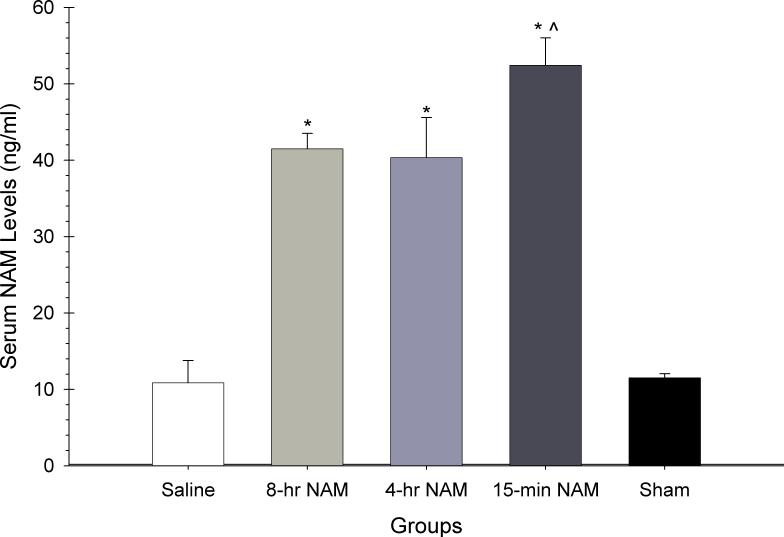
There were significant serum NAM concentration differences between groups as analyzed by a one-way ANOVA [F(4,27) = 34.52, p < 0.05]. Post hoc analyses revealed that all NAM treatment groups had significantly higher serum concentrations than saline or sham groups (p < 0.05).
Bilateral Tactile Adhesive Removal Task
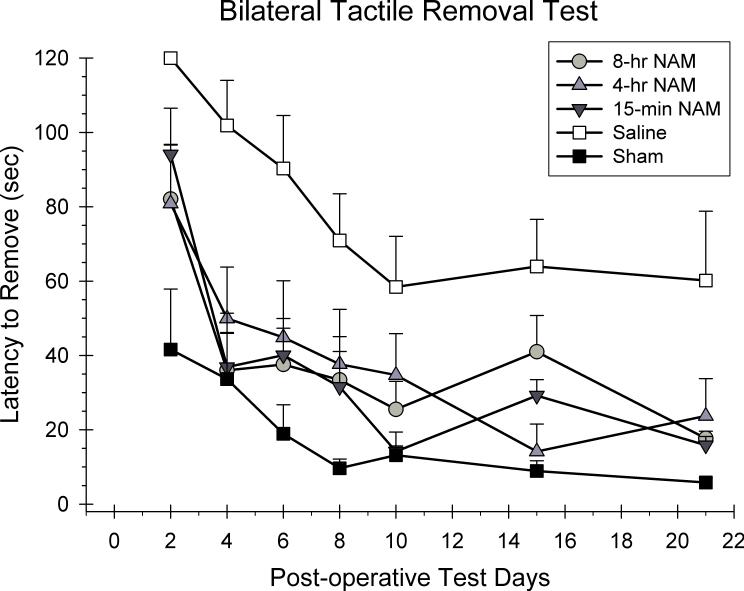
The latency to remove both stimuli from each trial was averaged together for analysis using a 5 × 7 ANOVA with repeated measures. Group (15-min NAM, 4-hr NAM, 8-hr NAM, CCI-saline, sham) and day (2, 4, 6, 8, 10, 14, and 21 post-CCI) were included as the between and within group factors in the analysis. A significant group effect was observed in the latency to remove adhesive patches from the forelimbs [F(4,29) = 8.98, p < 0.05]. All of the latencies to remove the stimuli improved over the course of testing as reflected in the significant day effect [F(3.97,154.01) = 25.55, p < 0.05]. However, the group × day interaction was not significant [F(21.24,154.01) = 0.90, p > 0.05]. Post hoc analyses of the group main effect revealed that the 15-min, 4-hr, and 8-hr NAM treatment conditions were significantly different from saline-treated animals (p < 0.05). In addition, the 15-min and 8-hr groups were not significantly different from the sham group (p < 0.05).
Vibrissae - Forelimb Placing
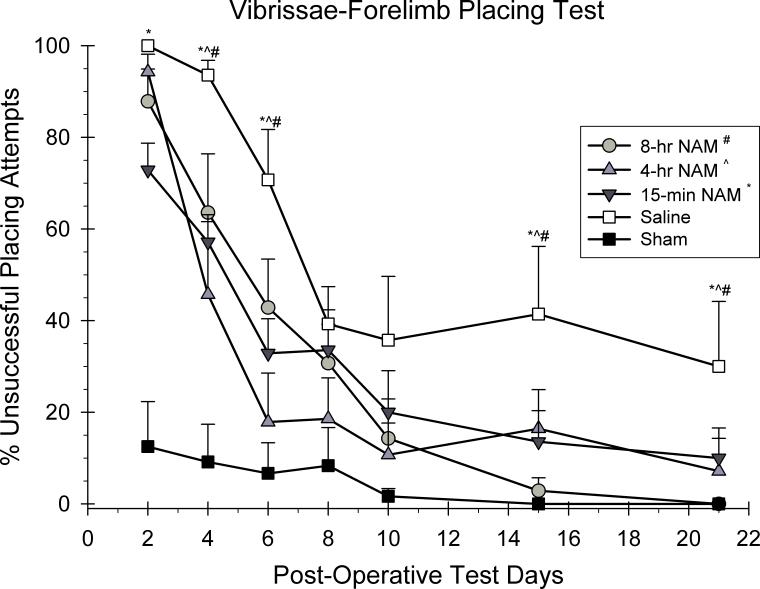
The ability to place with the forelimbs following vibrissae stimulation was analyzed using a 5 × 7 ANOVA with repeated measures. Group (15-min NAM, 4-hr NAM, 8-hr NAM, CCI-saline, sham) and day (2, 4, 6, 8, 10, 14, and 21 post-CCI) were included as the between and within group factors in the analysis. A significant group effect was observed in the placing reaction in both forelimbs [F(4,29) = 8.53, p < 0.05]. All forelimb placing responses improved over the course of testing as reflected in the significant day effect [F(4.14,120.09) = 64.56, p < 0.05]. In addition, the group × day interaction was also significant suggesting that the recovery rates for the groups were different across days [F(16.56,120.09) = 3.94, p < 0.05]. Post hoc analyses of the interaction effect revealed that the 15-min group was significantly different from the saline-treated group on days 2, 4, 6, 15, and 21 (p < 0.05). The 4-hr group was significantly different than the saline group on days 4, 6, 15, and 21 (p < 0.05). The 8-hr groups was also significantly different from the saline-treated group on days 4 , 6, 15, and 21 (p < 0.05).
Cognitive Assessment: Reference Memory
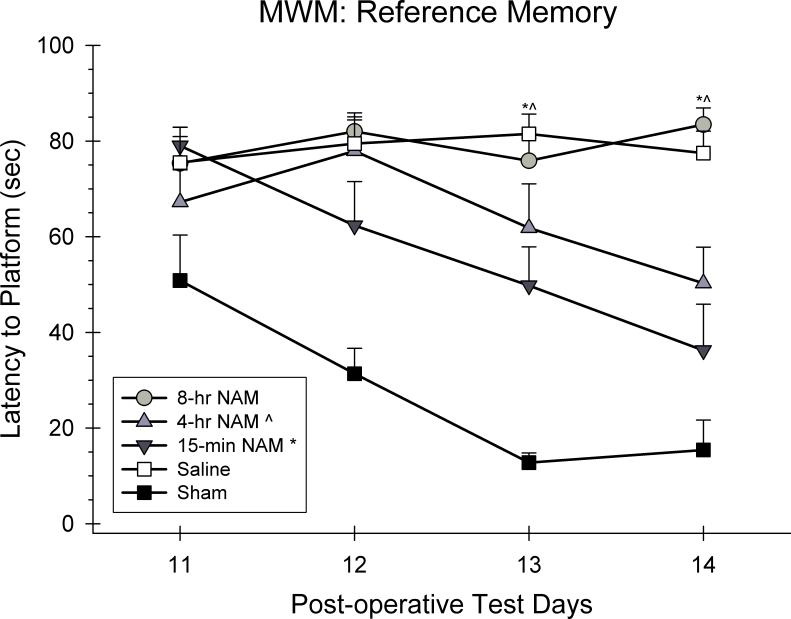
Swim latencies were analyzed using a 5 × 7 ANOVA with repeated measures. Group (15-min NAM, 4-hr NAM, 8-hr NAM, CCI-saline, sham) and day (11, 12, 13, and 14 post-CCI) were included as the between and within group factors in the analysis. A significant group effect was observed in the swim latencies [F(4,29) = 21.41, p < 0.05]. Swim latencies generally improved over the course of testing as reflected in the significant day effect [F(3.00,87.00) = 10.41, p < 0.05]. In addition, the group × day interaction was also significant suggesting that the recovery rates for the groups were different across days [F(12.00, 87.00) = 3.67, p < 0.05]. Post hoc analyses of the interaction effect revealed that the 15-min group was significantly different from the saline-treated group on days 13 and 14 (p < 0.05). The 4-hr group was significantly different than the saline group on days 13 and 14 (p < 0.05). However, the 8-hr group was not significantly different from the saline-treated group on any day (p > 0.05).
Cognitive Assessment: Working Memory
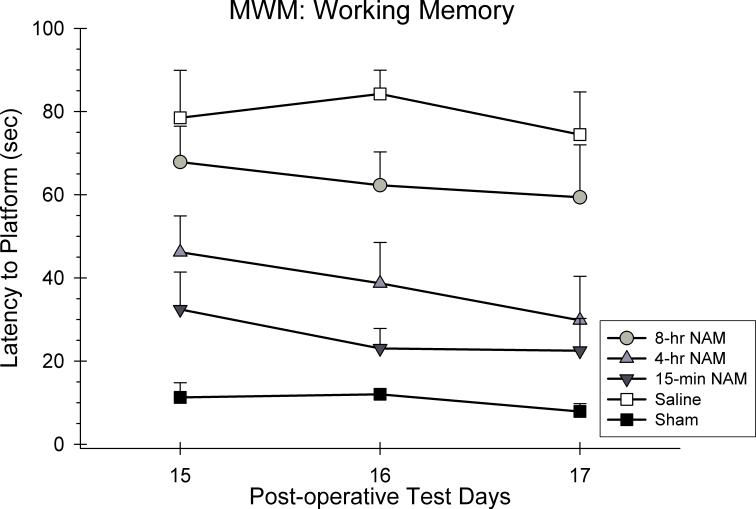
Swim latencies were analyzed using a 5 × 7 ANOVA with repeated measures. Group (15-min NAM, 4-hr NAM, 8-hr NAM, CCI-saline, sham) and day (15, 16, and 17 post-CCI) were included as the between and within group factors in the analysis. A significant group effect was observed in the swim latencies [F(4,29) = 14.90, p < 0.05]. Swim latencies generally did not improve over the course of testing as reflected in the non-significant day effect [F(1.72,49.75) = 2.70, p > 0.05]. In addition, the group x day interaction was also not significant suggesting that the recovery rates for the groups were not different across days [F(6.86, 49.75) = 0.41, p > 0.05]. Post hoc analyses of the group main effect revealed that the 15-min and 4-hr groups were significantly different from the saline-treated group (p < 0.05). However, the 8-hr group was not significantly different from the saline-treated group (p > 0.05). The 15-min group was also found to not be significantly different than the sham group (p < 0.05).
Lesion Analysis
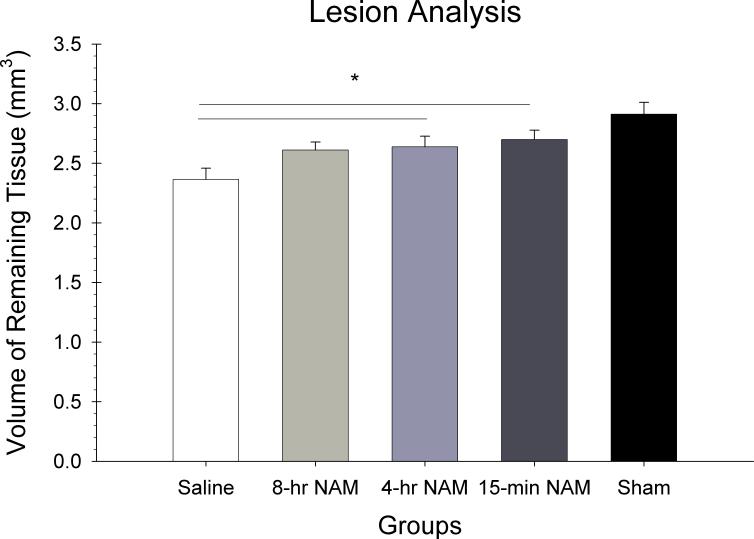
The volume of the remaining cortex calculated through the injury site was analyzed in a one way ANOVA with group (15-min NAM, 4-hr NAM, 8-hr NAM, CCI-saline, sham) as the between factor in the analysis. There were significant differences in cortical reduction as reflected in the main effect for group [F(4,25) = 7.42, p < 0.05]. Post hoc analysis revealed that the 15-min and 4-hr treatment conditions had significantly larger volumes of remaining cortex compared to saline (p < 0.05).
DISCUSSION
This study demonstrated that administration of a 6-dose regimen of NAM following frontal CCI had a dose-dependent effect on the level of circulating serum NAM. Administration of NAM starting at any of the initial time points post-CCI (15-min, 4-hr, or 8-hr) significantly elevated serum NAM compared to the animals not receiving NAM administration. Interestingly, the 15-min injection resulted in significantly higher levels of serum NAM than either of the two administration time points.
The main purpose of the present study was to establish the window of opportunity for NAM administration post-CCI. It was found that administration of NAM following frontal CCI had a task dependent effect on recovery of function. The 6-dose regimen of NAM significantly lessened the behavioral impairments observed following injury and led to a more rapid and sustained improvement in functional recovery. Specifically, the data from the bilateral tactile removal test showed that administration of NAM at the 15-min, 4-hr, and 8-hr post-CCI time points significantly improved the recovery of function on this test. Toward the end of the 21 day testing period the saline-treated injured animals showed a plateau in their recovery around 60 seconds. In all of the NAM-treated animals their performance recovered to around 30 seconds, compared to the sham controls which showed a latency around 10 seconds to remove the stimuli. A similar level of performance was also observed on the vibrissae-forelimb placing test. All NAM-treated animals showed significant recovery compared to the saline-treated animals. Treatment with NAM at any of the time points resulted in approximately a 90% improvement in placing performance compared to the saline-treated group which showed only a 70% improvement. In addition, the 15-min NAM group demonstrated a significant reduction in the initial impairment of the placing test. We have previously observed this effect with the 15-min administration time point following FPI (Hoane et al., 2006c). In general, on both sensorimotor tests examined in the current study, it was found that NAM administered as last as 8 hrs post-CCI significantly improved recovery of function. Furthermore, there was little difference in the recovery curves between any of the NAM administration groups. Thus, on the sensorimotor tests it appears that there is a relatively wide window of opportunity.
Cognitive assessment in the present study included the acquisition of a reference memory task in the MWM; as well as, the assessment of working memory in the MWM. Administration of NAM at either the 15-min or 4-hr time points significantly improved the acquisition of a reference memory task. Administration of NAM at 8 hrs post-CCI had no observable benefit compared to saline treatment. In fact, both the 8-hr NAM group and the saline-group showed virtually no improvement in performance from day 11 to day 14. In contrast, both the 15-min and 4-hr groups showed approximately a 50 percent improvement across the 4 testing days. A very similar effect was also observed with the assessment of working memory performance following frontal CCI. Both the 15-min and 4-hr treatment groups showed significant improvements in working memory compared to the saline-treated group. With the exception on day 16 there was very little difference in performance between the 8-hr and the saline-treated groups. Given these results, it appears that the current regimen of NAM treatment only has a window of opportunity out to 4 hrs when cognitive performance is assessed.
The results of the anatomical analysis also showed a time-dependent effect for NAM treatment. Both the 15-min and 4-hr treatment groups showed a preservation of cortical tissue loss following CCI compared to the saline-treated group. In contrast, the 8-hr group was not significantly different compared to the saline-treated group. Thus, it appears that the performance on the cognitive tests mirrors the results found in the anatomical data. However, in the lesion analysis it was found that none of the NAM-treated groups were significantly different from the non-injured sham group. In this regard, the treatment window would match the sensorimotor tests better than the cognitive tests. This paradoxical effect may suggest that the sensorimotor tests are more sensitive at detecting slight differences in cortical tissue preservation compared to the cognitive assessments. These findings demonstrate that it is important to assess across different behavioral categories and to include measures of lesion analysis when examining the preclinical efficacy of novel therapies.
The present study is the first study to establish the window of opportunity for NAM following TBI. However, several studies have examined the window of opportunity for NAM in models of ischemia. A single injection of NAM at a dose of 500 mg/kg was administered at either 30 mins, 2, 3, or 4 hrs following induction of permanent MCAo. Significant reductions in infarct volume were found with the 30 min and 2 hr groups, but not the 3 or 4 hrs groups (Ayoub et al., 1999). Significant functional improvement has been demonstrated on the neuroscore battery with the 500 mg/kg dose of NAM administered 4 hrs after stroke induction (Ayoub and Maynard, 2002). The results of the current study with NAM have shown a much different window of opportunity compared to what was seen in the stroke models. A more clinically relevant treatment window was shown in the present study. The FDA approved dose of 50 mg/kg, administered in a 6 dose regimen over the first 5 post-CCI days, resulted in significant improvements in functional recovery when administered 4−8 hrs post-injury.
Although the mechanism by which NAM provides neuroprotection is not entirely understood, it does appear to act at multiple levels. As a result of severe cellular injury there is increased poly (ADP-ribose) polymerase (PARP) activity leading to NAD+ depletion and thus apoptosis (Burkle, 2001). NAM, being an essential precursor to NAD+, has been shown to replenish NAD+ stores and allow for DNA repair (Ayoub et al., 1999, Maiese and Chong, 2003). NAM also exerts protective effects by inhibiting poly-ADP-ribose polymerase (PARP). Following breaks in DNA strands caused by oxidative damage, PARP binds to single or double strand breaks and cleaves NAD to catalyze the transfer of ADP-ribose, which binds to acceptor proteins and to PARP itself reducing amount of free NAD for normal energy metabolism (Virag and Szabo, 2002). Chronic PARP activation quickly depletes cellular NAD stores, administration of NAM has been shown to inhibit PARP, increase levels of NAD in cortical areas affected by ischemic events, and restore ATP levels (Chang et al., 2002, Yang et al., 2002, Sadanaga-Akiyoshi et al., 2003, Tam et al., 2005). NAM has also been shown to prevent cellular injury, due to inflammation, by maintaining membrane asymmetry and inhibiting several cytokines (Li et al., 2006). NAM activates protein kinase B and inhibits forkhead transcription factor's transcription of proapoptotic genes. A downstream effect of protein kinase B is closing of the mitochondrial transition pores opened by excessive Ca2+ influx. The mitochondrion is able to regain ionic homeostasis and bring cytochrome C back to physiological conditions thereby inhibiting caspase 9 mediated cell death (Maiese and Chong, 2003). In addition, NAM also reduces acute neuronal death and edema in the brain (Hoane et al., 2006a), as well as attenuates blood-brain barrier breach and apoptosis in the acute period post CCI (Hoane et al., 2006b). It has also been demonstrated that NAM significantly reduces GFAP expression 1 month post-FPI and CCI injuries (Hoane et al., 2003, Hoane et al., 2006c).
The results of this study indicate that a 6-dose regimen of NAM significantly improved performance on all behavioral measures of sensorimotor and cognitive function when administered as late as 4 hrs post-CCI. Furthermore, significant improvements in sensorimotor performance were observed 8 hrs post-CCI. NAM administration also significantly reduced tissue loss in the injured cortex when administered 4 hrs following injury. This initial attempt to establish the window of opportunity for NAM following TBI has shown that that window is between 4−8 hrs. Given the task dependent nature of the behavioral results it is likely that increasing the dosing regimen may also further increase the preclinical window of opportunity. Although the initial preclinical studies of NAM following TBI used a clinically impossible treatment time point (15-min post-injury), the current study has demonstrated a clinically relevant treatment window which supports NAM's translation to clinical studies.
Figure 1.
The effects of a 6-dose regimen of NAM (50 mg/kg, ip) or vehicle administered over 5 days on serum concentration of NAM. The graph shows the plotted mean (±SEM) serum NAM concentrations. Administration of NAM significantly elevated serum NAM levels compared to the saline-treated group (* = p<0.05). Interestingly, the 15-min injection regimen showed the highest levels of serum NAM compared to the other NAM groups (^ = p<0.05)
Figure 2.
The effects of a regimen of NAM (50 mg/kg, ip) or vehicle administered following frontal CCI or sham surgery on the bilateral tactile removal test. The graph shows the plotted mean (±SEM) latencies to remove the stimuli from both forelimbs. Treatment with the 15-min, 4-hr, and 8-hr NAM groups significantly improved performance compared to the saline-treated group.
Figure 3.
The effects of a regimen of NAM or vehicle administered following frontal CCI or sham surgery on the vibrissae-forelimb placing test. The graph shows the plotted mean (±SEM) percentage of unsuccessful placing attempts. Treatment with the 15-min (* = p<0.05), 4-hr (^ = p<0.05), and 8-hr (# = p<0.05) NAM groups significantly improved the placing performance compared to the saline-treated group. Treatment with the 15-min NAM group significantly reduced the magnitude of the injury compared to the saline-treated group on the first day of testing.
Figure 4.
The effects of a regimen of NAM or vehicle administered following frontal CCI or sham surgery on the acquisition of reference memory in the MWM. The graph shows the plotted mean (±SEM) swim latencies to the submerged platform. Treatment with the 15-min and 4-hr NAM groups significantly improved the acquisition of reference memory compared to the saline-treated group.
Figure 5.
The effects of a regimen of NAM or vehicle administered following frontal CCI or sham surgery on working memory performance in the MWM. The graph shows the plotted mean (±SEM) swim latencies to the submerged platform. Treatment with the 15-min and 4-hr NAM groups significantly improved working memory compared to the saline-treated group.
Figure 6.
Lesion analysis. Plotted is the mean (±SEM) remaining cortical volume for each group. NAM at the 15-min and 4-hr time points reduced the amount of injury-induced tissue loss compared to the vehicle group (* = p < 0.05).
Figure 7.
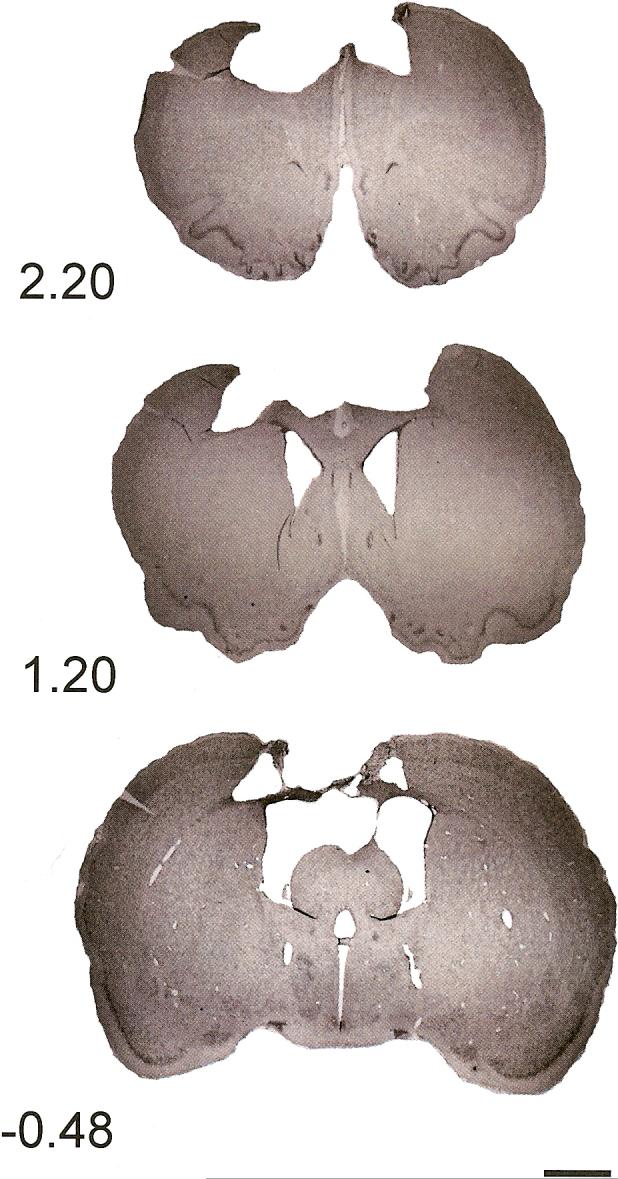
Histology plate. Shown are representative cresyl violet images (4 μm) of sections in the saline-treated injured brain at coordinates 2.20 mm, 1.20 mm, and −0.48 mm relative to bregma (0.44x, scale bar = 2 mm).
Acknowledgements
Research supported by NINDS (NS045647-03) to MRH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ayoub IA, Lee EJ, Ogilvy C, Flint Beal M, Maynard KI. Nicotinamide reduces infarction up to two hours after the onset of permanent focal cerebral ischemia in wistar rats. Neurosci Lett. 1999;259:21–24. doi: 10.1016/s0304-3940(98)00881-7. [DOI] [PubMed] [Google Scholar]
- Ayoub IA, Maynard KI. Therapeutic window for nicotinamide following transient focal cerebral ischemia. Neuroreport. 2002;11:213–216. doi: 10.1097/00001756-200202110-00008. [DOI] [PubMed] [Google Scholar]
- Burkle A. PARP-1: a regulator of genomic stability linked with mammalian longevity. Chembiochem. 2001;2:725–728. doi: 10.1002/1439-7633(20011001)2:10<725::AID-CBIC725>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Chang ML, Yang J, Kem S, Klaidman L, Sugawara T, Chan PH, Adams JD., Jr Nicotinamide and ketamine reduce infarct volume and DNA fragmentation in rats after brain ischemia and reperfusion. Neurosci Lett. 2002;322:137–140. doi: 10.1016/s0304-3940(01)02520-4. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. TINS. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Gilligan JE, Griggs WM, Jelly MT, Morris DG, Haslam RR, Matthews NT, Everest ER, Bryce RL, Marshall PB, Peisach RA. Mobile intensive care services in rural South Australia. The Medical journal of Australia. 1999;171:617–620. doi: 10.5694/j.1326-5377.1999.tb123822.x. [DOI] [PubMed] [Google Scholar]
- Gillmor HA, Bolton CH, Hopton M, Moore WP, Perrett D, Bingley PJ, Gale EA. Measurement of nicotinamide and N-methyl-2-pyridone-5-carboxamide in plasma by high performance liquid chromatography. Biomed Chromatogr. 1999;13:360–362. doi: 10.1002/(SICI)1099-0801(199908)13:5<360::AID-BMC893>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hoane M, Wolyniak J, Akstulewicz S. Administration of riboflavin improves behavioral outcome and reduces edema formation and GFAP expression following traumatic brain injury. J Neurotrauma. 2005:1112–1122. doi: 10.1089/neu.2005.22.1112. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Akstulewicz SL, Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in the rat. J Neurotrauma. 2003;20:1189–1198. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Gilbert DR, Holland MA, Pierce JL. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci Lett. 2006a:35–39. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006b;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Pierce JL, Holland MA, Birky ND, Dang T, Vitek MP, McKenna SE. The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J Neurotrauma. 2007;24:1108–1118. doi: 10.1089/neu.2006.0254. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Tan AA, Pierce JL, Anderson GD, Smith DC. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J Neurotrauma. 2006c;23:1535–1548. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- Holland MA, Tan AA, Smith DC, Hoane MR. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J Neurotrauma. 2008;25:140–152. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Cell Life versus cell longevity: the mysteries surrounding the NAD+ precursor nicotinamide. Curr Med Chem. 2006;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner MD, Plone MA, Cain CK, Frydel BR, Francis JM, Emerich DF, Sutton RL. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J Neurotrauma. 1998;15:199–216. doi: 10.1089/neu.1998.15.199. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier; New York: 2005. [DOI] [PubMed] [Google Scholar]
- Sadanaga-Akiyoshi F, Yao H, Tanuma S, Nakahara T, Hong JS, Ibayashi S, Uchimura H, Fujishima M. Nicotinamide attenuates focal ischemic brain injury in rats: with special reference to changes in nicotinamide and NAD+ levels in ischemic core and penumbra. Neurochem Res. 2003;28:1227–1234. doi: 10.1023/a:1024236614015. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y, Mitha AP, Ogilvy C, Maynard KI. Post-treatment with nicotinamide (vitamin B3) reduces the infarct volume following permanent focal cerebral ischemia in female sprague-dawley and wistar rats. Neurosci Lett. 2000;281:111–114. doi: 10.1016/s0304-3940(00)00854-5. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Woodlee MT. Orienting and Placing. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat. Oxford University Press; New York: 2005. pp. 129–140. [Google Scholar]
- Tam D, Tam M, Maynard KI. Nicotinamide modulates energy utilization and improves functional recovery from ischemia in the in vitro rabbit retina. Ann N Y Acad Sci. 2005;1053:258–268. doi: 10.1196/annals.1344.023. [DOI] [PubMed] [Google Scholar]
- Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- Yang J, Klaidman LK, Adams JD. Medicinal chemistry of nicotinamide in the treatment of ischemia and reperfusion. Mini Rev Med Chem. 2002;2:125–134. doi: 10.2174/1389557024605483. [DOI] [PubMed] [Google Scholar]