Abstract
Purpose
We have demonstrated that administration of heparin-binding EGF-like growth factor (HB-EGF) protects the intestines from injury. The aim of the current study was to evaluate the effect of HB-EGF gene disruption on intestinal restitution, angiogenesis and long term survival after intestinal ischemia/reperfusion (I/R) injury.
Methods
HB-EGF (−/−) and wild-type HB-EGF (+/+) litter mate mice were subjected to 45 min of superior mesenteric artery (SMA) occlusion followed by reperfusion. Functional recovery of the gut permeability barrier was evaluated with Ussing chamber studies, and microvessel density (MVD) was evaluated immunohistochemically. Animal survival was evaluated using the Kaplan-Meier method.
Results
Histological damage after ischemia was significantly higher in HB-EGF (−/−) mice compared to HB-EGF (+/+) mice, associated with a significantly higher number of incompetent (non-healed, non-resurfaced) villi indicative of delayed structural healing by restitution. HB-EGF (−/−) mice had increased intestinal permeability after intestinal I/R. HB-EGF (−/−) mice had significantly lower MVD at 3 and 7 days after I/R, indicating that HB-EGF gene deletion resulted in delayed onset of angiogenesis. Two week mortality rates were significantly higher in HB-EGF (−/−) mice.
Conclusions
Endogenous HB-EGF significantly enhances healing by restitution, prolongs survival and enhances angiogenesis in mice subjected to I/R. These findings support our hypothesis that HB-EGF administration may improve outcome in patients with intestinal I/R injury, including necrotizing enterocolitis.
Keywords: Heparin-binding EGF-like growth factor, Intestine, Ischemia/reperfusion injury, Angiogenesis
INTRODUCTION
The gut mucosa is constantly subjected to injury and is continuously undergoing renewal. In addition, the gut is highly susceptible to hypoperfusion injury due to its higher critical oxygen requirement compared to the whole body (1) and due to the mucosal countercurrent microcirculation (2). Restitution, a process dependant upon intestinal epithelial cell (IEC) migration, is a critical form of intestinal healing that rapidly restores the gut permeability barrier, resulting in protection of the interior milieu from the hostile external environment.
Heparin-binding EGF-like growth factor (HB-EGF) was initially identified in the conditioned medium of cultured human macrophages (3), and later found to be a member of the epidermal growth factor (EGF) family (4). We have shown that administration of HB-EGF promotes IEC migration in vitro (5) and enhances restitution in rats subjected to I/R or hemorrhagic shock and resuscitation (HS/R) in vivo (5; 6). Additionally, blocking of endogenous HB-EGF using the HB-EGF-specific inhibitor CRM197 or blocking of EGFR in vitro resulted in suppression of intrinsic IEC migration and healing in vitro (5). These studies suggest that endogenous HB-EGF is involved in IEC migration and restitution.
Healing by restitution is efficient for superficial intestinal injuries. However, deep mucosal injuries are more clinically relevant, with recovery from deep injuries requiring remodeling of the extracellular matrix and the submucosa as well as angiogenesis (7). In addition to being important for deep injuries, angiogenesis is also essential for restitution. Despite significant progress in our understanding of intestinal healing by restitution, very little is known about the role of angiogenesis in the healing of deeper intestinal injuries.
Expression of HB-EGF is significantly increased in response to tissue damage (5; 8), hypoxia (9), oxidative stress (10) and wounding of intestinal epithelial monolayers (11). This pattern of expression is consistent with a pivotal role for HB-EGF in I/R injury and repair processes. The critical role for HB-EGF in post-ischemic regeneration has been demonstrated in various organs including the brain, kidney, and heart (9; 12; 13). HB-EGF is also a potent chemoattractant for various cell types (14; 15). Exogenous HB-EGF promotes rabbit corneal angiogenesis and neovascularisation in mouse skin (16), and recent studies have shown that HB-EGF is involved in tumor angiogenesis (17), catecholamine-induced vascular trophic effects (18) and angiopoietin-induced angiogenesis (19). We have recently demonstrated that HB-EGF administration preserves the mesenteric microcirculation in rats subjected to HS/R (6).
Taken together, it is conceivable that HB-EGF may be involved in angiogenesis during healing from deep intestinal injuries. The current study was designed to demonstrate the role of endogenous HB-EGF gene expression on healing by restitution, angiogenesis and overall animal survival in HB-EGF null mice subjected to various degrees of ischemic injuries.
MATERIALS AND METHODS
Mouse model of intestinal ischemia/reperfusion (I/R) injury
HB-EGF (−/−) and HB-EGF (+/+) mice were a kind gift from Dr. David Lee (Chapel Hill, NC) (20). In HB-EGF (−/−) mice, HB-EGF exons 1 and 2 were replaced with PGK-Neo, thus deleting the signal peptide and propeptide domains. The desired targeting events were verified by Southern blots of genomic DNA and exon-specific PCR, with Northern blots confirming absence of the respective transcripts (20).
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Children’s Research Institute (Protocol #00903 AR). Ten week-old male HB-EGF (−/−) or HB-EGF (+/+) mice were fasted for 16–18 h with access to water only. Under inhalation anesthesia using 2% isoflurane, a midline laparotomy was performed, followed by ligation of collaterals between the SMA, celiac, and inferior mesenteric arteries. The SMA was then occluded using two microvascular clamps for 45 min followed by various durations of reperfusion. Changes in arterial flow were assessed by morphological intestinal changes, and by intravital microscopy in randomly selected animals. Control HB-EGF (−/−) and HB-EGF (+/+) mice were subjected to identical procedures with the exception of vascular manipulation. Throughout the procedure the abdominal cavity was kept temporarily closed, with body temperature maintained at 37ºC. Animals were euthanized by exsanguination under anesthesia. All animals survived the initial ischemic injury with complete recovery from the procedure for at least 12 hours. Three mice were used per group, with each experiment performed in duplicate.
For evaluation of HB-EGF gene deletion on animal survival, both HB-EGF (−/−) and HB-EGF (+/+) mice were subjected to a pilot study to determine a suitable duration of ischemia. These studies showed that 45 min of ischemia was not enough to cause significant mortality in either group, and that 90 min of ischemia resulted in over 70% mortality in both groups. Accordingly, 70 min of ischemia was selected in order to evaluate the tolerance of HB-EGF (−/−) and HB-EGF (+/+) mice to prolonged ischemia. After the 70 min ischemic period, animals were allowed to recover and were kept in a heat controlled environment for two weeks after surgery.
Histologic injury score
Tissue sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E). Histologic scoring of the depth of tissue injury was performed according to Chiu et al.(21) with modifications as follows: score 0, no damage; score 1, subepithelial space at villous tip; score 2, loss of mucosal lining of the villous tip; score 3, loss of less than half of the villous structure; score 4, loss of more than half of the villous structure; and score 5, transmural necrosis. Sections were evaluated blindly.
Morphological criteria indicative of in vivo restitution
Whereas the histologic injury score evaluates the depth of intestinal injury, quantification of incompetent villi was used to evaluate the structural intestinal barrier as we have previously described (5). An incompetent villous was identified as a villous with an incomplete mucosal lining, regardless of the depth of injury. Any epithelial gap was considered as a potential port of bacterial or macromolecular translocation into the submucosal space, and hence was considered a breach in the intestinal barrier. We have previously demonstrated that the number of incompetent villi reflects both the functional integrity of the intestinal barrier as well as early healing by restitution (5). The degree of in vivo restitution was evaluated using our previously outlined criteria (5). These criteria are applied to well-aligned villi in PAS-stained sections and include: 1) histologic features indicative of prior loss of mucosa resulting in subsequent villous contraction, with short, blunted, or concave villous tips compared to non-damaged or less injured villi in the same histologic section; 2) restoration of the mucosal surface of injured villi with a single layer of flat, squamous enterocytes resulting from migration and flattening during restitution; and/or 3) restoration of mucosal continuity with a single cell layer containing four or more goblet cells in continuity, without intervening enterocytes. The average numbers of incompetent villi per cross section were quantified as an indicator of intestinal restitution.
Intestinal permeability assay using Ussing chambers
Intestinal permeability in Ussing chambers was used as an index of recovery of the gut barrier. Ileum was opened along the mesenteric border to produce a flat sheet and rinsed with ice-cold HEPES buffer solution (HBS) at a constant pH of 7.4. HBS containing 1.25 mmol/L Ca was used as the bathing solution for the mucosal component during the experiment and as a stabilizing solution for both the mucosal and the serosal sides between experiments. The bathing solution was maintained at 37°C with water jacketed reservoirs connected to a constant temperature circulating pump gassed with 95% O2/5%CO2. Full-thickness ileal specimens were mounted onto Ussing chambers to expose a circular area of epithelium (0.25 cm2). The serosal and mucosal sides of the segments were bathed in 1ml of HBS containing 1.25 mmol/L Ca and continuously exposed to 95% O2. Mucosal barrier function was determined by measurement of unidirectional mucosal-to-serosal flux of FITC-dextran for 30 min. After dilution of the FD-4muc, both serosal and mucosal FD-4 were measured using a Bioassay Reader HTS 7000 (Perkin Elmer) with excitation wave of 485 nm and emission measured at 520 nm. The mucosal-to-serosal FD-4 clearance was calculated using the following modified formula:
Evaluation of microvascular density (MVD) during intestinal healing from I/R injury
Immunohistochemistry was performed on paraffin-embedded tissue sections using the avidin-biotin peroxidase complex method, as described previously (22). Deparaffinized rehydrated sections were treated in 0.1% trypsin for 30 min at room temperature (RT). Vascular endothelial cells (EC) were stained with rabbit polyclonal anti-Von Willebrand factor antibody (Abcam Inc., Cambridge, MA) at a final dilution of 1:800. The incubation time for the primary antibody was 1 h at RT. Immunohistochemistry was conducted using a Mouse to Mouse HRP staining kit (ScyTeck Laboratories, Logan, UT) according to the recommendations of the manufacturer. V-W antibody staining was labeled using 3-Amino-9-Ethylcarbzole (AEC) as chromogenic substrate. Stained sections were scanned and captured using Metamorph computer software to evaluate villous MVD. Immunoreactive (dark staining) EC in longitudinally oriented villi were quantified in at least 12 sections per animal. Average EC staining was then estimated per animal group. Three animals were evaluated per group with the entire experiment performed in duplicate.
Statistical analyses
All results are presented as mean ± SD. Statistical comparisons between all groups were performed using one-way ANOVA. When significant differences were detected between groups, further comparisons between two groups were performed using Student t test. Animal survival was assessed using the Kaplan-Meyer method with comparisons made using the Log-rank test. A level of p<0.05 was considered statistically significant.
RESULTS
HB-EGF gene disruption is associated with increased intestinal injury in mice subjected to ischemia/reperfusion injury
To evaluate the role of endogenous HB-EGF on intestinal tolerance to I/R injury, HB-EGF (−/−) and HB-EGF (+/+) mice were subjected to 45 min of ischemia and a variable duration of reperfusion. Changes in histological injury in the ileum within the first 3 h after I/R were determined. Immediately after ischemia, histologic injury scores in HB-EGF(+/+) mice (1.5 ± 0.18) were not significantly different from those in HB-EGF (−/−) mice (2.15 ± 0.45) (Figure 1). One hour after reperfusion the degree of intestinal injury increased slightly but not significantly in both animal groups. By 3 h of reperfusion the depth of intestinal damage significantly decreased in HB-EGF (+/+) mice (1.5 ± 0.2) but not in HB-EGF (−/−) mice, which continued to have a high depth of injury (2.36 ± 0.26; p<0.05). Thus, early healing results in rapid tissue recovery in HB-EGF (+/+) mice but not in HB-EGF (−/−) mice, suggesting that endogenous HB-EGF enhances early recovery from post-ischemic injury.
Figure 1. Deletion of the HB-EGF gene is associated with higher intestinal injury scores in mice subjected to moderate intestinal ischemia/reperfusion.
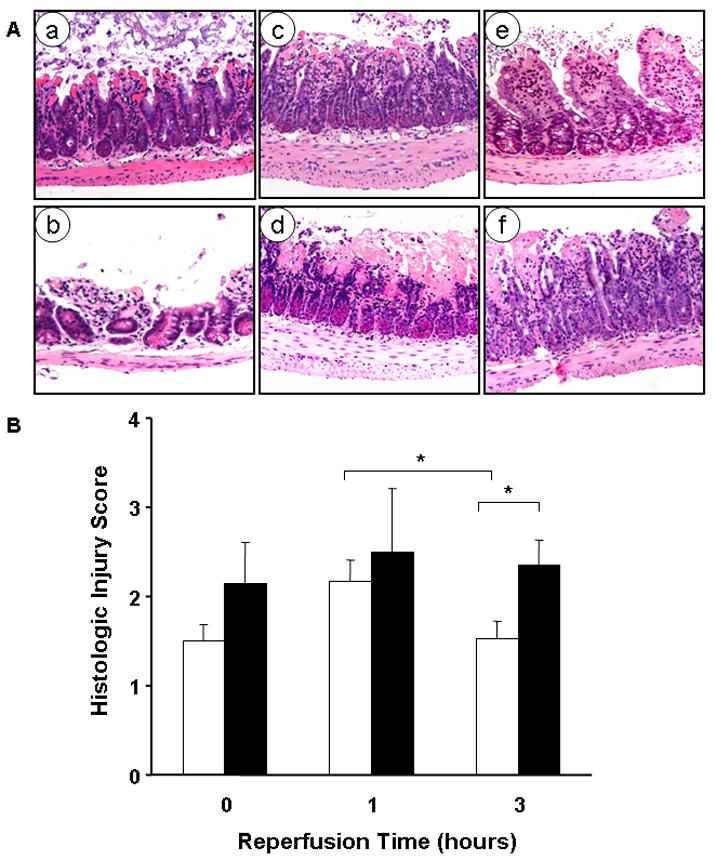
A) Representative examples of histologic injury in the distal 5 cm of ileum at the end of ischemia in HB-EGF (+/+) mice (a) and HB-EGF (−/−) mice (b); one hour after reperfusion in HB-EGF (+/+) mice (c) and HB-EGF (−/−) mice (d); and 3 hours after reperfusion in HB-EGF (+/+) mice (e) and HB-EGF (−/−) mice (f).
B) At the end of ischemia there was no significant difference in injury score between HB-EGF (+/+) (□) and HB-EGF(−/−) (■) mice. Histological damage reached a higher level in HB-EGF (+/+) mice one hour after injury and was significantly reduced at three hours of reperfusion. However, in HB-EGF(−/−) mice, the injury score at three hours of reperfusion was significantly higher than that in HB-EGF (+/+) mice. *p<0.05.
HB-EGF gene disruption is associated with delayed structural recovery of the gut barrier after ischemia/reperfusion injury
We have demonstrated that restitution occurs during the first 3 h after I/R and that restitution is responsible for early recovery of the gut mucosal barrier in rats subjected to I/R (5). To assess structural restitution quantitatively, we determined the average number of structurally incompetent villi (villi which can serve as a portal of entry for macromolecules due to incomplete mucosal epithelial lining regardless of the depth of injury). At the end of ischemia, the average number of incompetent villi was 8.5 ± 3.4 in HB-EGF (+/+) mice compared to 22.0 ± 12 in HB-EGF (−/−) mice (Figure 2A). One hour after reperfusion, the number of incompetent villi increased to 19.2 ± 4.0 in HB-EGF (+/+) mice and to 44.4 ± 16 in HB-EGF (−/−) mice, coinciding with changes in the intestinal injury score. Three hours after reperfusion, the number of incompetent villi declined significantly in HB-EGF (+/+) mice (8.0 ± 2.6) compared to HB-EGF (−/−) mice (31.9 ± 5.2; p<0.05), with the latter having a significantly higher number of incompetent villi. This indicates that endogenous HB-EGF is essential for early restitution and structural recovery of the gut barrier after I/R, and that deletion of the HB-EGF gene delays the occurrence of restitution.
Figure 2. Endogenous HB-EGF promotes intestinal healing by restitution after I/R in mice.
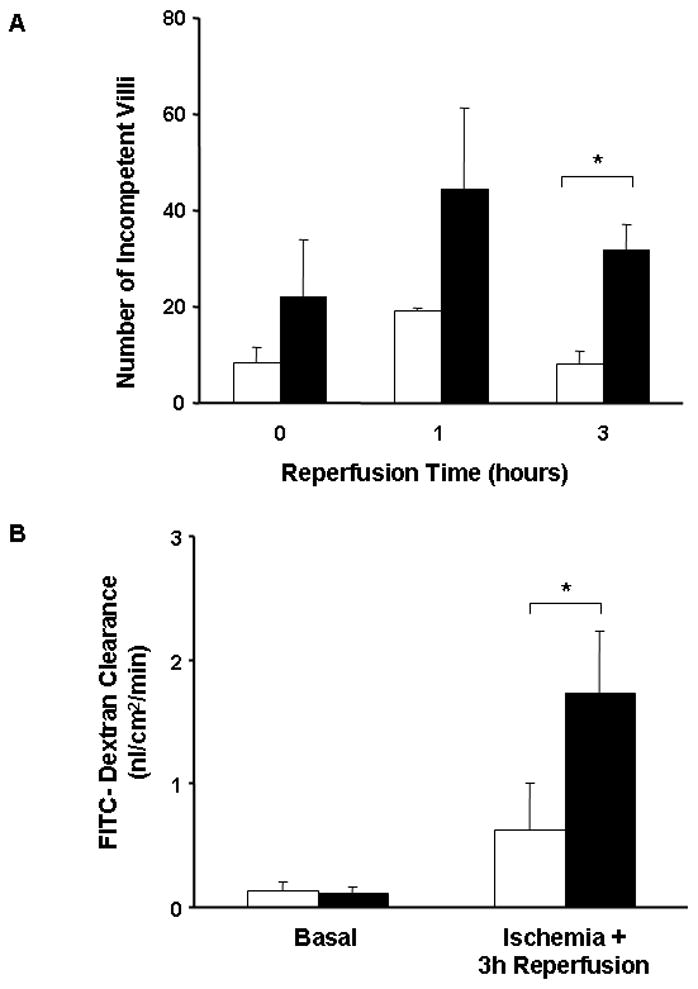
A) Quantification of restitution as determined by the number of structurally incompetent villi. HB-EGF (−/−) mice (■) had a higher number of incompetent villi compared to HB-EGF(+/+) mice (□) at all time points. Three hours after reperfusion, HB-EGF (−/−) mice continued to have a significantly higher number of incompetent villi compared to HB-EGF (+/+) mice. *P<0.05.
B) Quantification of the mucosa-to-serosa unidirectional clearance of FITC-dextran as an index of functional gut barrier recovery using the Ussing system. Under basal conditions, there was no significant difference in FITC-dextran clearance between HB-EGF (+/+) (□) and HB-EGF (−/−) (■) mice. Three hours after reperfusion, HB-EGF (+/+) mice had a significantly lower clearance compared to HB-EGF (−/−) mice, indicating a better functional recovery of the gut barrier in wild-type mice. *p<0.05.
HB-EGF gene disruption is associated with delayed functional recovery of the intestinal permeability barrier in vivo
To evaluate the effect of endogenous HB-EGF on the functional gut permeability barrier, we investigated the mucosa-to-serosa unidirectional clearance to FD-4 using the Ussing system. The basal level of FD-4 clearance in HB-EGF (+/+) mice (0.13 ± 0.07 nl/cm2/min) was comparable to that of HB-EGF (−/−) mice (0.11 ± 0.05 nl/cm2/min) (Figure 2B). Three hours after I/R, the level of FD-4 clearance in HB-EGF (+/+) mice (0.62 ± 0.07 nl/cm2/min) was significantly lower than that in HB-EGF (−/−) mice (1.7 ± 0.49 nl/cm2/min; p<0.05), indicating that endogenous HB-EGF gene expression is essential for functional recovery of the gut barrier.
HB-EGF gene disruption is associated with delayed angiogenesis during intestinal regeneration after ischemia/reperfusion injury
Regeneration after ischemic damage occurs as a two stage process. While restitution occurs in the early stages resulting in reestablishment of mucosal continuity and barrier function, complete regeneration and restoration of villous height requires regrowth of connective tissue and angiogensis. To evaluate the angiogenic changes after I/R in mice, villous MVD was evaluated 3 and 7 days after I/R by immunohistochemistry using Von Willibrand factor related antibody. Under basal conditions, EC immunoreactivity to VW factor in HB-EGF (+/+) mice (1.56 ± 0.41%) was not significantly different from that in HB-EGF (−/−) mice (1.33 ± 0.33%) (Figure 3). Three days after I/R, villous MVD in HB-EGF (+/+) mice decreased significantly to 1.2 ± 0.45%. At this time point, the MVD in HB-EGF (−/−) mice (0.59 ± 0.29%) was significantly lower than basal levels, and significantly lower than that of HB-EGF (+/+) mice. Seven days after I/R, villous MVD increased in both animal groups but was still significantly lower in HB-EGF (−/−) mice (1.06 ± 0.31%) compared to HB-EGF (+/+) mice (1.35± 0.21%, p<0.05).
Figure 3. Endogenous HB-EGF gene expression promotes angiogenesis after I/R in mice.
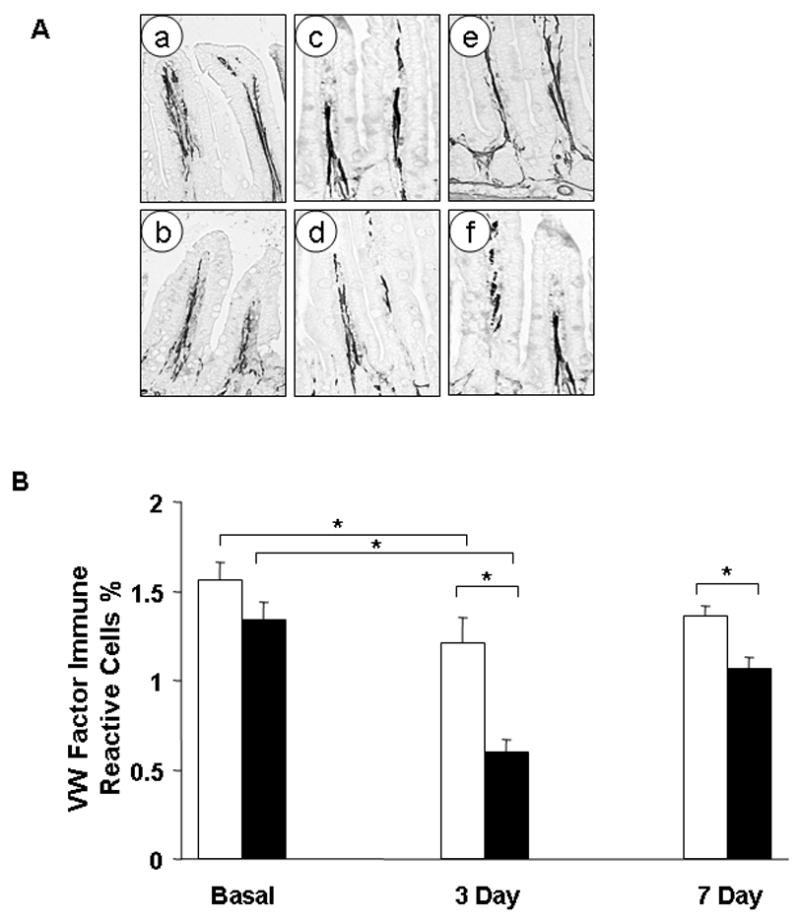
A) Representative examples of immunoreactivity to VW factor related antibody which stains endothelial cells. Staining is seen as black color for computer analysis purposes. Shown are representative examples of the terminal 5 cm of ileum at: basal levels in HB-EGF (+/+) mice (a) and HB-EGF (−/−) mice (b); three days after I/R in HB-EGF (+/+) mice (c) and HB-EGF (−/−) mice (d); and 7 days after I/R in HB-EGF (+/+) mice (e) and HB-EGF (−/−) mice (f).
B) Quantification of the extent of VW factor related antibody (MVD) immunoreactivity/20X scanned section was evaluated using a Metamorph computer analysis software. At basal levels, there was no significant difference between MVD in HB-EGF (+/+) (□) and HB-EGF(−/−) (■) mice. Three days after I/R, the MVD decreased significantly in HB-EGF (+/+) mice compared to the basal levels, but then approached basal levels at 7 days after I/R. In HB-EGF (−/−) mice however, MVD was significantly lower than that in HB-EGF (+/+) mice at both 3 and 7 days after I/R. *p<0.05.
HB-EGF gene disruption is associated with significantly lower survival after prolonged intestinal ischemia in mice
To elucidate the importance of endogenous HB-EGF to survival after I/R, both HB-EGF (+/+) and HB-EGF (−/−) mice were subjected to severe intestinal ischemia for 70 min. Whereas the overall 2 week survival in HB-EGF (+/+) animals was 63.6%, survival in HB-EGF (−/−) mice was significantly reduced to 30.8% (p<0.05) (Figure 4). This indicates that endogenous HB-EGF expression plays an axial role in both healing and survival in animals subjected to intestinal I/R.
Figure 4. Deletion of the HB-EGF gene is associated with decreased survival in mice subjected to prolonged intestinal ischemia/reperfusion.
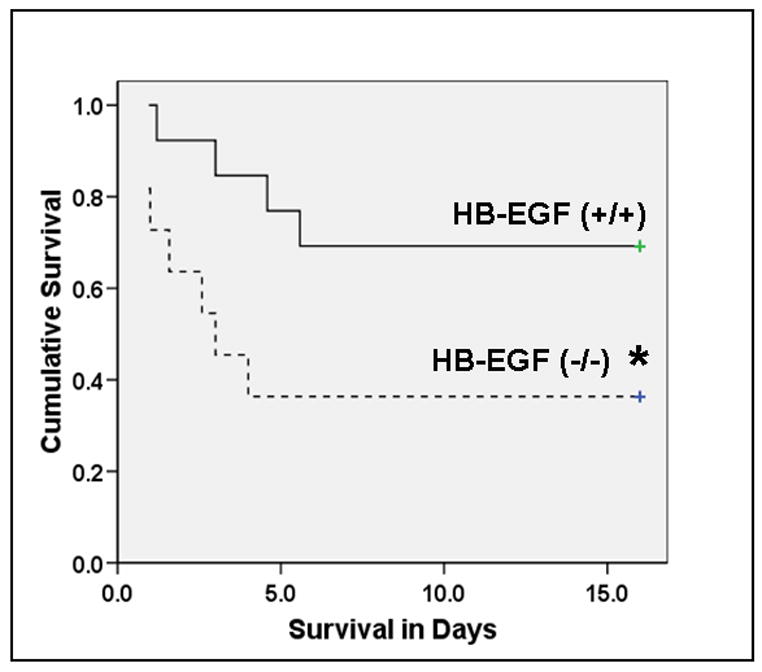
Mice were subjected to 70 min of ischemia and were observed for 2 weeks for evaluation of overall survival. Survival curves were evaluated using the Kaplan Meyer method and survival rates were compared using the Log Rank test. HB-EGF (+/+) animals (solid line) had significantly improved survival compared to HB-EGF(−/−) mice (dotted line). *p<0.05.
DISCUSSION
Our results demonstrate that endogenous HB-EGF is important for intrinsic restitution, preservation of gut barrier function and survival after intestinal I/R injury. These results clearly demonstrate that endogenous HB-EGF expression plays an axial role in both structural and functional recovery of the gut barrier by restitution in mice subjected to I/R. It is well established that the mortality rate after intestinal ischemia often exceeds 90% (23). In HB-EGF (−/−) mice subjected to 70 min of ischemia the mortality rate was significantly higher than that in HB-EGF (+/+) mice. Most animal mortality occurred between 12–48 h after injury, suggesting that acute complications such as bacterial toxemia or multiorgan dysfunction syndrome are the most probable causes of death. These results are not surprising since it has been known for decades that loss of the barrier and immune functions of the intestine are major contributing factors leading to deleterious complications and death after I/R. These results support the crucial role of HB-EGF in the recovery of gut barrier function after I/R, and explain our previous results showing that administration of exogenous HB-EGF increases survival in rats subjected to I/R injury in vivo (24).
Despite the critical role of healing by restitution in the rapid restoration of the mucosal barrier, full recovery of deeper intestinal injuries requires connective tissue remodeling, regeneration of the submucosa and effective angiogenesis. In the current study, we found that 45 min of ischemia was enough to produce intestinal damage involving more than half of the villous height in mice, indicating that the distal microvascular villous structures had been damaged or lost. This depth of injury has been consistently observed in our previous studies in rats subjected to intestinal I/R or to HS/R (5; 6) indicating that even moderate degrees of intestinal injury will require angiogenesis for complete intestinal regeneration. Surprisingly, despite a fair understanding of angiogenesis regulators in inflammatory bowel disease and healing of GI ulcers, neither angiogenesis nor angiogenesis regulators have been studied during intestinal regeneration from injuries such as ischemia or hypoperfusion.
To understand the changes in angiogenesis during intestinal healing, we evaluated MVD beginning on the third day after I/R. This time point has been suggested to be the initiation point of angiogenesis (25; 26). In HB-EGF (+/+) mice we found that MVD was significantly lower than basal pre-ischemic levels, consistent with the initial degree of injury with damage to distal villous structures. Seven days after intestinal I/R, MVD approached basal levels in HB-EGF (+/+) mice, indicating that angiogenesis occurs between 3 and 7 days after injury. Three days after I/R, MVD was significantly lower in HB-EGF (−/−) mice compared to HB-EGF (+/+) mice, and continued to be significantly lower 7 days after I/R. This suggests that endogenous HB-EGF is involved in angiogenesis during intestinal healing and that deletion of the HB-EGF gene results in delayed onset of angiogenesis.
HB-EGF is a potent mitogen and chemotactic factor for fibroblasts and smooth muscle cells (27) both of which play important roles in angiogenesis and reconstruction of connective tissue structures. Although HB-EGF is not a direct mitogen for EC, it is a potent chemoattractant for these cells. We have demonstrated that HB-EGF stimulates tube formation in EC in vitro, and that HB-EGF stimulates angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways (28). Thus, HB-EGF can induce angiogenesis via enhancing migration of fibroblasts, EC and vascular smooth muscle cells, all of which are important elements of angiogenesis.
As part of its role in promoting angiogenesis, HB-EGF may protect vascular EC from hypoperfusion/hypoxia-induced vascular injury. We have recently shown that HB-EGF significantly reduces post-resuscitation deterioration of mesenteric microcirculation in rats subjected to HS/R (6). A common sequellae after hypoperfusion is damage to EC, most likely due to polymorphonuclear leukocyte (PMN)/EC interactions (29). Mice deficient in leukocytes or endothelial adhesion molecules demonstrate improved capillary blood flow after intestinal I/R (30). This suggests that PMN/EC interactions play an important role in microvascular damage after hypoperfusion states. We have recently demonstrated that pretreatment of PMN with HB-EGF significantly reduces PMN/EC adhesion and PMN transendothelial migration after anoxia/reoxygenation injury in vitro (31). We also demonstrated that HB-EGF down-regulates the expression of adhesion molecules and the infiltration of inflammatory cells after SMA occlusion in rats (32). Taken together, it is possible that HB-EGF improves the microcirculation via multiple mechanisms including induction of angiogenesis as well as protection of EC from damage during hypoperfusion states.
Deletion of the HB-EGF gene results in abnormal embryonic cardiac cushion development resulting in defective valvulogenesis, with stenosis of the semilunar and atrioventricular valves leading to cardiac hypertrophy (20). Although the use of HB-EGF (−/−) mice allows assessment of the effects of loss of HB-EGF signaling, the effects of potential cardiac failure must be taken into consideration. Chalothorn et al. used 4 month old HB-EGF(−/−) mice in studies of the effect of HB-EGF on angiogenesis in a hindlimb ischemia model (33). The authors demonstrated that HB-EGF (−/−) mice had cardiac hypertrophy without evidence of heart failure, with normal hemodynamic parameters both awake and under anesthesia. Furthermore, 6 month old HB-EGF (−/−) mice only have a 25% reduction in left ventricular fractional shortening (20). This is less than the mildest level of human heart failure which is characterized by an ejection fraction that is reduced by at least 40%. Take together, these results suggest that HB-EGF (−/−) mice have compensated cardiac hypertrophy without heart failure, arguing against the presence of hemodynamic disturbances affecting our observed results. In addition, the fact that our studies were performed in HB-EGF KO mice that were only 10 weeks old makes it even less likely that HB-EGF KO-related hemodynamic disturbances would affect our results. Interestingly, Chalothorn et al. found no differences in angiogenesis after hindlimb ischemia in HB-EGF (−/−) compared to HB-EGF (+/+) mice (33), as opposed to the results in the intestine reported here.
In conclusion, deletion of the HB-EGF gene results in decreased intestinal healing by restitution in mice subjected to moderate I/R. In mice subjected to prolonged intestinal ischemia, deletion of HB-EGF results in significantly higher mortality indicating its essential role in tolerance to ischemic injury and its critical role in restoration of barrier function of the gut. More importantly the current study highlights the importance of endogenous HB-EGF in angiogenesis during intestinal recovery from deep mucosal injuries secondary to I/R. Despite the multiplicity of EGF family members, the effect of loss of HB-EGF is not compensated for by other EGF family members. These results support the therapeutic use of HB-EGF in the prevention or treatment of disease processes involving intestinal I/R injury, including necrotizing enterocolitis.
Acknowledgments
This work was supported by a grant from the National Institutes of Health R01 GM 61193-05 (GEB), by the Children’s Hospital Firefighter’s Endowment (ONE) and by the Children’s Research Institute (CRI). The authors would like to kindly thank Dr. David Lee (Chapel Hill, NC) for supplying HB-EGF knockout mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marik PE. Total splanchnic resuscitation, SIRS, and MODS. Crit Care Med. 1999;27:257–258. doi: 10.1097/00003246-199902000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Chiara O, Pelosi P, Segala M, Turconi MG, Brazzi L, Bottino N, Taccone P, Zambelli M, Tiberio G, Boswell S, Scalea TM. Mesenteric and renal oxygen transport during hemorrhage and reperfusion: evaluation of optimal goals for resuscitation. J Trauma. 2001;51:356–362. doi: 10.1097/00005373-200108000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 5.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 6.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Podolsky DK. Mucosal immunity and inflammation V Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. 1999;277:G495–499. doi: 10.1152/ajpgi.1999.277.3.G495. [DOI] [PubMed] [Google Scholar]
- 8.Cribbs RK, Harding PA, Luquette MH, Besner GE. Endogenous production of heparin-binding EGF-like growth factor during murine partial-thickness burn wound healing. J Burn Care Rehabil. 2002;23:116–125. doi: 10.1097/00004630-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Jin K, Mao XO, Sun Y, Xie L, Jin L, Nishi E, Klagsbrun M, Greenberg DA. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank GD, Mifune M, Inagami T, Ohba M, Sasaki T, Higashiyama S, Dempsey PJ, Eguchi S. Distinct mechanisms of receptor and nonreceptor tyrosine kinase activation by reactive oxygen species in vascular smooth muscle cells: role of metalloprotease and protein kinase C-delta. Mol Cell Biol. 2003;23:1581–1589. doi: 10.1128/MCB.23.5.1581-1589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis PD, Hadfield KM, Pascall JC, Brown KD. Heparin-binding epidermal-growth-factor-like growth factor gene expression is induced by scrape-wounding epithelial cell monolayers: involvement of mitogen-activated protein kinase cascades. Biochem J. 2001;354:99–106. doi: 10.1042/0264-6021:3540099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai M, Zhang M, Homma T, Garrick B, Abraham JA, McKanna JA, Harris RC. Production of heparin binding epidermal growth factor-like growth factor in the early phase of regeneration after acute renal injury. Isolation and localization of bioactive molecules. J Clin Invest. 1997;99:2128–2138. doi: 10.1172/JCI119386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka N, Masamura K, Yoshida M, Kato M, Kawai Y, Miyamori I. A role of heparin-binding epidermal growth factor-like growth factor in cardiac remodeling after myocardial infarction. Biochem Biophys Res Commun. 2002;297:375–381. doi: 10.1016/s0006-291x(02)02197-6. [DOI] [PubMed] [Google Scholar]
- 14.Faull RJ, Stanley JM, Fraser S, Power DA, Leavesley DI. HB-EGF is produced in the peritoneal cavity and enhances mesothelial cell adhesion and migration. Kidney Int. 2001;59:614–624. doi: 10.1046/j.1523-1755.2001.059002614.x. [DOI] [PubMed] [Google Scholar]
- 15.Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, Hanakawa Y, Ohmoto H, Yoshino K, Shirakata Y, Matsuzawa Y, Hashimoto K, Taniguchi N. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abramovitch R, Neeman M, Reich R, Stein R, Keshet E, Abraham J, Solomon A, Marikovsky M. Intercellular communication between vascular smooth muscle and endothelial cells mediated by heparin-binding epidermal growth factor-like growth factor and vascular endothelial growth factor. FEBS Lett. 1998;425:441–447. doi: 10.1016/s0014-5793(98)00283-x. [DOI] [PubMed] [Google Scholar]
- 17.Ongusaha PP, Kwak JC, Zwible AJ, Macip S, Higashiyama S, Taniguchi N, Fang L, Lee SW. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res. 2004;64:5283–5290. doi: 10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Koivisto L, Heino J, Uitto VJ. Bacterial heat shock protein 60 may increase epithelial cell migration through activation of MAP kinases and inhibition of alpha6beta4 integrin expression. Biochem Biophys Res Commun. 2004;319:1088–1095. doi: 10.1016/j.bbrc.2004.04.202. [DOI] [PubMed] [Google Scholar]
- 19.Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, Sundvall M, Maatta JA, Laine VJ, Yla-Herttuala S, Higashiyama S, Alitalo K, Elenius K. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. Faseb J. 2003;17:1609–1621. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- 20.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. Embo J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu C-J, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 22.El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–1562. doi: 10.1002/hep.510270613. [DOI] [PubMed] [Google Scholar]
- 23.Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. American Gastrointestinal Association. Gastroenterology. 2000;118:954–968. doi: 10.1016/s0016-5085(00)70183-1. [DOI] [PubMed] [Google Scholar]
- 24.Pillai SB, Hinman CE, Luquette MH, Nowicki PT, Besner GE. Heparin-binding epidermal growth factor-like growth factor protects rat intestine from ischemia/reperfusion injury. J Surg Res. 1999;87:225–231. doi: 10.1006/jsre.1999.5764. [DOI] [PubMed] [Google Scholar]
- 25.Brasken P, Lehto M, Renvall S. Changes in the connective tissue composition of the submucosal layer of colonic anastomosis. An immunohistologic study in rats. Acta Chir Scand. 1989;155:413–419. [PubMed] [Google Scholar]
- 26.Wise L, McAlister W, Stein T, Schuck P. Studies on the healing of anastomoses of small and large intestines. Surg Gynecol Obstet. 1975;141:190–194. [PubMed] [Google Scholar]
- 27.Kume N, Gimbrone MA., Jr Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest. 1994;93:907–911. doi: 10.1172/JCI117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta V, Besner G. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007 doi: 10.1080/08977190701773070. in press. [DOI] [PubMed] [Google Scholar]
- 29.Zakaria el R, Garrison RN, Kawabe T, Harris PD. Role of neutrophils on shock/resuscitation-mediated intestinal arteriolar derangements. Shock. 2004;21:248–253. doi: 10.1097/01.shk.0000111824.07309.19. [DOI] [PubMed] [Google Scholar]
- 30.Horie Y, Wolf R, Anderson DC, Granger DN. Hepatic leukostasis and hypoxic stress in adhesion molecule-deficient mice after gut ischemia/reperfusion. J Clin Invest. 1997;99:781–788. doi: 10.1172/JCI119224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocourt DV, Mehta VB, Wu D, Besner GE. Heparin-binding EGF-like growth factor decreases neutrophil-endothelial cell interactions. J Surg Res. 2007;141:262–266. doi: 10.1016/j.jss.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Xia G, Martin AE, Besner GE. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434–439. doi: 10.1053/jpsu.2003.50075. [DOI] [PubMed] [Google Scholar]
- 33.Chalothorn D, Moore SM, Zhang H, Sunnarborg SW, Lee DC, Faber JE. Heparin-binding epidermal growth factor-like growth factor, collateral vessel development, and angiogenesis in skeletal muscle ischemia. Arterioscler Thromb Vasc Biol. 2005;25:1884–1890. doi: 10.1161/01.ATV.0000175761.59602.16. [DOI] [PubMed] [Google Scholar]


