Abstract
Background
Older adults are at increased risk of dehydration, yet water balance is understudied in this population.
Objective
This controlled diet study assessed the effect of age on water input, output, and balance in healthy adults. Hydration status (plasma osmolality and urine specific gravity) and body composition were also measured.
Design
Eleven men and 14 women aged 23–46 y and 10 men and 11 women aged 63–81 y were subjects. Water balance was assessed during days 7–10 of three 18-d controlled feeding trials with protein intakes of 0.50, 0.75, and 1.00 g · kg−1 · d−1. Total water input included water from the provided foods and beverages, ad libitum intake, and metabolic production. Water output included the losses in urine and stool and the insensible losses from respiration and nonsweating perspiration.
Results
Ad libitum water consumption, total water intake, water output through urine, total water output, and net water balance were not different in the older subjects than in the younger subjects. Markers of hydration status were within the range of clinical normalcy for all groups. Total body water (TBW) was not significantly different, fat-free mass (FFM) was significantly lower (P < 0.05), and FFM hydration (TBW:FFM) was significantly higher (P < 0.05) in the older subjects than in the younger subjects. Dietary protein intake did not influence any of these results.
Conclusions
These results show that healthy older adults maintain water input, output, and balance comparable to those of younger adults and have no apparent changes in hydration status. The results support that the hydration of FFM is increased in older men and women.
Keywords: Fluid intake, urine and stool water, thirst, dietary protein, plasma osmolality
Introduction
Water is fundamental to existence. Total body water (TBW) is tightly regulated within ±0.2% of body weight each day (1). Water balance is achieved and maintained by matching the input and output of water from the body. The Food and Nutrition Board Panel on Dietary Reference Intakes for Electrolytes and Water (2) has established the adequate intake for water of adults aged between 19 and >70 y as the median total water intake (from a combination of drinking water, beverages, and food) of 19–30-y-old men (3.7 L/d) and women (2.7 L/d) from the National Health and Nutrition Examination Survey III (NHANES III) data (3). The ad libitum consumption of drinking water, water in foods and beverages, and metabolic water production contribute to water input, and water output occurs in urine, stool, sweat, and insensible respiration and perspiration. Thirst and hormonal mechanisms are responsible for keeping TBW within a narrow range. Thirst is stimulated by an increase in plasma osmolality, a decrease in plasma volume, or a decrease in blood pressure (4). The hormone vasopressin is responsible for controlling water balance on a daily basis (4, 5). A net fluid loss of as little as 1% of body weight will increase plasma osmolality, and a 2% loss will negatively affect exercise performance (6). A water deficit of 20% can be life-threatening (7).
The aging process is associated with several physiologic changes that may affect one's ability to maintain water balance. These changes include a decrease in TBW associated with a loss of fat-free mass (FFM) (8), a decrease in the sensation of thirst (9), and alterations in plasma vasopressin concentration or effectiveness (or both), which influence the ability of the kidneys to concentrate urine (5, 10, 11). Dehydration is the most frequent fluid or electrolyte disorder in older adults (12). It is important to ensure that older adults consume enough fluids to maintain euhydration and that the current fluid intake recommendations (2, 13–16) are adequate to offset any age-associated changes in how water balance is achieved.
Only a few studies exist on water intake and excretion in older humans. De Castro et al (17) reported that age did not influence ad libitum oral water intake, based on 7-d food diaries from 262 healthy, community-living 20–80-y-old men and women. Water excretion was not documented. Raman et al (18) reported findings from 458 community-living 40–79-y-old adults who completed an evaluation of water turnover with the use of the 2H oxide technique. Water intake, derived as the difference between water turnover and nonoral water input, was highly variable among the subjects and lower in the 70–79-y-old men and women, compared with men and women aged 40–49 y. Urinary water excretion was also highly variable and not influenced by age, except for a small increase in urine output with advancing age in men only. Direct measurements of ad libitum drinking and food and beverage water intakes, stool water excretion, and hydration status were not obtained, and subjects aged <40 y were not included in that study. We are unaware of any research study that has directly measured ad libitum oral water input, water output in both urine and stool, and water balance in older adults; incorporated measurements of hydration status, body composition, and perceived thirst; and included younger adults as a comparison group.
The purpose of this study was to use a strictly controlled diet protocol to assess the effect of age on markers of water input, output, and balance in healthy men and women. Markers of hydration status (plasma osmolality and urine specific gravity), perceived thirst, and body composition (including FFM and TBW) were also assessed.
Subjects and Methods
Subjects
Fifty-eight adults were recruited to participate in this study through advertisements in local newspapers and community postings. The recruits included 13 younger men (YM), 12 older men (OM), 21 younger women (YW), and 12 older women (OW). Forty-eight adults completed the study: 12 YM, 10 OM, 15 YW, and 11 OW. Data from 1 YM and 1 YW were excluded because of incomplete stool and urine collections, respectively. Thus, results are presented from 11 YM, 10 OM, 14 YW, and 11 OW. Each subject completed first a telephone interview and then a screening to determine whether he or she was qualified to participate. The screening consisted of a self-reported medical history questionnaire, a resting-state electrocardiogram, routine clinical blood and urine chemistries, and an interview with the study coordinator. Eligible participants were required to have clinically normal kidney, heart, liver, and thyroid functions; to have complete bladder control; not to smoke; to have clinically normal blood pressure; and not to have diabetes mellitus. Subjects who consumed physician-prescribed medications to achieve and maintain clinically normal organ functions and blood pressure were included in the study. All the YW who participated in the study had normal menstrual function and were not lactating, and testing was done the week after the beginning of menstrual bleeding. All of the OW in this study were postmenopausal and aged ≥70 y.
Written informed consent was obtained from all subjects. The protocol was approved by the Human Research Advisory Committee at the University of Arkansas for Medical Sciences and the Committee on the Use of Human Research Subjects at Purdue University. Each subject received a monetary stipend for participation.
Experimental design
Each subject completed three 18-d periods in random order. Each of the 3 trials was conducted with the same experimental conditions, except for the macronutrient contents of the diets. The subjects consumed their habitual diet for a minimum of 1 wk between trials as a dietary washout. This experimental design was used to assess the effect of age on the dietary protein requirement of adults. The number of subjects recruited for the study was deemed adequate to assess the hypothesized age-associated difference in protein requirement. The strict dietary control and complete urine and stool collections associated with the nitrogen balance assessment provided a unique opportunity to also assess water balance.
Diet
A rotating schedule of 3 menus was used to provide each subject with all of the foods and beverages in precisely portioned quantities throughout each of the 3 trials. No foods or beverages (other than water) were provided ad libitum. The total energy intake of each subject was individually set at 1.75 times the resting energy expenditure, estimated from the Harris-Benedict equation for men or women (19). The protein intake during each trial was 0.50 (inadequate), 0.75 (marginal), or 1.00 (adequate) g · kg−1 · d−1. Nonprotein energy consisted of 65% carbohydrate and 35% fat among all 3 trials. Adjustment to protein intake among the trials was accomplished with both vegetable- and animal-based proteins (20). A diet that contained 0.2 g protein · kg−1 · d−1 was provided to each subject on the first day of each trial to enhance adaptation to the subsequent protein intake. The energy and macronutrient contents of the subjects' daily menus were calculated with the use of NUTRITIONIST V software (version 1.5; First Databank Inc, San Bruno, CA).
Subjects consumed breakfast at the research kitchen each weekday morning; weekday lunch and dinner and all weekend meals were packaged for consumption away from the laboratory. Instructions were provided for each subject to eat all the foods provided, to scrape all containers, and to use water to clean out any food left in the containers and to drink that water. The subjects were allowed to consume water ad libitum and were instructed not to consume any additional food or beverages and not to consume any alcohol or added salt during the 3 trials. No caffeine was allowed during the trials, but the subjects were allowed to consume decaffeinated tea and coffee provided to them on request. Selected menu items had to be changed because of availability, which resulted in a higher sodium intake among the men than women. All of the subjects were provided with one multivitamin and mineral supplement tablet (Advanced Formula Centrum; Lederle Laboratories, Pearl River, NY) to consume each day and were asked to discontinue use of all other nonprescription or physician-recommended dietary supplements. Each person was asked to maintain his or her usual amount of physical activity and to not start any new exercise programs.
Water input
Water input included ad libitum water consumption, water consumption from foods and beverages, and metabolic water production. From days 7–10 of each trial, each subject was instructed to exclusively consume their ad libitum water intake from bottled water provided to them. The subjects were instructed to drink only the bottled water, to use the bottled water to make any decaffeinated tea or coffee consumed, and to use the bottled water to clean out any excess food in their containers. The subjects were not given any guidelines about water intake and were uninformed of the research aim to determine water balance. The bottles were weighed before and after the subjects returned them to quantify the total amount of water consumed per day. The ad libitum water consumed by the subjects is denoted as WAL.
Duplicate food composites were made for each of the trials' 3 menus (a total of 9 composites). Foods and beverages (not including ad libitum water intake) were combined and homogenized in a blender, and total weight was recorded. An aliquot of each composite was frozen and analyzed for water content. A 3-g sample of each aliquot thawed and mixed by vortex was dried at 80 °C (Isotemp, model 516G; Fisher Scientific International Inc, Hampton, NH) for 3 d to a constant weight to eliminate the water in the sample and was reweighed immediately on removal from the oven. Water content of foods and beverages was determined from the proportion of the wet sample that was water multiplied by the total composite weight and is denoted as WFB.
Metabolic water production was estimated from the macronutrient intakes of each subject, assuming that 41, 55, and 107 g water was produced for every 100 g protein, carbohydrate (starch), and fat consumed, respectively (21). These general factors approximate the water production from the complete oxidation of a mixture of macronutrients consumed. Quantification of the sources and quantities of all proteins, carbohydrates, and fats consumed would be needed to more precisely estimate water production according to the theoretical stoichiometry of the oxidation reactions. Water produced through metabolic processes is denoted as WM.
Water output
Water output includes urinary water, stool water, insensible water loss by respiration and through the skin (ie, perspiration or transepidermal diffusion), and sweat water loss. From days 7–10, each subject completed 24-h urine collections. The water volume of each 24-h collection was calculated as the total weight of the collection divided by its density, which was determined by weighing a precisely portioned volume of the collection. The amount of water in the urine is denoted as WU.
Subjects completed a 72-h stool collection from days 7–9 of each trial. The beginning and end of the collection were identified with the use of stool dye markers. All stools collected during the 72-h period were combined, homogenized in a blender dedicated to this task, and weighed. An aliquot of each sample was frozen and later analyzed for water content by using the same method as for the food composites. Stool water is denoted as WS.
Insensible water losses through respiration and the skin were estimated according to the proportions of 13 and 30 mL water, respectively, given off for every 100 kcal energy consumed (22). These factors are considered reasonable estimates of these water losses, with the understanding that the insensible water loss of an individual is proportionate to heat production and will vary as a result of body size, the level of physical activity, and environmental conditions. The day-to-day indoor and outdoor environmental conditions that the subjects resided in were not recorded. The estimations were based on the assumption that total dietary energy provided matched each subject's energy requirement. The insensible water loss by respiration and perspiration is denoted as WI. Water loss by sweat was not estimated but may be closely approximated as the difference between total water input and output from urine, stool, respiration, and insensible perspiration.
Calculation of water balance
Net water balance was calculated for each trial with the following formula and was expressed on a daily basis (in g water/d):
| (1) |
The assessment of net water balance assumes that body water volume is constant during the measurement period; ie, water is not retained or released from the body. The retention or release of body water was estimated from the day-to-day changes in body weight during the balance periods.
Comparison of fluid intake to recommendations
Fluid intake, including water consumed ad libitum and through food and beverages, was compared with 3 recommendations. The fluid intake recommendations were 1) 30 mL/kg body wt (13,14); 2) 1 mL/kcal energy consumed (15); and 3) 100 mL/kg for the first 10 kg, 50 mL/kg for next 10 kg, and 15 mL for each additional kilogram of body weight (16).
Body composition
Fasting-state nude body weight was measured to the nearest 0.1 kg each weekday morning on an electronic digital scale (model ES200L; Ohaus Corporation, Pine Brook, NJ). Whole-body density was measured with the use of an air-displacement plethysmography system (BOD POD; Life Measurement Inc, Concord, CA) (23), which has been validated for use with older adults (24). Percentage body fat and FFM were estimated from body density with the use of the two-compartment equation of Siri (25). TBW was measured by using a deuterium oxide dilution method (26, 27). The body density and TBW measurements were usually done on day 14 of each of the 3 trials, and each subject's results from the 3 trials were averaged for presentation.
Assessment of hydration status
Hydration status was assessed by evaluating urine specific gravity and plasma osmolality. Urine specific gravity was assumed to equal urine density, which was determined by weighing a specified volume of urine from each 24-h collection. Fasting-state blood samples were collected on day 12 of each trial by venipuncture of an antecubital vein. The blood was placed into a heparin-coated tube, and the tube was centrifuged (1000 × g for 10 min at 4 °C) to obtain plasma. Osmolality was determined with the use of an osmometer either at our laboratory (The Advanced Osmometer, Model 3D3; Advanced Instruments Inc, Norwood, MA) or at a commercial clinical chemistry laboratory (LabCorp, Burlington, NC).
Assessment of physical activity
To estimate the energy expenditure associated with habitual physical activity, each subject completed the Yale Physical Activity Survey (28) once per trial. Values from the 3 trials were averaged to obtain an overall estimate of daily energy expenditure (in kcal/d) and total time (in h/wk) for work, exercise, and recreational activity. A categorical listing of different types of activities (ie, vigorous activity, leisurely walking, moving, standing, and sitting) was calculated as the product of the frequency and duration of each activity and a weighting factor. These activity dimension indexes are reported as the sum of the 5 activity categories.
Questionnaires
To assess the subject's perceptions of appetite, during 1 d of each trial, the men in the study completed a Visual Analog Scale immediately on waking and every hour thereafter (29). These data were not collected for the women because this assessment tool was added to the protocol after most of the women had finished the study. The following questions were included on the questionnaires: “How strong is your feeling of thirst?” and “How strong is your desire to eat something salty?” Subjects were instructed to mark a single horizontal line on a 100-mm vertical labeled magnitude scale to indicate their feeling about the question at each time point. Data were scored to the nearest millimeter with the use of a ruler. One value was obtained for each subject by averaging the values obtained over the course of the day and then averaging the values from each of the 3 trials.
Statistical analysis
Data are reported as mean ± SD for each age and sex group. The main effects of trial (ie, dietary protein intake; within-subjects effect), age (between-subjects effect), and sex (between-subjects effect) and the interactions among these independent variables were assessed by three-factor repeated-measures analysis of variance. These analyses established that no significant age-by-sex, trial-by-sex, trial-by-age, or trial-by-sex-by-age interactions existed. A P value < 0.05 was considered significant. Data were analyzed with the use of JMP STATISTICAL DISCOVERY software (version 3; SAS Institute, Cary, NC).
Results
Independent of sex, the older subjects were not significantly different in height and weight from the younger subjects, although body mass index was higher for the older subjects (Table 1). The older subjects also had lower FFM, no significant difference in TBW, and higher percentage of body fat. The TBW:FFM was lower in the younger subjects than in the older subjects. TBW was 53 ± 5% (YM), 50 ± 6% (OM), 47 ± 5% (YW), and 39 ± 4% (OW) of body weight. For all 4 groups, fasting body weight on the first and last days of the 4-d balance periods was not significantly different, with variability < 0.1 kg/d. For all subjects combined, the daily fluctuations represented ±0.4% of fasting body weight. The CV of the daily body weight changes was 0.29%.
Table 1.
Subject characteristics1
| Group | ||||
|---|---|---|---|---|
| YM
(n = 11) |
OM
(n = 10) |
YW
(n = 14) |
OW
(n = 11) |
|
| Age (y)2 | 30 ± 73 (23–43)4 | 72 ± 4 (63–79) | 31 ± 8 (21–46) | 75 ± 4 (70–81) |
| Height (cm)5 | 177.6 ± 5.0 | 173.2 ± 4.5 | 169.1 ± 5.9 | 162.8 ± 5.3 |
| Weight (kg)5 | 78.6 ± 15.7 | 79.6 ± 11.7 | 64.9 ± 8.5 | 72.9 ± 13.2 |
| BMI (kg/m2)2 | 24.8 ± 4.4 | 26.5 ± 3.3 | 22.7 ± 2.5 | 27.4 ± 4.2 |
| Body fat (%)2,5 | 22.4 ± 6.7 | 28.3 ± 6.7 | 28.8 ± 5.9 | 45.4 ± 6.7 |
| FFM (kg)2,5 | 59.9 ± 8.9 | 56.0 ± 6.5 | 44.6 ± 2.5 | 38.6 ± 5.8 |
| TBW (L)5 | 41.1 ± 5.9 | 40.8 ± 5.8 | 30.2 ± 2.3 | 28.1 ± 3.2 |
| TBW:FFM (%)2 | 68.7 ± 4.0 | 73.3 ± 11.0 | 67.5 ± 3.1 | 72.5 ± 6.9 |
| Urine specific gravity2,5 | 1.013 ± 0.003 (1.010–1.018) | 1.010 ± 0.003 (1.003–1.015) | 1.009 ± 0.003 (1.007–1.013) | 1.007 ± 0.001 (1.005–1.011) |
| Plasma osmolality (mOsm/kg) | 281 ± 11 (275–315) | 291 ± 12 (281–321) | 288 ± 4 (276–294) | 291 ± 4 (282–297) |
YM, younger men; OM, older men; YW, younger women; OW, older women; FFM, fat-free mass; TBW, total body water.
Younger significantly different from older, P < 0.05 (between-subjects main effect of age, three-factor repeated-measures ANOVA).
x̄ ± SD (all such values) for the adequate protein intake trial of 1.00 g protein · kg−1 · d −1.
Range (all such values).
Men significantly different from women, P < 0.05 (between-subjects main effect of sex, three-way repeated-measures ANOVA).
The dietary intakes of energy, carbohydrate, fat, and fiber were higher for the younger subjects than for the older subjects and were not appreciably different among the 3 trials (Table 2). Independent of trial, total protein intake was not different between the younger and older subjects (because protein was provided on a per-kilogram body weight basis, and body weight was not different between the younger and older subjects). Sodium and potassium intakes were not different between the younger and older subjects.
Table 2.
Controlled dietary intakes of younger and older men and women during water balance study1
| Group | |||||
|---|---|---|---|---|---|
| Intake | Trial | YM
(n = 11) |
OM
(n = 10) |
YW
(n = 14) |
OW
(n = 11) |
| Energy (kcal/d)2,3 | IPro | 3140 ± 594 | 2756 ± 451 | 2455 ± 165 | 2225 ± 210 |
| MPro | 3076 ± 465 | 2752 ± 387 | 2460 ± 167 | 2227 ± 214 | |
| APro | 3083 ± 519 | 2836 ± 455 | 2460 ± 162 | 2229 ± 214 | |
| Protein (g/d)3 | IPro | 40 ± 8 | 40 ± 6 | 33 ± 6 | 37 ± 6 |
| MPro | 60 ± 12 | 60 ± 9 | 48 ± 7 | 55 ± 9 | |
| APro | 80 ± 16 | 81 ± 11 | 64 ± 9 | 73 ± 12 | |
| Fat (g/d)2,3 | IPro | 115 ± 22 | 100 ± 17 | 90 ± 6 | 77 ± 7 |
| MPro | 109 ± 16 | 98 ± 15 | 88 ± 6 | 77 ± 7 | |
| APro | 106 ± 18 | 98 ± 17 | 86 ± 5 | 74 ± 6 | |
| Carbohydrate (g/d)2,3 | IPro | 484 ± 96 | 422 ± 71 | 380 ± 25 | 330 ± 32 |
| MPro | 462 ± 71 | 407 ± 56 | 370 ± 25 | 313 ± 29 | |
| APro | 452 ± 79 | 408 ± 72 | 358 ± 23 | 302 ± 28 | |
| Fiber (g/d)2,3 | IPro | 26.5 ± 4.7 | 25.6 ± 3.7 | 20.4 ± 2.0 | 19.1 ± 1.6 |
| MPro | 29.7 ± 3.8 | 28.3 ± 2.1 | 22.1 ± 1.4 | 18.1 ± 1.8 | |
| APro | 34.0 ± 4.9 | 34.7 ± 4.4 | 23.7 ± 2.0 | 17.7 ± 1.5 | |
| Sodium (mg/d)3,4 | IPro | 2622 ± 510 | 2420 ± 335 | 3747 ± 542 | 4156 ± 471 |
| MPro | 2888 ± 435 | 2747 ± 403 | 4398 ± 445 | 4447 ± 408 | |
| APro | 3206 ± 524 | 3155 ± 437 | 4858 ± 323 | 4513 ± 460 | |
| Potassium (mg/d) | IPro | 2414 ± 369 | 2274 ± 319 | 2105 ± 189 | 1603 ± 213 |
| MPro | 2467 ± 276 | 2279 ± 221 | 2292 ± 183 | 1598 ± 218 | |
| APro | 2492 ± 350 | 2441 ± 368 | 2386 ± 243 | 1689 ± 212 | |
All values are x̄ ± SD. YM, younger men; OM, older men; YW, younger women; OW, older women; IPro, inadequate protein intake (0.50 g protein · kg−1 · d−1); MPro, marginal protein intake (0.75 g protein · kg−1 · d−1); APro, adequate protein intake (1.00 g protein · kg−1 · d−1).
Younger significantly different from older, P < 0.05 (between-subjects main effect of age, three-way repeated-measures ANOVA).
Men significantly different from women, P < 0.05 (between-subjects main effect of sex; three-way repeated-measures ANOVA).
Different sodium intakes of the men and women were a result of using different food items to achieve the controlled macronutrient intakes and of the need to switch these items because of product availability.
Dietary protein intake did not influence ad libitum water intake or urine and stool water outputs (Table 3). Although food and beverage water decreased significantly with increased protein intake, because of the somewhat higher water content of the different foods used to manipulate protein intake, the difference in water was only 132 mL between the 0.5 and 1.0 g protein · kg−1 · d−1 trials. The estimates of metabolic water production and respiratory and perspiration insensible water losses were not affected by protein intake.
Table 3.
Water input and output of younger and older men and women during water balance study1
| Group | |||||
|---|---|---|---|---|---|
| Variable | Trial | YM
(n = 11) |
OM
(n = 10) |
YW
(n = 14) |
OW
(n = 11) |
| Water input (mL/d) | |||||
| Ad libitum2 | IPro | 1694 ± 759 | 1936 ± 578 | 1134 ± 667 | 1635 ± 413 |
| MPro | 1714 ± 533 | 1704 ± 440 | 1382 ± 661 | 1670 ± 385 | |
| APro | 1758 ± 537 | 1849 ± 689 | 1452 ± 661 | 1612 ± 236 | |
| Food and beverages2,3 | IPro | 2231 ± 386 | 1910 ± 415 | 1773 ± 177 | 1716 ± 255 |
| MPro | 2095 ± 301 | 1850 ± 302 | 1749 ± 233 | 1670 ± 172 | |
| APro | 1997 ± 419 | 1785 ± 328 | 1686 ± 224 | 1611 ± 180 | |
| Metabolic2,3 | IPro | 406 ± 77 | 356 ± 59 | 317 ± 20 | 278 ± 28 |
| MPro | 396 ± 60 | 353 ± 50 | 318 ± 22 | 276 ± 27 | |
| APro | 394 ± 67 | 362 ± 59 | 313 ± 19 | 275 ± 26 | |
| Total2 | IPro | 4331 ± 961 | 4202 ± 864 | 3224 ± 749 | 3628 ± 458 |
| MPro | 4204 ± 758 | 3906 ± 488 | 3448 ± 695 | 3617 ± 437 | |
| APro | 4150 ± 804 | 3996 ± 943 | 3451 ± 744 | 3498 ± 337 | |
| Water output | |||||
| Urine | IPro | 2278 ± 878 | 2432 ± 770 | 1929 ± 672 | 2347 ± 546 |
| MPro | 2162 ± 539 | 2246 ± 391 | 2060 ± 732 | 2385 ± 552 | |
| APro | 2095 ± 514 | 2302 ± 803 | 2057 ± 681 | 2186 ± 333 | |
| Stool2,3 | IPro | 117 ± 56 | 138 ± 76 | 76 ± 41 | 152 ± 82 |
| MPro | 137 ± 51 | 147 ± 101 | 84 ± 60 | 102 ± 55 | |
| APro | 138 ± 37 | 146 ± 49 | 81 ± 34 | 128 ± 85 | |
| Insensible2,3 | IPro | 1351 ± 255 | 1185 ± 194 | 1073 ± 83 | 957 ± 90 |
| MPro | 1323 ± 200 | 1183 ± 167 | 1058 ± 72 | 958 ± 92 | |
| APro | 1325 ± 223 | 1220 ± 196 | 1044 ± 60 | 959 ± 92 | |
| Total2 | IPro | 3746 ± 1013 | 3755 ± 835 | 3077 ± 732 | 3455 ± 498 |
| MPro | 3622 ± 682 | 3576 ± 462 | 3202 ± 782 | 3445 ± 547 | |
| APro | 3558 ± 613 | 3667 ± 912 | 3183 ± 678 | 3273 ± 352 | |
| Net water balance2 | IPro | 586 ± 634 | 446 ± 688 | 146 ± 365 | 173 ± 174 |
| MPro | 583 ± 410 | 330 ± 291 | 247 ± 437 | 172 ± 359 | |
| APro | 592 ± 675 | 329 ± 481 | 268 ± 579 | 226 ± 372 | |
All values are x̄ ± SD. YM, younger men; OM, older men; YW, younger women; OW, older women; IPro, inadequate protein intake (0.50 g protein · kg−1 · d−1); MPro, marginal protein intake (0.75 g protein · kg−1 · d−1); APro, adequate protein intake (1.00 g protein · kg−1 · d−1).
Men significantly different from women, P < 0.05 (between-subjects main effect of sex; three-way repeated-measures ANOVA).
Younger significantly different from older, P < 0.05 (between-subjects main effect of age, three-way repeated-measures ANOVA).
Independent of age, the men had significantly higher food and beverage water input (P = 0.001), ad libitum water intake (P = 0.034), and metabolic water production (P < 0.001) than did the women (Table 3). Total water input was significantly higher in the men than in the women (P = 0.001). Water output in stool (P = 0.026) and insensible losses (P < 0.001) were significantly higher for the men than for the women, and urinary water excretion was not different between the men and the women. The percentages of water content of stool were 78 ± 4% (YM), 77 ± 6% (OM), 77 ± 6% (YM), and 83 ± 6% (OW). Total water output was significantly higher in the men (P = 0.031). Net water balance, which did not include sweat water loss, was significantly higher in the men than in the women (P = 0.020).
Independent of sex, the younger subjects had significantly higher food and beverage water input (P = 0.042) and metabolic water production (P = 0.004) than did the older subjects, but there was no significant difference in ad libitum water intake (P = 0.111) (Table 3). Total water intake was not different between the younger and the older subjects. Water output in urine was not influenced by age, whereas stool water output was significantly lower (P = 0.042) and insensible water loss was significantly higher (P = 0.010) in the younger subjects than in the older subjects. Net water balance was not different between the younger and the older subjects.
All 4 groups were adequately hydrated, based on normalcy ranges of 1.006–1.020 for urine specific gravity (30) and 280–300 mOsm/kg for plasma osmolality (31) (Table 1). Although all 4 groups were within the clinically normal range, urine specific gravity was significantly higher in the men than in the women (P < 0.001) and in the younger subjects than in the older subjects (P < 0.001). Plasma osmolality was not influenced by age and sex. Individually, 43 subjects were adequately hydrated, and 3 subjects (1 OM and 2 OW) had above-normal hydration. On the basis of plasma osmolality, 42 subjects were adequately hydrated, 3 subjects (1 YM and 2 OM) had below-normal hydration, and 1 OM had above-normal hydration.
Total water intake (food, beverage, and ad libitum water intakes combined) exceeded each of the 3 intake recommendations, as well as the 50th percentile for water intake from NHANES III (Figure 1). It is important to note that the 50th percentile values of for the YM and YW are the “adequate intakes” set by the Food and Nutrition Board for adults of all ages, and that these 3.7 and 2.7 L/d values, respectively, are higher than the NHANES III 50th percentile water intakes for OM (2.9 L/d) and OW (2.5 L/d).
Figure 1.
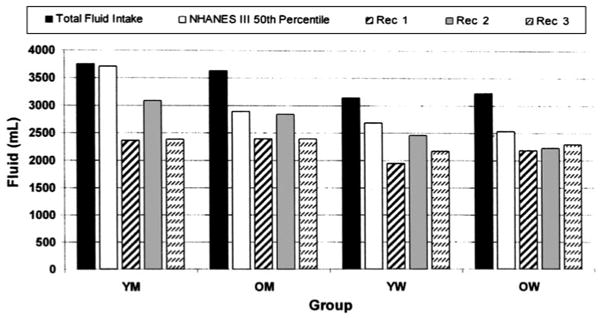
Total fluid intake of younger men (YM), older men (OM), younger women (YW), and older women (OW) with comparisons to recommended (Rec) fluid intakes. The total fluid intake included water consumed ad libitum and from the foods and beverages provided. The third National Health and Nutrition Examination Survey (NHANES III) 50th percentile is the median total fluid intake for the respective groups. These values for the YM and YW are used as the “adequate intake” of water set by the Food and Nutrition Board for all adults, independent of age (2). Rec 1 = 30 mL/kg body wt (13, 14); Rec 2 = 1 mL/kcal energy consumed (15); Rec 3 = 100 mL/kg for first 10 kg, 50 mL/kg for next 10 kg, and 15 mL for each additional kilogram of body weight (16).
No significant differences in daily energy expenditure for physical activity, total time in activity, and the activity indexes sum were found among groups. For the YM, OM, YW, and OW, daily energy expenditure for physical activity was 1069 ± 635, 973 ± 480, 724 ± 340, and 817 ± 443 kcal/d; total time in activity was 28 ± 15, 27 ± 13, 22 ± 11, and 26 ± 14 h/wk; and the activity indexes sum was 48 ± 11, 46 ± 21, 44 ± 11, and 36 ± 14 total units, respectively.
Average perceived thirst was not different between the younger and the older men. Average perceived desire to eat something salty was significantly higher in the older men than in the younger men (P = 0.006). Similar data were not collected from the women.
Discussion
This comprehensive water balance study is the first to document water input and output in younger and older men and women. The results indicate that chronologic age does not influence the ability of a healthy person to consume sufficient fluid to achieve water balance and to maintain indexes of hydration within clinical normalcy.
The observation that age did not influence total water intake is consistent with some previous research. De Castro et al (17), using 7-d food diary data from 262 healthy men and women grouped into 20–34 y, 35–49 y, 50–64 y, and 65–80 y age ranges, reported that total water intake, regardless of source, was not different among the 4 age groups. They also reported that age did not influence water intake from “drinks,” defined as an item ingested in liquid form and not normally considered a food (ie, items such as soups and instant breakfast beverages were excluded). In contrast, Raman et al (18), using 2H-labeled water turnover data from 458 men and women grouped into 40–49 y, 50–59 y, 60–69 y, and 70–79 y age ranges, reported that preformed water intake, defined as water consumed orally from beverages and food, was lower in the 70–79-y-old men than in the 40–49-y-old men and lower in the 70–79-y-old women than in the 40–59-y-old women. The dietary total water intakes of ≈3300 mL/d in older women and 3700 mL/d in older men in the current study are somewhat higher than those Raman et al (18) reported for the 70–79-y-old women (2330 mL/d) and men (2750 mL/d) and are 135–160% of recommended intakes (13–15). The strict dietary control of the present study provides an opportunity to document that age did not influence ad libitum water consumption and that it accounted for ≈44–50% of dietary total water intake among the 4 groups when water intake from food and beverage sources was controlled. The ≈1720, 1830, 1350, and 1640 mL/d ad libitum water intake by the YM, OM, YW, and OW, respectively, represents 91%, 96%, 71%, and 86% of the recommendation to consume 8 glasses (8 oz or 237 mL each) of water each day (≈1900 mL/d) (32, 33).
Improved education of older adults about guidelines for water intake is required. For example, the modified food guide pyramid for adults aged ≥70 y (32) depicts 8 glasses of water as the foundation of the pyramid. However, Russell et al (32) indicated in the description of the pyramid that older adults are encouraged to consume ≥8 servings of noncaffeine, nonalcohol “fluids” per day, but not specifically water. Nutrition education directed toward older adults should continue to emphasize the importance of adequate water intake, but it should more clearly state that this need may be met by consuming a variety of fluids, not exclusively 8 glasses of water. Indeed, little scientific support is found for the recommendation to consume 8 (8-oz or 237 mL) glasses of water (33). The overconsumption of water may cause water intoxication and nonfatal hyponatremia, especially in older adults as a result of an age-associated decline in the kidneys to dilute urine (2, 33).
The current study and previous research by de Castro et al (17, 34) and Raman et al (18) used healthy subjects who were not purposefully exposed to conditions known to alter fluid balance, such as water deprivation, overhydration, altered metabolic state (eg, induced hypertonicity), altered environmental conditions (eg, high temperature or altitude), and prolonged exercise (5, 9, 35–39). Under stressed conditions, thirst responses are controlled by homeostatic mechanisms (36). Older adults exhibit a different thirst response and reduced fluid intake (36), which are associated with an age-related shift in the set point that controls body fluid volume and composition (37, 39). The ability of the older subjects in the current and past research studies (17, 18, 34) to maintain water intakes comparable to those of groups of younger subjects suggests that water consumption was not controlled by homeostatic mechanisms (17, 36). That is, the experimental conditions were such that the main determiners of water intake were unregulated factors, such as the amount and timing of food intake, food and beverage preferences, food and beverage availability, and the consequences of food and beverage intake (eg, urine production). De Castro et al (17) emphasized that the apparent lack of homeostatic control of fluid intake under unstressed conditions does not imply that homeostatic mechanisms are not important in older adults, but only that they are not likely called on when water intake exceeds requirement.
The findings that dietary protein intakes that spanned the range of adequacy did not influence water input, output, and balance corroborate those of Luft et al (40). They reported that water intake (food, beverage, and ad libitum consumption) and urinary excretion were not different when 8 young men consumed either 80 or 180 g protein/d during 7-d controlled feeding periods. De Castro et al (17) observed that, for 20–80-y-old men and women, protein intake positively correlated with total fluid intake. However, this apparent relation did not remain when other dietary factors (ie, carbohydrate, fat, and sodium) were considered with multivariate analyses, and the researchers concluded that the primary determinant of fluid ingestion was the amount of solid ingested, not a specific macronutrient. The current findings that urine specific gravity and plasma osmolality were not influenced by protein intake or subject age, coupled with the lack of change in ad libitum water consumption, support the conclusion that healthy older adults with clinically normal kidney function are able to successfully respond to changes in urea production. These conclusions should not be generalized to high-protein diets (40) or protein deficiency (41) without further research.
The apparent positive net water balance (Table 3) was expected, because water output by sweat was not accounted for in the water balance equation. The accurate assessment of 24-h sweat loss is notoriously difficult to quantify and will vary considerably from person to person. The greater net water balance in men than in women may be attributed to greater sweat losses in men during resting and exercise states (42). The questionnaire-based assessment of physical activity suggested that the 4 groups performed comparable amounts of work, activities, and exercise. This finding would support the conclusion that the differential net water balance between men and women was not due to differences in habitual activities. This conclusion should be drawn cautiously, because the questionnaire was designed to assess patterns of physical activities during the previous month (28) and not on the specific days of water balance assessment. In addition, this questionnaire was designed for use in older adults and is better suited to evaluating the energy expenditure of physical activity for groups of subjects than for individual subjects (43). The stability of body weight during the water balance periods suggests that the positive water balance was not due to body water retention and that these subjects were able to tightly regulate their body water.
The difference in water balance between the men and the women is not likely due to the difference in sodium intake. Luft et al (40) reported that water intake and urinary water excretion in young men were not influenced when sodium intake was set at 10, 200, or 400 mEq/d, and de Castro et al (17, 34) showed that sodium intake did not relate to fluid intake among 262 men and women aged 20–80 y.
The observation that urinary water output was not different in the younger and older men and women is generally consistent with the observation of Raman et al (18), who reported no age-related difference in urinary excretion among women aged 40–69 y, but they reported ≈20% greater urine excretion in 60–69-y-old men than in 40–59-y-old men. In the current study, urinary water excretion accounted for ≈60, 63, 64, and 68% of total water output in the YM, OM, YW, and OW, respectively. These values are comparable to the 66% value reported by Raman et al (18) and higher than the 50% value previously assumed (15). Stool water excretion accounted for ≈4% of total water output among the 4 groups in the present study. The measured stool moisture content was 79%, which is higher than the 72% assumed by Raman et al (18).
The assumed constant hydration of FFM is among the most widely known and applied body-composition constants (44, 45). The biological importance of an age-related change in FFM hydration, as observed in this study, is not well established, but it may relate to age-associated shifts in body composition (including osteoporosis and sarcopenia). The biological importance of the FFM hydration is also applicable to growth, sex, body size, and acute or chronic catabolic illness (44). As reviewed by Wang et al (44), controversy exists as to whether FFM hydration is influenced by age in the adult human. The current observation that FFM hydration (TBW:FFM; Table 1) was higher in the older men and women than in the younger men and women supports previous findings in men (46) and women (47). In contrast, some studies showed no age-related change in FFM hydration (8, 48–51). Wang et al (44) commented that these contradictory findings may relate to differences in health status, levels of physical activity, body mass, and other differential population characteristics. The distribution of body fluid within the intracellular and extracellular compartments is important physiologically but was not assessed in this study.
In summary, the results of this comprehensive water balance study in younger and older men and women suggest that chronologic age does not compromise the ability of an apparently healthy person to consume sufficient water to maintain hydration status. These data provide the first documentation that ad libitum water intake is comparable among younger and older adults who are adapted to controlled diets that provide protein intakes ranging from 63% to 125% of the recommended dietary allowance. These findings are limited to healthy people who are not acutely exposed to stressors known to compromise hydration status. Further research into the effect of age on water balance with the direct measurement of water input and output in older adults under uncontrolled dietary conditions is needed.
Acknowledgments
We thank the volunteers for their dedication to the project. We also greatly appreciate the hard work of WWC's research staff, and we especially thank Heidi Iglay, John Apolzan, Zonda Birge, Jan Green, and Ryan Mercer for their technical assistance with the study.
MJB, NSC, and WWC participated in conducting the study, analyzing the samples, processing and interpreting the data, and writing the manuscript. WWC and NSC also participated in the conception and design of the experiment. None of the authors had any personal or financial conflicts of interest.
Footnotes
Supported by NIH (R01 AG15750 and M01 RR14288) and the US Department of Agriculture (98-35200-6151).
References
- 1.Adolph EF. Physiological regulations. Lancaster, PA: The Jacques Cattell Press; 1943. [Google Scholar]
- 2.Food and Nutrition Board. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: The National Academies Press; 2004. prepublication copy unedited proofs. [Google Scholar]
- 3.National Center for Health Statistics. The third National Health and Nutrition Examination Survey (NHANES III, 1988-94) reference manuals and reports. Hyattsville, MD: US Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics; 1996. [Google Scholar]
- 4.Naitoh M, Burrell LM. Thirst in elderly subjects. J Nutr Health Aging. 1998;2:172–7. [PubMed] [Google Scholar]
- 5.Stout NR, Kenny RA, Baylis PH. A review of water balance in ageing in health and disease. Gerontology. 1999;45:61–6. doi: 10.1159/000022063. [DOI] [PubMed] [Google Scholar]
- 6.Brooks GA. Exercise physiology: human bioenergetics and its applications. 3rd. Mountain View, CA: Mayfield; 2000. [Google Scholar]
- 7.Wardlaw GM, Kessel MW. Perspectives in nutrition. 5th. Boston, MA: McGraw-Hill; 2002. [Google Scholar]
- 8.Schoeller DA. Changes in total body water with age. Am J Clin Nutr. 1989;50:1176–81. doi: 10.1093/ajcn/50.5.1176. [DOI] [PubMed] [Google Scholar]
- 9.Phillips PA, Rolls BJ, Ledingham JG, et al. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311:753–9. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 10.Beck LH. The aging kidney. Defending a delicate balance of fluid and electrolytes. Geriatrics. 2000;55:26–8. 31–2. [PubMed] [Google Scholar]
- 11.Kositzke JA. A question of balance—dehydration in the elderly. J Gerontol Nurs. 1990;16:4–11. doi: 10.3928/0098-9134-19900501-04. [DOI] [PubMed] [Google Scholar]
- 12.Lavizzo-Mourey RJ. Dehydration in the elderly: a short review. J Natl Med Assoc. 1987;79:1033–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Chernoff R. Meeting the nutritional needs of the elderly in the institutional setting. Nutr Rev. 1994;52:132–6. doi: 10.1111/j.1753-4887.1994.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 14.Grant A, DeHoog S. Nutritional assessment and support. 4th. Seattle, WA: Grant/DeHoog; 1991. [Google Scholar]
- 15.National Research Council, Subcommittee on the Tenth Edition of the RDAs, National Institutes of Health, National Research Council, Committee on Dietary Allowances. Recommended dietary allowances. 10th. Washington, DC: National Academy Press; 1989. [Google Scholar]
- 16.Skipper A. Dietitian's handbook of enteral and parenteral nutrition. Rockville, MD: Aspen Publishers; 1989. [Google Scholar]
- 17.de Castro JM. Age-related changes in natural spontaneous fluid ingestion and thirst in humans. J Gerontol. 1992;47:P321–30. doi: 10.1093/geronj/47.5.p321. [DOI] [PubMed] [Google Scholar]
- 18.Raman A, Schoeller DA, Subar AF, et al. Water turnover in 458 American adults 40–79 yr of age. Am J Physiol Renal Physiol. 2004;286:F394–401. doi: 10.1152/ajprenal.00295.2003. [DOI] [PubMed] [Google Scholar]
- 19.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institute of Washington; 1919. Publication no. 279. [Google Scholar]
- 20.Morse MH, Haub MD, Evans WJ, Campbell WW. Protein requirement of elderly women: nitrogen balance responses to three levels of protein intake. J Gerontol A Biol Sci Med Sci. 2001;56:M724–30. doi: 10.1093/gerona/56.11.m724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Bois EF. Basal metabolism in health and disease. Philadelphia, PA: Lea & Febiger; 1924. [Google Scholar]
- 22.Ziegler EE, Filer LJ, International Life Sciences Institute-Nutrition Foundation . Present knowledge in nutrition. 7th. Washington, DC: ILSI Press; 1996. [Google Scholar]
- 23.McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27:1686–91. [PubMed] [Google Scholar]
- 24.Yee AJ, Fuerst T, Salamone L, et al. Calibration and validation of an air-displacement plethysmography method for estimating percentage body fat in an elderly population: a comparison among compartmental models. Am J Clin Nutr. 2001;74:637–42. doi: 10.1093/ajcn/74.5.637. [DOI] [PubMed] [Google Scholar]
- 25.Siri WE, editor. Body composition from fluid spaces and density: analysis of methods. Washington, DC: National Academy of Sciences; 1961. [PubMed] [Google Scholar]
- 26.Schloerb PR, Friis-Hansen BJ, Edelman IS, Solomon AK, Moore FD. The measurement of total body water in the human subject by deuterium oxide dilution; with a consideration of the dynamics of deuterium distribution. J Clin Invest. 1950;29:1296–310. doi: 10.1172/JCI102366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukaski HC, Johnson PE. A simple, inexpensive method of determining total body water using a tracer dose of D2O and infrared absorption of biological fluids. Am J Clin Nutr. 1985;41:363–70. doi: 10.1093/ajcn/41.2.363. [DOI] [PubMed] [Google Scholar]
- 28.DiPietro L, Caspesen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25:628–42. [PubMed] [Google Scholar]
- 29.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “labeled magnitude scale” for measuring sensations of taste and smell. Chem Senses. 1996;21:323–34. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 30.Popowski LA, Oppliger RA, Patrick Lambert G, Johnson RF, Kim Johnson A, Gisolf CV. Blood and urinary measures of hydration status during progressive acute dehydration. Med Sci Sports Exerc. 2001;33:747–53. doi: 10.1097/00005768-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Zeman FJ, Ney DM. Applications in medical nutrition therapy. 2nd. Englewood Cliffs; NJ: Merrill: 1996. [Google Scholar]
- 32.Russell RM, Rasmussen H, Lichtenstein AH. Modified food guide pyramid for people over seventy years of age. J Nutr. 1999;129:751–3. doi: 10.1093/jn/129.3.751. [DOI] [PubMed] [Google Scholar]
- 33.Valtin H. “Drink at least eight glasses of water a day.” Really? Is there scientific evidence for “8 × 8”? Am J Physiol Regul Integr Comp Physiol. 2002;283:R993–1004. doi: 10.1152/ajpregu.00365.2002. [DOI] [PubMed] [Google Scholar]
- 34.de Castro JM. The relationship of spontaneous macronutrient and sodium intake with fluid ingestion and thirst in humans. Physiol Behav. 1991;49:513–9. doi: 10.1016/0031-9384(91)90273-q. [DOI] [PubMed] [Google Scholar]
- 35.Consolazio CF, Matoush LO, Johnson HL, Daws TA. Protein and water balances of young adults during prolonged exposure to high altitude (4300 meters) Am J Clin Nutr. 1968;21:154–61. [Google Scholar]
- 36.Kenney WL, Chiu P. Influence of age on thirst and fluid intake. Med Sci Sports Exerc. 2001;33:1524–32. doi: 10.1097/00005768-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol. 1994;76:1615–23. doi: 10.1152/jappl.1994.76.4.1615. [DOI] [PubMed] [Google Scholar]
- 38.Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol. 1998;274:R187–95. doi: 10.1152/ajpregu.1998.274.1.R187. [DOI] [PubMed] [Google Scholar]
- 39.Stachenfeld NS, Mack GW, Takamata A, DiPietro L, Nadel ER. Thirst and fluid regulatory responses to hypertonicity in older adults. Am J Physiol. 1996;271:R757–65. doi: 10.1152/ajpregu.1996.271.3.R757. [DOI] [PubMed] [Google Scholar]
- 40.Luft FC, Fineberg NS, Sloan RS, Hunt JN. The effect of dietary sodium and protein on urine volume and water intake. J Lab Clin Med. 1983;101:605–10. [PubMed] [Google Scholar]
- 41.Jamison RL, Oliver RE. Disorders of urinary concentration and dilution. Am J Med. 1982;72:308–22. doi: 10.1016/0002-9343(82)90823-3. [DOI] [PubMed] [Google Scholar]
- 42.Kaciuba-Uscilko H, Grucza R. Gender differences in thermoregulation. Curr Opin Clin Nutr Metab Care. 2001;4:533–6. doi: 10.1097/00075197-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Kruskall LJ, Campbell WW, Evans WJ. The Yale Physical Activity Survey for older adults: predictions in the energy expenditure due to physical activity. J Am Diet Assoc. 2004;104:1251–7. doi: 10.1016/j.jada.2004.05.207. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am J Clin Nutr. 1999;69:833–41. doi: 10.1093/ajcn/69.5.833. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: new physiological modeling approach. Am J Physiol. 1999;276:E995–1003. doi: 10.1152/ajpendo.1999.276.6.E995. [DOI] [PubMed] [Google Scholar]
- 46.Hewitt MJ, Going SB, Williams DP, Lohman TG. Hydration of the fat-free body mass in children and adults: implications for body composition assessment. Am J Physiol. 1993;265:E88–95. doi: 10.1152/ajpendo.1993.265.1.E88. [DOI] [PubMed] [Google Scholar]
- 47.Bergsma-Kadijk JA, Baumeister B, Deurenberg P. Measurement of body fat in young and elderly women: comparison between a four-compartment model and widely used reference methods. Br J Nutr. 1996;75:649–57. doi: 10.1079/bjn19960170. [DOI] [PubMed] [Google Scholar]
- 48.Visser M, Gallagher D, Deurenberg P, Wang J, Pierson RN, Jr, Heymsfield SB. Density of fat-free body mass: relationship with race, age, and level of body fatness. Am J Physiol. 1997;272:E781–7. doi: 10.1152/ajpendo.1997.272.5.E781. [DOI] [PubMed] [Google Scholar]
- 49.Baumgartner RN, Heymsfield SB, Lichtman S, Wang J, Pierson RN., Jr Body composition in elderly people: effect of criterion estimates on predictive equations. Am J Clin Nutr. 1991;53:1345–53. doi: 10.1093/ajcn/53.6.1345. [DOI] [PubMed] [Google Scholar]
- 50.Goran MI, Poehlman ET, Danforth E, Jr, Nair KS. Comparison of body fat estimates derived from underwater weight and total body water. Int J Obes Relat Metab Disord. 1994;18:622–6. [PubMed] [Google Scholar]
- 51.Mazariegos M, Wang ZM, Gallagher D, et al. Differences between young and old females in the five levels of body composition and their relevance to the two-compartment chemical model. J Gerontol. 1994;49:M201–8. doi: 10.1093/geronj/49.5.m201. [DOI] [PubMed] [Google Scholar]


