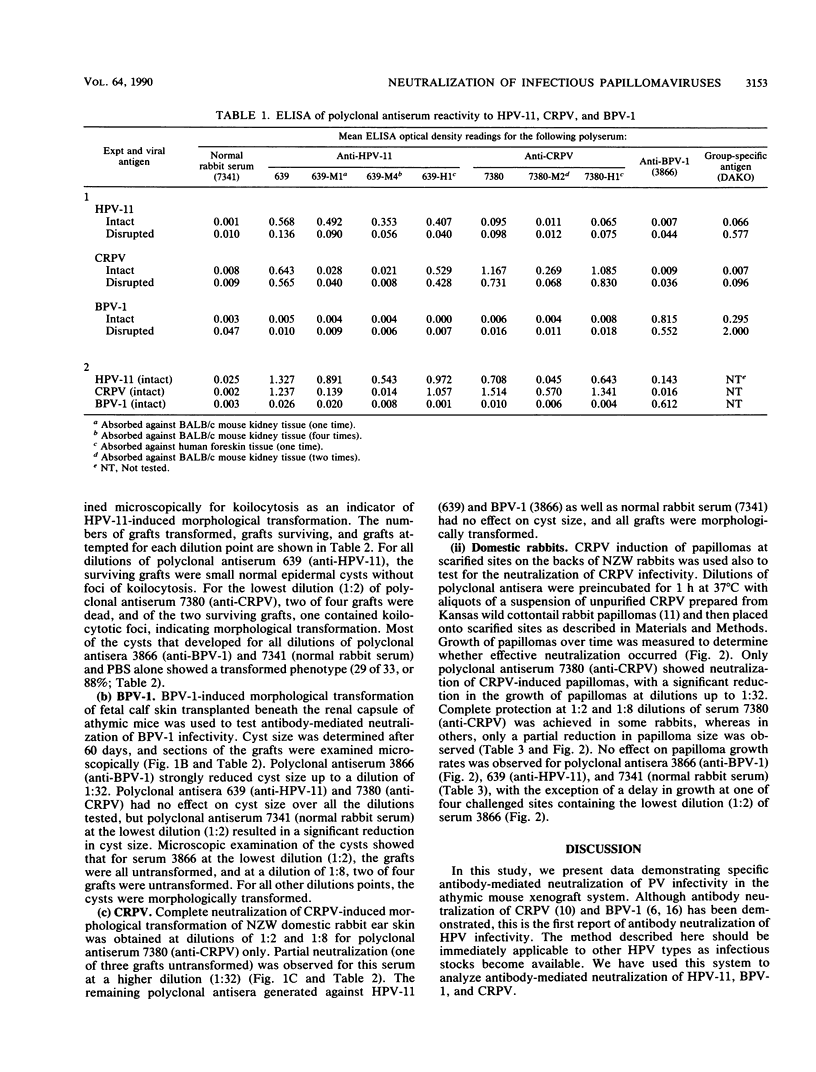
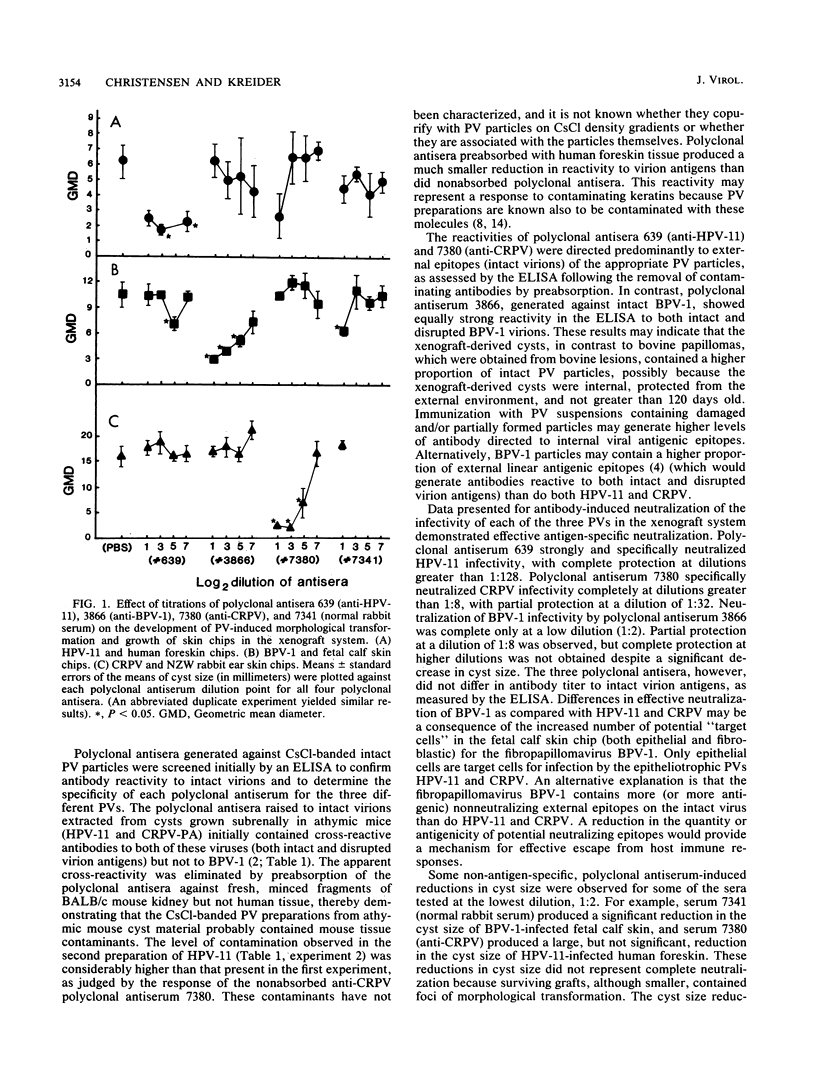
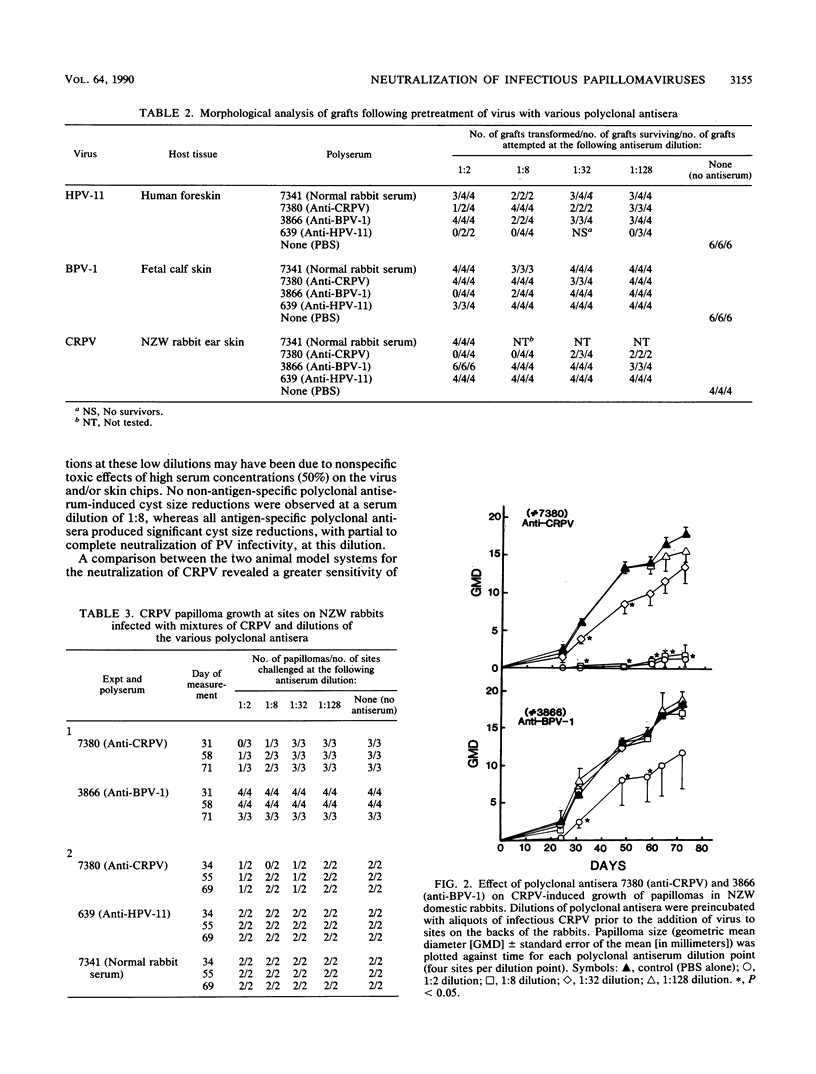
Abstract
Specific antibody-mediated neutralization of infectious human papillomavirus type 11 (HPV-11) was achieved in the athymic mouse xenograft system, in which HPV-11 induced morphological transformation of human foreskin. Virus-specific neutralization was demonstrated by the ability of an HPV-11-specific polyclonal antiserum to neutralize HPV-11 infectivity and not bovine papillomavirus type 1 (BPV-1) or cottontail rabbit papillomavirus (CRPV) infectivity. In all three virus infectivity systems, neutralization was detected by the failure of the virus suspension to induce morphological transformation of the appropriate skin xenografts placed under the renal capsule of athymic mice. Rabbit polyclonal antisera were also generated against intact virions of both BPV-1 and CRPV, and neutralizing activity was tested in the xenograft system with BPV-1 and fetal bovine skin and with CRPV and rabbit ear skin. The three polyclonal antisera contained virus-specific neutralizing antibodies, demonstrating that neutralizing epitopes existed on all three papillomaviruses and that these epitopes were antigenically non-cross-reactive. The athymic mouse xenograft system was a useful model for detecting papillomavirus-specific neutralizing antibodies and offers the only opportunity for the analysis of neutralizing antibodies to human papillomaviruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen N. D., Kreider J. W., Cladel N. M., Galloway D. A. Immunological cross-reactivity to laboratory-produced HPV-11 virions of polysera raised against bacterially derived fusion proteins and synthetic peptides of HPV-6b and HPV-16 capsid proteins. Virology. 1990 Mar;175(1):1–9. doi: 10.1016/0042-6822(90)90180-y. [DOI] [PubMed] [Google Scholar]
- Cowsert L. M., Lake P., Jenson A. B. Topographical and conformational epitopes of bovine papillomavirus type 1 defined by monoclonal antibodies. J Natl Cancer Inst. 1987 Nov;79(5):1053–1057. [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol. 1975 May;15(5):1239–1247. doi: 10.1128/jvi.15.5.1239-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey S. J., Nielsen T., Vetner M., Storgaard L., Smed V., Larsen P. M. Demonstration of in vitro synthesis of human papilloma viral proteins from hand and foot warts. J Invest Dermatol. 1989 Jun;92(6):817–824. doi: 10.1111/1523-1747.ep12696833. [DOI] [PubMed] [Google Scholar]
- GALLOWAY T. C., SOPER G. R., ELSEN J. Carcinoma of the larynx after irradiation for papilloma. Arch Otolaryngol. 1960 Sep;72:289–294. doi: 10.1001/archotol.1960.00740010297001. [DOI] [PubMed] [Google Scholar]
- KREIDER J. W. STUDIES ON THE MECHANISM RESPONSIBLE FOR THE SPONTANEOUS REGRESSION OF THE SHOPE RABBIT PAPILLOMA. Cancer Res. 1963 Oct;23:1593–1599. [PubMed] [Google Scholar]
- Kreider J. W., Bartlett G. L., Sharkey F. E. Primary neoplastic transformation in vivo of xenogeneic skin grafts on nude mice. Cancer Res. 1979 Jan;39(1):273–276. [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Leure-Dupree A. E., Zaino R. J., Weber J. A. Laboratory production in vivo of infectious human papillomavirus type 11. J Virol. 1987 Feb;61(2):590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider J. W., Howett M. K., Wolfe S. A., Bartlett G. L., Zaino R. J., Sedlacek T., Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature. 1985 Oct 17;317(6038):639–641. doi: 10.1038/317639a0. [DOI] [PubMed] [Google Scholar]
- Larsen P. M., Storgaard L., Fey S. J. Proteins present in bovine papillomavirus particles. J Virol. 1987 Nov;61(11):3596–3601. doi: 10.1128/jvi.61.11.3596-3601.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



