Abstract
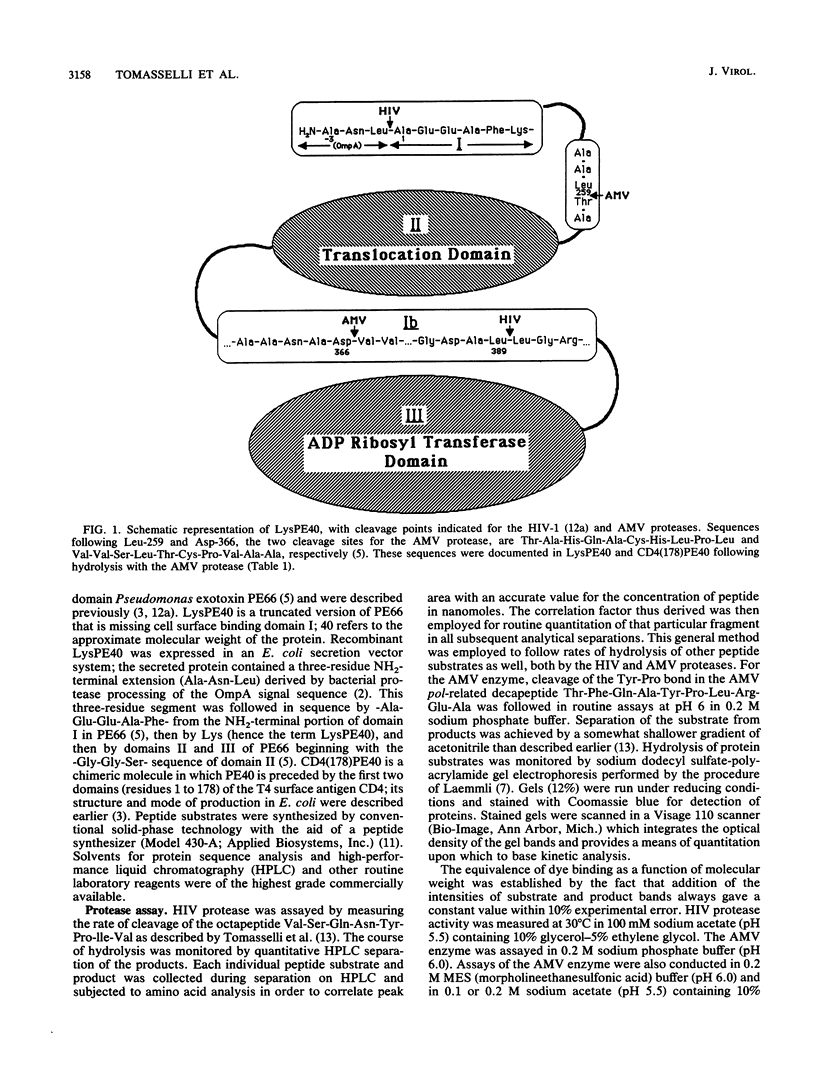
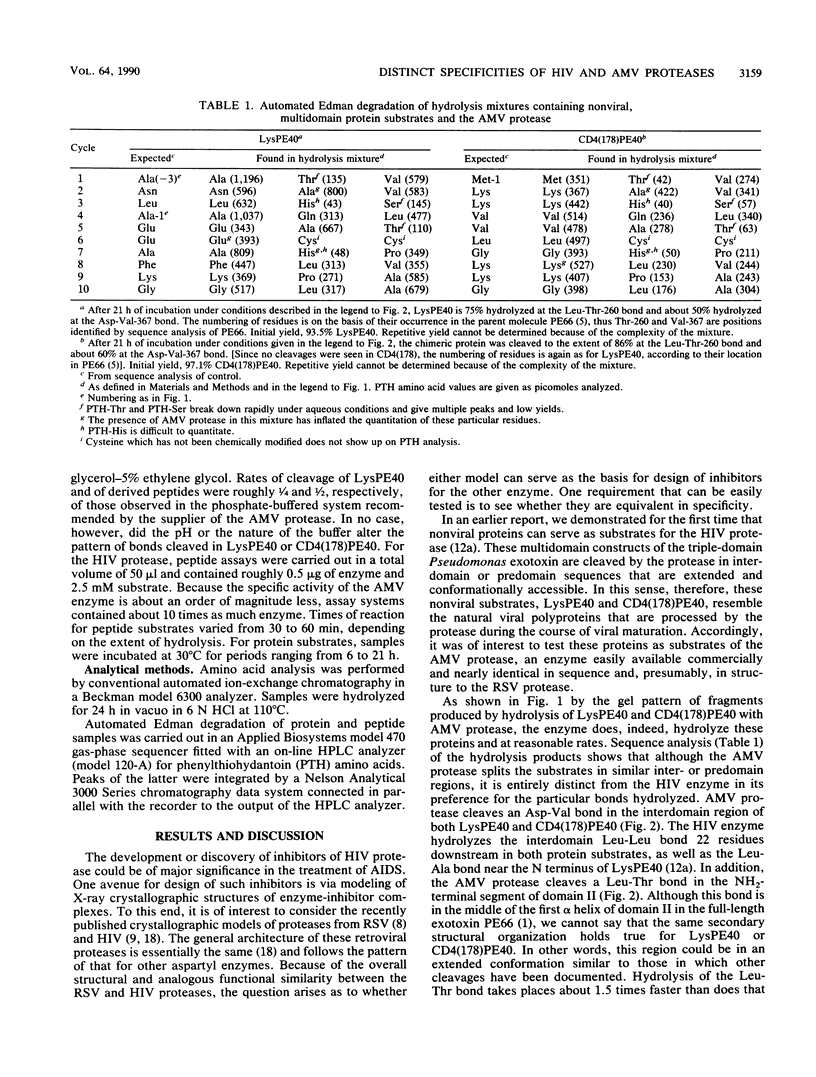
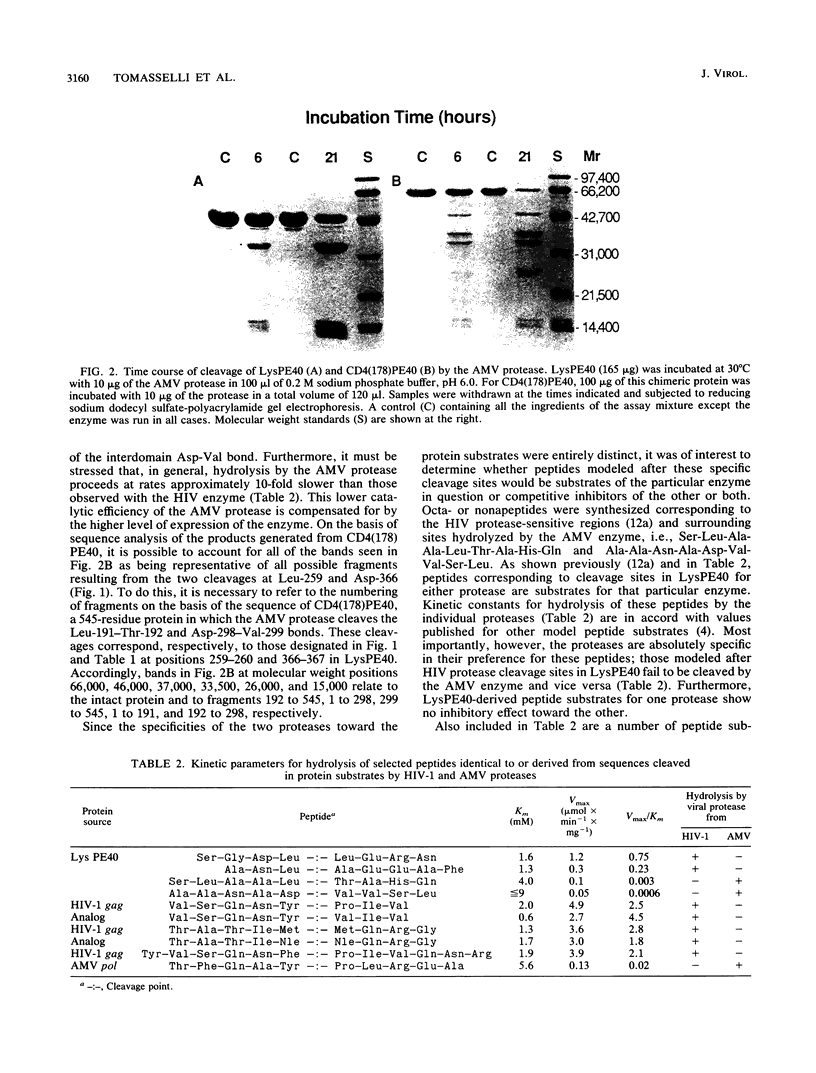
The virally encoded proteases from human immunodeficiency virus (HIV) and avian myeloblastosis virus (AMV) have been compared relative to their ability to hydrolyze a variant of the three-domain Pseudomonas exotoxin, PE66. This exotoxin derivative, missing domain I and referred to as LysPE40, is made up of a 13-kilodalton NH2-terminal translocation domain II connected by a segment of 40 amino acids to enzyme domain III of the toxin, a 23-kilodalton ADP-ribosyltransferase. HIV protease hydrolyzes two peptide bonds in LysPE40, a Leu-Leu bond in the interdomain region and a Leu-Ala bond in a nonstructured region three residues in from the NH2-terminus. Neither of these sites is cleaved by the AMV enzyme; hydrolysis occurs, instead, at an Asp-Val bond in another part of the interdomain segment and at a Leu-Thr bond in the NH2-terminal region of domain II. Synthetic peptides corresponding to these cleavage sites are hydrolyzed by the individual proteases with the same specificity displayed toward the protein substrate. Peptide substrates for one protease are neither substrates nor competitive inhibitors for the other. A potent inhibitor of HIV type 1 protease was more than 3 orders of magnitude less active toward the AMV enzyme. These results suggest that although the crystallographic models of Rous sarcoma virus protease (an enzyme nearly identical to the AMV enzyme) and HIV type 1 protease show a high degree of similarity, there exist structural differences between these retroviral proteases that are clearly reflected by their kinetic properties.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra J. K., Jinno Y., Chaudhary V. K., Kondo T., Willingham M. C., FitzGerald D. J., Pastan I. Antitumor activity in mice of an immunotoxin made with anti-transferrin receptor and a recombinant form of Pseudomonas exotoxin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8545–8549. doi: 10.1073/pnas.86.21.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Mizukami T., Fuerst T. R., FitzGerald D. J., Moss B., Pastan I., Berger E. A. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature. 1988 Sep 22;335(6188):369–372. doi: 10.1038/335369a0. [DOI] [PubMed] [Google Scholar]
- Darke P. L., Nutt R. F., Brady S. F., Garsky V. M., Ciccarone T. M., Leu C. T., Lumma P. K., Freidinger R. M., Veber D. F., Sigal I. S. HIV-1 protease specificity of peptide cleavage is sufficient for processing of gag and pol polyproteins. Biochem Biophys Res Commun. 1988 Oct 14;156(1):297–303. doi: 10.1016/s0006-291x(88)80839-8. [DOI] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler M., Katz R. A., Danho W., Leis J., Skalka A. M. Synthetic peptides as substrates and inhibitors of a retroviral protease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4185–4189. doi: 10.1073/pnas.85.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller M., Jaskólski M., Rao J. K., Leis J., Wlodawer A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature. 1989 Feb 9;337(6207):576–579. doi: 10.1038/337576a0. [DOI] [PubMed] [Google Scholar]
- Navia M. A., Fitzgerald P. M., McKeever B. M., Leu C. T., Heimbach J. C., Herber W. K., Sigal I. S., Darke P. L., Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989 Feb 16;337(6208):615–620. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- Richards A. D., Roberts R., Dunn B. M., Graves M. C., Kay J. Effective blocking of HIV-1 proteinase activity by characteristic inhibitors of aspartic proteinases. FEBS Lett. 1989 Apr 10;247(1):113–117. doi: 10.1016/0014-5793(89)81251-7. [DOI] [PubMed] [Google Scholar]
- Sawyer T. K., Pals D. T., Mao B., Staples D. J., de Vaux A. E., Maggiora L. L., Affholter J. A., Kati W., Duchamp D., Hester J. B. Design, structure-activity, and molecular modeling studies of potent renin inhibitory peptides having N-terminal Nin-For-Trp (Ftr): angiotensinogen congeners modified by P1-P1' Phe-Phe, Sta, Leu psi[CH(OH)CH2]Val or leu psi[CH2NH]Val substitutions. J Med Chem. 1988 Jan;31(1):18–30. doi: 10.1021/jm00396a006. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. Retroviral proteases: first glimpses at the anatomy of a processing machine. Cell. 1989 Mar 24;56(6):911–913. doi: 10.1016/0092-8674(89)90621-1. [DOI] [PubMed] [Google Scholar]
- Tomasselli A. G., Hui J. O., Sawyer T. K., Staples D. J., FitzGerald D. J., Chaudhary V. K., Pastan I., Heinrikson R. L. Interdomain hydrolysis of a truncated Pseudomonas exotoxin by the human immunodeficiency virus-1 protease. J Biol Chem. 1990 Jan 5;265(1):408–413. [PubMed] [Google Scholar]
- Tomasselli A. G., Olsen M. K., Hui J. O., Staples D. J., Sawyer T. K., Heinrikson R. L., Tomich C. S. Substrate analogue inhibition and active site titration of purified recombinant HIV-1 protease. Biochemistry. 1990 Jan 9;29(1):264–269. doi: 10.1021/bi00453a036. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Miller M., Jaskólski M., Leis J., Skalka A. M., Wlodawer A. Molecular modeling of the HIV-1 protease and its substrate binding site. Science. 1989 Feb 17;243(4893):928–931. doi: 10.1126/science.2537531. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]