Abstract
Cardiac hypertrophy and dilatation can result from stimulation of signal transduction pathways mediated by heterotrimeric G proteins, especially Gq, whose α subunit activates phospholipase Cβ (PLCβ). We now report that transient, modest expression of a hemagglutinin (HA) epitope-tagged, constitutively active mutant of the Gq α subunit (HAα*q) in hearts of transgenic mice is sufficient to induce cardiac hypertrophy and dilatation that continue to progress after the initiating stimulus becomes undetectable. At 2 weeks, HAα*q protein is expressed at less than 50% of endogenous αq/11, and the transgenic hearts are essentially normal morphologically. Although HAα*q protein declines at 4 weeks and is undetectable by 10 weeks, the animals develop cardiac hypertrophy and dilatation and die between 8 and 30 weeks in heart failure. As the pathology develops, endogenous αq/11 rises (2.9-fold in atria; 1.8-fold in ventricles). At 2 weeks, basal PLC activity is increased 9- to 10-fold in atria but not ventricles. By 10 weeks, it is elevated in both, presumably because of the rise in endogenous αq/11. We conclude that the pathological changes initiated by early, transient HAα*q expression are maintained in part by compensatory changes in signal transduction and other pathways. Cyclosporin A (CsA) prevents hypertrophy caused by activation of calcineurin [Molkentin, J. D., Lu, J.-R., Antos, C. L., Markham, B., Richardson, J., Robbins, J., Grant, S. R. & Olson, E. N. (1998) Cell 93, 215–228]. Because HAα*q acts upstream of calcineurin, we hypothesized that HAα*q might initiate additional pathways leading to hypertrophy and dilatation. Treating HAα*q mice with CsA diminished some, but not all, aspects of the hypertrophic phenotype, suggesting that multiple pathways are involved.
Many kinds of stresses can lead to cardiac hypertrophy and dilatation; some are mechanical, others are the result of stimulation of signal transduction pathways mediated by heterotrimeric G proteins, especially Gq, whose α subunit stimulates phospholipase Cβ (PLCβ). For example, activation of receptors that are coupled to Gq (such as receptors for endothelin, angiotensin II, and α1-adrenergic agonists) leads to hypertrophy in isolated neonatal cardiomyocytes (reviewed in ref. 1) and in vivo (2–4). Although each of these receptors activates Gαq, the hypertrophic phenotype differs among the different models in age of onset, severity, chamber-specificity, and histology. Some of the variation may be the result of receptors coupling to more than one G protein and initiating several signaling pathways. The importance of Gαq is emphasized further by the observation that interfering with the interface between Gq-coupled receptors and Gαq by overexpressing a carboxyl-terminal peptide of Gαq in hearts of transgenic mice caused the mice to develop significantly less hypertrophy in response to pressure overload (5). While the present work was in progress, D’Angelo et al. (6) reported that a 4-fold increase in the expression of wild-type Gαq in the mouse heart led to cardiac hypertrophy at 12–14 weeks. The authors carefully described the cardiac function of transgenic hearts by echocardiography and hemodynamic studies. However, the previous studies have not reported whether continuous transgene expression is necessary for the development and progression of cardiac pathology.
We now report that transient, modest cardiac expression of a hemagglutinin (HA) epitope-tagged, constitutively active mutant of the Gq α subunit (HAα*q) in transgenic mice is sufficient to cause hypertrophy and dilatation both of atria and ventricles that proceed inexorably to death in cardiac failure. While it is not unusual for short but complex stimuli (for example, myocardial infarction) to lead to progressive cardiac pathology, it has not been shown previously that transient expression of a single protein can do so. The internally placed HA epitope does not interfere with HAα*q function (7), but enabled us to distinguish transgenic HAα*q from endogenously expressed αq and to determine the time course of its expression. The mutant protein has diminished GTPase activity, so it remains in its active, GTP-bound state and does not depend on receptor-mediated activation (8). Therefore, it should be effective at a low level of expression. Furthermore, since HAα*q interacts poorly with G protein βγ subunits, it is unlikely to bind to endogenous βγ subunits and therefore shift the equilibrium of other α subunits to the dissociated, active state.
Recently, Molkentin et al. (9) demonstrated that calcineurin, a Ca2+-activated phosphatase, regulates cardiac hypertrophy in mice through activation of NFAT (nuclear factor of activated T cells), which then binds to GATA, forming a transcription factor that may control the hypertrophic gene response. Mice expressing an active form of calcineurin had a 2- to 3-fold increase of heart weight/body weight ratios compared with control littermates. However, treatment with cyclosporin A (CsA) prevented hypertrophy, presumably through inhibition of calcineurin and prevention of the dephosphorylation of NFAT. Because HAα*q acts upstream of calcineurin, activating additional signaling pathways (such as protein kinase C), we expected that CsA treatment might blunt some effects of HAα*q expression, but not prevent the cardiac pathology. Indeed, our results suggest that pathways in addition to activation of calcineurin may contribute to the development of the hypertrophic, dilated phenotype initiated by HAα*q.
MATERIALS AND METHODS
Plasmid Construction and Generation of Transgenic Mice.
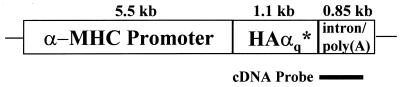
The cDNA encoding for mouse HA epitope-tagged, constitutively active G protein αq subunit (HAαqQ209L or HAα*q) was kindly provided by Paul T. Wilson and Henry R. Bourne (University of San Francisco, CA). A pGEM-9Zf vector containing the murine 5.5-kb α-myosin heavy chain promoter and the simian virus 40 (SV40) intron/polyadenylation signal (0.85 kb) were the gift of Walter J. Koch (Duke University Medical Center, Durham, NC). The 1.1-kb HindIII-NotI fragment containing the coding sequence for HAα*q was ligated into the blunted SalI sites of pGEM-9Zf and confirmed by restriction mapping and nucleotide sequencing. A linear 7.45-kb DNA fragment was released with KpnI and NotI. Transgenic FVB mice were generated by the Transgenic Mouse Facility of Harvard Medical School. The transgene was identified by PCR with transgene-specific primers and by genomic Southern analysis using a random-primed 32P-labeled DNA probe consisting of part of the SV40 intron sequence (750 bp). We used strain α*q52.
Histology.
Hearts were fixed in 4% neutral-buffered formalin or 0.1 M cacodylate-buffered 2.5% glutaraldehyde/2% paraformaldehyde at pH 7.4 (Karnovsky fixative), cut either transversely at the midventricular level or bisected in a plane that exposed both atria and ventricles, and embedded in paraffin. Sections were made from the cut surfaces at 4–5 μm and stained with the hematoxylin and eosin stain for overall morphology and Massons’ trichrome stain for collagen.
Northern Blot Analysis.
Total RNA from ventricles of α*q52 and wild-type littermates was extracted with RNAzol B (Tel-Test, Friendswood, TX). Total RNA (5–10 μg) was size-fractionated and blotted by using standard techniques. cDNA probes consisting of part of the SV40 intron sequence (750 bp), rat atrial natriuretic factor (ANF, base pair 1–580; kindly provided by Christine E. Seidman, Harvard Medical School, Boston, MA), or human glyceraldehyde-3-phosphate dehydrogenase (GAPDH, CLONTECH) were random-primed 32P-labeled. Oligonucleotide probes for mouse α-myosin heavy chain (α-MHC) and β-MHC (as described in ref. 10) were synthesized and labeled with [γ-32P]ATP by T4 polynucleotide kinase (GIBCO/BRL). Blots were prehybridized in 50% formamide/10× Denhardt’s solution/1% SDS/5× SSC (20× SSC: 3 M NaCl/0.3 M sodium citrate dihydrate)/100 μg/ml salmon sperm DNA, and hybridized with 3 × 106 cpm/ml of cDNA probes or 3 × 107 cpm/ml of oligonucleotide probes. After two washes each with 1× SSC/0.1% SDS and 0.25× SSC/0.1% SDS for 15 min at room temperature, followed by a 10 min wash at 55°C with 0.25× SSC/0.1% SDS (cDNA probes) or 0.5× SSC/0.1% SDS (oligonucleotide probes), the blots were exposed to x-ray film at −70°C.
Western Blot Analysis.
Hearts were obtained from α*q52 and wild-type littermates at 14–16 days, 30 days, and 62–75 days (designated as 2, 4, and 10 weeks, respectively). Atrial appendages‖ and ventricles were isolated and quickly frozen in liquid nitrogen. On the day of homogenization, left and right atria from 8–10 2-week-old wild-type or α*q52 mice were pooled. Only right atria from 10-week-old mice were pooled (eight wild-type mice and two to three α*q52 mice), since the left atrium often contained thrombi. The heart tissue was homogenized on ice in 50 mM Tris⋅HCl, pH 7.6/6 mM MgCl2/75 mM sucrose/1 mM DTT/1 mM EDTA plus proteinase inhibitors (3 mM benzamidine and 1 μg/ml each soya and lima bean trypsin inhibitor and leupeptin). Particulate and soluble fractions were separated by ultracentrifugation at 48,500 × g for 30 min at 4°C. The pellet was resuspended with a glass–teflon Potter–Elvehjem tissue grinder. The protein was determined by Bradford assay (11) with BSA as standard.
Seventy-five micrograms of protein from both fractions was separated on 9% SDS/PAGE (12), followed by wet blotting (13). Nitrocellulose strips were blocked with 5% nonfat dry milk in PBS, incubated with 12CA5 recognizing the HA epitope (1:1,000, Babco) and C-19 recognizing Gαq/11 (1:500; Santa Cruz Biotechnology), washed with PBS plus 0.1% Tween 20, and developed with horseradish peroxidase-conjugated secondary antibodies and chemiluminescence. Quantitation was done by computerized densitometry by using nih image 1.61. When endogenous αq/11 levels were measured, every gel contained wild-type samples of the appropriate age that were averaged and set at 100%. Transgenic samples were expressed as percentage of wild type.
Measurement of [3H]Inositol Phosphate Formation.
PLC activity was measured by a modification of the method of Brown and Brown (14). Small pieces (1–3 mg) of left and right atria and of the free right ventricular wall from 2- and 10-week-old α*q52 and wild-type littermates were quickly prepared, blotted, weighed, and placed into a 24-well culture dish with 500 μl oxygenated (95% O2/5% CO2) Krebs–Henseleit buffer containing 118 mM NaCl/4.7 mM KCl/3 mM CaCl2/1.2 mM KH2PO4/25 mM NaHCO3/1.2 mM MgSO4/0.5 mM EDTA/10 mM glucose. After 1.5 hr of labeling with 4 μCi/ml myo[3H]inositol (16.3 Ci/mmol, Amersham; Life Science, Arlington Heights, IL), LiCl was added to a final concentration of 10 mM for another 30 min. Extraction and separation of inositol phosphates were performed as described in Li et al. (15). The amount of protein in each tissue piece was determined according to Bradford (11) after solubilization of the extracted tissue in 0.5 M NaOH. The results were the same whether referred to mg protein or tissue wet weight. All tissue pieces were viable since, in agreement with ref. 14, they were able to respond to carbamylcholine with a 3- to 5-fold increase in PLC activity (data not shown).
Treatment with CsA.
Beginning at 17–18 days of age, pups in two litters each (containing α*q52 mice and wild types) were injected twice daily s.c. in the back of the neck with either CsA (15 mg/kg body weight) or vehicle (Cremophor EL and ethanol in PBS). The body weight of all mice was monitored every other day. At 43–45 days, mice were sacrificed, hearts and lungs were weighed, and the apex of the ventricles was quickly frozen in liquid nitrogen for subsequent RNA analysis. The right tibia of each mouse was isolated and the length was measured.
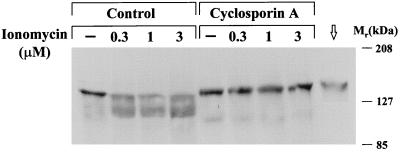
To demonstrate the efficiency of CsA treatment, Ca2+-induced NFAT-1/p dephosphorylation was studied in Western blots of whole spleen cell lysates from CsA-treated mice compared with control mice. Spleens were removed from animals sacrificed 5 hr after the last injection, placed in ice-cold DMEM (supplemented with 10 mM l-glutamine and 10% fetal calf serum), and dissociated by using a 70-μm mesh nylon cell strainer. Cells were counted immediately, adjusted to 10 × 106 cells/ml, and incubated for 10 min with or without ionomycin (0.3–3 μM). Cells were pelleted, resuspended in 1× electrophoresis sample buffer (ref. 12; supplemented with 20 mM sodium pyrophosphate and 10 mM EDTA), and boiled for 1 hr or until the lysates were no longer viscous. Whole spleen lysates (10 × 106 cell equivalents per lane) were resolved on 7% SDS/PAGE and analyzed with an anti-NFAT-1/p (anti-67.1) antibody (16).
RESULTS
Production of Transgenic Mice.
Transgenic mice were created that express a HA epitope-tagged, constitutively active mutation of αq, HAα*q, under the control of the α-MHC promoter (Fig. 1). Replacing amino acids 125–130 in αq with part of the HA epitope, does not interfere with its function (7). The ability of the α-MHC promoter to drive cardiac-specific transgene expression has been well established (for example, refs. 2 and 4). This promoter is active throughout development in the atria and turns on around birth in the ventricle (17, 18). Two independent transgenic lines (α*q44 and α*q52) were established from two male founders. The analyses in this report focus on the development of hypertrophy in the α*q52 line. Mice from the α*q44 line express low levels of HAα*q mRNA and only begin to reexpress ventricular ANF at 5 months of age, whereas α*q52 mice die between 2 and 7 months with massive cardiac dilatation and hypertrophy (see below).
Figure 1.
Schematic representation of the transgenic vector.
Cardiac Pathology in α*q52 Mice.
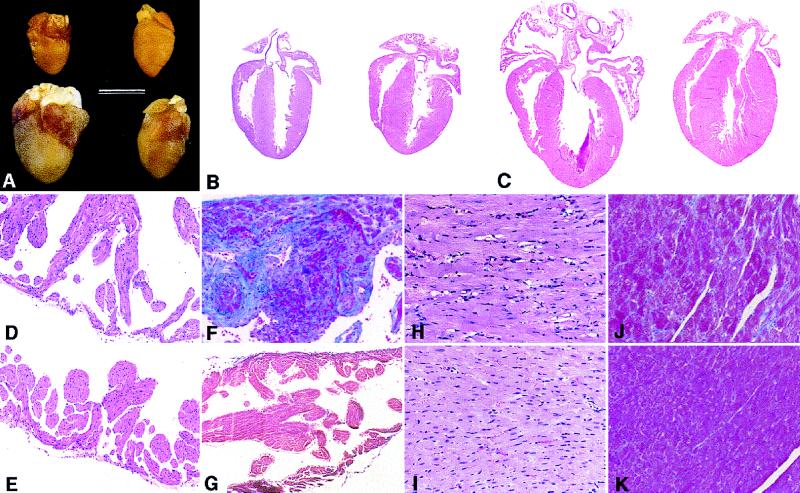
At 2 weeks, the hearts from α*q52 mice were essentially normal and barely distinguishable from wild types. The ventricular weight/body weight (VW/BW) ratio in α*q52 mice compared with wild-type mice was only slightly increased (Table 1). The atrial weight/body weight (AW/BW) ratio was not changed significantly (Table 1), although there was a suggestion of mild, variable atrial enlargement (Fig. 2A). No consistent pathologic findings were noted upon histological examination of any chamber (not shown). By 4 weeks, the AW/BW and VW/BW ratios were increased, and by 10 weeks, α*q52 mice revealed massive enlargement and dilatation of all four chambers (Fig. 2 A and C). Histological examination showed atrial thrombi in various stages of organization. The atria were dilated with wall thinning and fibrosis (Fig. 2 D and F, respectively). Since there were often thrombi in the left atria, the degree of hypertrophy is better reflected by the VW/BW ratio that was increased to 121% of wild type (Table 1). The cardiomyocytes of the hypertrophied, dilated ventricles were enlarged with big hyperchromatic nuclei and degenerative changes (including intracellular vacuolization) (Fig. 2H). There was diffuse interstitial fibrosis (Fig. 2J), but no evidence of myocyte disarray, necrosis, or myocarditis. Similar to our model, expression of unactivated Gαq (6), α1-adrenergic receptors (2), or angiotensin AT1 receptors (4) caused cardiomyopathy with myocyte hypertrophy and vacuolization without myocyte disarray or necrosis. In contrast, we observed diffuse atrial and ventricular fibrosis, not reported in those studies. The α*q52 mice died spontaneously between 8 and 30 weeks of age, often with pulmonary congestion reflected in an increased lung weight/body weight ratio (Table 1). There was intense hepatic congestion in some mice, occasionally with early centrolobular necrosis (not shown), indicating bilateral cardiac dysfunction. No organ infarcts were detectable in autopsies, suggesting that the deaths were not caused by thromboemboli.
Table 1.
Comparison of body, heart, and lung weight in α*q52 and wild-type mice at 2, 4, and 10 weeks of age
| 2 weeks
|
4 weeks
|
10 weeks
|
|||||
|---|---|---|---|---|---|---|---|
| WT, n = 33 | α*q52, n = 33 | WT, n = 5 | α*q52, n = 7 | WT, n = 18 | α*q52, n = 30 | ||
| Body weight (BW, g) | 6.8 ± 1.4 | 6.5 ± 1.5 | 16.1 ± 2.4 | 15.6 ± 6.1 | 22.1 ± 1.6 | 22.0 ± 2.0 | |
| Atria | |||||||
| Weight (AW, mg) | 3.6 ± 0.6 | 3.6 ± 0.6 | 7.8 ± 2.4 | 15.2 ± 5.3† | (LA) | 3.7 ± 0.6 | 18.3 ± 14.7§ |
| (RA) | 3.5 ± 0.6 | 9.0 ± 3.6§ | |||||
| AW/BW, mg/g | 0.55 ± 0.07 | 0.57 ± 0.09 | 0.48 ± 0.08 | 1.00 ± 0.22§ | (LA) | 0.17 ± 0.03 | 0.84 ± 0.72§ |
| (RA) | 0.16 ± 0.03 | 0.41 ± 0.18§ | |||||
| Ventricles | |||||||
| Weight (VW, mg) | 34 ± 6 | 34 ± 7 | 65 ± 8 | 71 ± 17 | 85 ± 7 | 103 ± 17§ | |
| VW/BW, mg/g | 5.00 ± 0.3 | 5.20 ± 0.3‡ | 4.05 ± 0.15 | 4.79 ± 0.98 | 3.88 ± 0.26 | 4.7 ± 0.8§ | |
| Lungs | |||||||
| Lung W/BW, mg/g | 16.9 ± 3.7 | 17.4 ± 2.8 | 10.8 ± 2.0 | 10.0 ± 1.6 | 7.5 ± 0.9 | 9.7 ± 2.2§ | |
For the 2-week time point, data were obtained from eight litters containing equal numbers of heterozygous and wild-type mice. At 4 and 10 weeks, only data for females are shown. At 10 weeks, data for left and right atria (LA and RA) are shown separately. Ten-week-old male α*q52 mice gave similar results compared with wild-type mice (data not shown). Values given are mean ± SD.
P < 0.02;
P < 0.005; and
P < 0.0005 (Student’s t-test) relative to age-matched wild type.
Figure 2.
Gross and microscopic pathology of hearts from α*q52 mice. (A) Gross morphology of hearts from 2-week-old (Upper) and 10-week-old (Lower) α*q52 mice (Left) and wild-type mice (Right). (Bar = 6 mm.) (B and C) Four-chamber sections of hearts from 2-week-old (B) and 10-week-old (C) α*q52 mice (Left) and wild-type mice (Right). (D–K) Comparison of myocardial histology in α*q52 (Upper) and wild-type mice (Lower) at 10 weeks. Atria from α*q52 mice showed thickened trabeculae and external wall thinning (D) with fibrosis (F), shown by collagen staining (blue). In ventricles from α*q52 mice hearts, there are myocyte (and nuclear) hypertrophic and degenerative changes (including intracellular vacuolization) (H) and diffuse interstitial fibrosis (J). (B–E, H, and I) Stained with hematoxylin and eosin. (F, G, J, and K) Stained with Masson’s trichrome stain. [×4 (B and C); ×33 (D–G); ×66 (H–K).]
HAα*q Transgene Is Transiently Expressed at a Low Level.
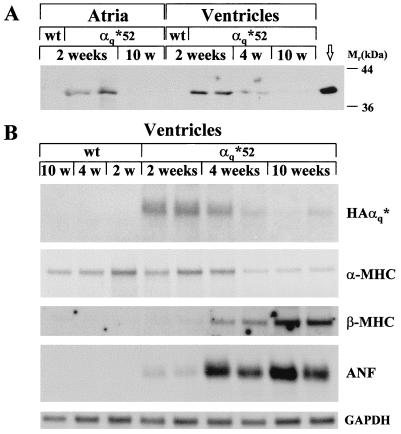
HAα*q protein, detected with antibody against the HA epitope, was present in the particulate fraction of atrial appendages and ventricles of 2-week-old α*q52 mice (Fig. 3A). No HAα*q was found in the soluble fraction (data not shown). By 4 weeks, the level of HAα*q dropped in ventricles to 28 ± 8% (SEM, n = 5, P < 0.001) of the level at 2 weeks. By 10 weeks, HAα*q protein was undetectable in atria and in ventricles. At 2 weeks, total αq/11 (endogenous αq/11 plus HAα*q) was increased in the particulate fraction from atria by 30 ± 3% (range, n = 2 pools of left and right atria from 8–10 mice) compared with wild type and in ventricles by 56 ± 15% (SEM, n = 8, P < 0.02) compared with wild type. Although we cannot ascribe all of the increase in total αq/11 to transgene expression, these data set an upper limit for the ratio of HAα*q to endogenous αq/11 at 2 weeks. Surprisingly, by 10 weeks, there were major compensatory changes in the level of endogenous αq/11. Compared with control, endogenous αq/11 was increased by 82 ± 16% (SEM, n = 10, P < 0.005) in ventricles and by 194 ± 27% (SEM, n = 4 pools from two to three mice, P < 0.001) in right atria.
Figure 3.
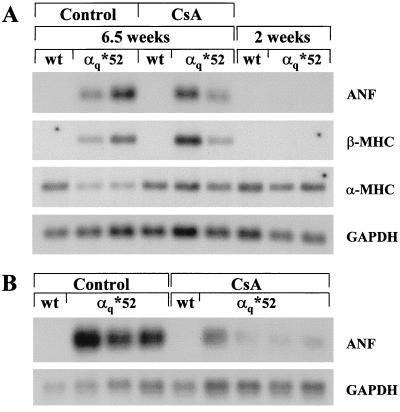
Expression and down-regulation of HAα*q protein and mRNA in α*q52 mice. (A) Western blot of 75 μg of the particulate fraction from atria and ventricles obtained from 2-, 4-, and 10-week-old α*q52 and wild-type mice. The blot was incubated with the HA-antibody 12CA5 and exposed for 5 min. Crude homogenate (1.25 μg) from HAα*q-expressing COS-7 cells, which were transiently transfected with HAα*q in pcDNA3, was used as positive control (marked with an arrow). (B) Northern blots of 10 μg total RNA extracted from ventricles from 2-, 4-, and 10-week-old week α*q52 and wild-type mice. The blots were probed with cDNA probes for HAα*q, ANF, or GAPDH and oligonucleotide probes for α-MHC and β-MHC. Exposure times were 20 hr (ANF and GAPDH), 24 hr (α-MHC), 4 days (β-MHC), and 11 days (SV40).
Down-regulation of the α-MHC promoter-driven HAα*q transgene occurs either transcriptionally or by increased RNA degradation, because the decrease in steady-state protein level was accompanied by a decrease in the steady-state level of the corresponding mRNA, as shown for ventricles in Fig. 3B. Expression of α-MHC itself may go down or be unchanged in response to cardiac hypertrophy (for example, refs. 19–21). In α*q52 mice, the level of α-MHC mRNA was reduced by 10 weeks of age (Fig. 3B), suggesting that the decrease in HAα*q was a result of decreased α-MHC promoter activity.
Phospholipase C Activity in Hearts of α*q52 Mice.
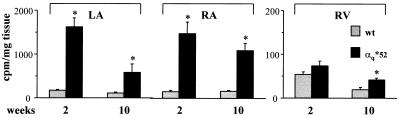
In 2-week-old α*q52 mice, basal PLC activity in the left and right atria was increased 9- to 10-fold over controls (Fig. 4), confirming that HAα*q stimulates PLC. A large phenotypic effect from such a small amount of transgene expression is what one would expect for a constitutively active protein. In contrast, there was only a minimal rise in ventricular PLC activity (Fig. 4) despite a similar level of HAα*q expression in atria and ventricles (see Fig. 3A). At 10 weeks, when HAα*q protein was not detectable, PLC activity continued to be elevated in left and right atria (5- and 6.5-fold, respectively) and was elevated in ventricles (2-fold). We attribute the elevated basal PLC activity at 10 weeks to the up-regulation of endogenous αq/11.
Figure 4.
Basal phospholipase C activity in hearts from α*q52 and wild-type mice. PLC activity was determined in tissue pieces from left atria (LA), right atria (RA), and right ventricles (RV) from 2- and 10-week-old α*q52 (solid bars) and wild-type mice (shaded bars). Data are expressed in cpm/mg tissue and represent mean ± SEM for 4–7 (2-week-old) and 10–14 (10-week-old) mice. Similar results were obtained when the cpm obtained were normalized to the amount of protein in each tissue piece (data not shown). Note the difference in scale for atria and ventricles.
Cardiac Hypertrophy and Dilatation Proceed Despite the Down-Regulation of HAα*q.
As described above in detail (see paragraph on pathology), α*q52 mice show only minimal pathology at 2 weeks when the transgene is expressed. The progression of the cardiomyopathy despite the disappearance of the transgene by 10 weeks is confirmed by the reexpression of fetal genes such as β-MHC and ANF in ventricles from α*q52 mice (Fig. 3B). The β-MHC mRNA was detectable at 4 weeks and increased further by 10 weeks. ANF mRNA was detectable at very low levels at 2 weeks. It reached high levels by 4 weeks and continued to be elevated by 10 weeks.
HAα*q-Induced Myocardial Hypertrophy Is Not Prevented by CsA.
To test whether inhibition of calcineurin activity affects cardiac hypertrophy in α*q52 mice, we analyzed two vehicle-treated and two CsA-treated litters (each containing α*q52 and wild-type mice). The treatment began at 17 or 18 days of age, when α*q52 mice still express the HAα*q protein but have not yet developed cardiac hypertrophy, and was carried out until they reached the age of 6.5 weeks, when they usually have developed hypertrophy. We chose a dose of 15 mg/kg body weight (twice daily s.c.) since a higher dose of 25 mg/kg used by Molkentin et al. (9) was lethal to most of our pups. The dose used in this study was sufficient to block calcineurin activity as shown by the lack of dephosphorylation of NFAT-1/p upon incubation with increasing concentrations of the Ca2+ ionophore, ionomycin, in spleen cells from CsA-treated mice compared with controls taken 5 hr after the last injection (Fig. 5).
Figure 5.
Effect of CsA treatment on Ca2+-induced NFAT-1/p dephosphorylation in lysates from spleen cells. Western blot of whole spleen cell lysates from mice treated with or without CsA after incubation with ionomycin. The blot was incubated with an NFAT-1/p antibody that recognizes both the phosphorylated and dephosphorylated form of NFAT-1 (upper and lower band, respectively). Exposure time was 5 min. For control, a lysate (from 0.3 × 106 cells) of a mouse T cell line (Cl. 7W2; ref. 24) expressing NFAT-1/p was used (marked with an arrow).
The effect of treatment with CsA on body weight, tibia length, and heart weights is shown for males in Table 2. Treatment with CsA affected the growth rate of both wild-type and α*q52 mice but had a much more profound effect on transgenic mice than on wild types. The body weight of CsA-treated α*q52 mice was 31% less than vehicle-treated α*q52 controls, while the CsA-treated wild-type mice lagged behind their vehicle-treated wild-type controls by only 10%. Although the transgenic mice had slightly shorter tibia lengths than wild-type controls, there was no significant difference in tibia length between vehicle-treated and CsA-treated animals.
Table 2.
Effect of CsA treatment on body weight, tibia length, and heart weights in male α*q52 and wild-type mice
| Vehicle-treated
|
CsA-treated
|
CsA vs. vehicle‡
|
||||
|---|---|---|---|---|---|---|
| WT, n = 4 | α*q52, n = 6 | WT, n = 3 | α*q52, n = 5 | P value, WT | P value, α*q52 | |
| Body weight (BW, g) | 22.5 ± 0.7 | 20.6 ± 1.9 | 20.6 ± 0.7 | 14.2 ± 2.7† | .02 | .001 |
| Tibia length (TL, mm) | 16.7 ± 0.2 | 16.2 ± 0.9 | 16.6 ± 0.2 | 15.7 ± 0.1† | NS | NS |
| Ventricles | ||||||
| Weight (VW, mg) | 90 ± 3 | 107 ± 10† | 77 ± 1 | 76 ± 11 | .002 | .0006 |
| VW/BW, mg/g | 3.99 ± 0.04 | 5.23 ± 0.32† | 3.76 ± 0.09 | 5.41 ± 0.37† | .005 | NS |
| VW/TL, mg/mm | 5.4 ± 0.15 | 6.63 ± 0.41† | 4.67 ± 0.01 | 4.84 ± 0.7 | .0005 | .0005 |
| Right atrium | ||||||
| Weight (RA, mg) | 3.5 ± 0.4 | 10.9 ± 3.0† | 3.8 ± 0.4 | 7.7 ± 1.2† | NS | .05 |
| RA/BW, mg/g | 0.16 ± 0.02 | 0.53 ± 0.15† | 0.18 ± 0.02 | 0.55 ± 0.09† | NS | NS |
| RA/TL, mg/mm | 0.21 ± 0.02 | 0.67 ± 0.17† | 0.23 ± 0.02 | 0.49 ± 0.08† | NS | .05 |
In addition to the data presented, two male CsA-treated α*q52 mice died before the end of the study (at 33 and 35 days of age) with increased heart weights (data not shown). The CsA-treated litters contained only four female α*q52, which had a similar phenotype as the males (data not shown). The four litters examined contained a total of 15 wild-type and 24 α*q52 mice, an unusual distribution. Most of the litters generated by heterozygote/wild-type matings contain about 50% of each genotype (see, for example, Table 1, 2 weeks). Values given are mean ± SD. NS, not significant.
P < 0.01 (Student’s t-test) for unpaired comparisons between α*q52 and wild-type mice that have been subjected to the same treatment.
The P values are assessed by Student’s t-test for unpaired comparisons between CsA- and vehicle-treated α*q52 or wild-type mice.
The absolute weight of the ventricles from vehicle-treated 6.5-week-old α*q52 mice was significantly larger than that of the wild type. The difference was reflected in increased VW/BW ratio and ventricular weight/tibia length (VW/TL) ratio. On CsA treatment, the ventricular weights of both transgenes and wild types dropped significantly. The drop in ventricular weight was almost proportional to the body weight since the VW/BW ratio did not change for α*q52 mice and was only slightly reduced in wild types. Thus, the transgenic animals continued to have a high VW/BW ratio after CsA treatment. However, the results calculated on the basis of tibia length were different. The VW/TL ratio dropped significantly on CsA treatment both in wild-type and α*q52 mice and became essentially the same in both. Thus, by one measure (VW/BW), CsA had no effect on the relative ventricular weight, but by another (VW/TL), it made the α*q52 ventricle indistinguishable from wild type. Because of the ambiguity in interpreting the results of the effect of CsA on ventricular weight, we used two other criteria to determine whether or not CsA affects the hypertrophy present in the transgenic animals: atrial weights and gene expression.
First, we considered the effect of CsA treatment on the atrial pathology. We do not know for certain whether the atrial pathology is a primary result of expression of the transgene, or whether it is secondary to a contractile defect in the ventricle. Both the right and left atria were massively enlarged in older α*q52 mice. Some of the increase in weight of the left atrium is a result of the formation of organized thrombi, making analysis of left atrial weights harder to interpret. Nevertheless, the enlargement and thrombus formation appeared to be equal with or without CsA treatment (data not shown). The absolute weight of the right atrium (that did not contain thrombi) dropped slightly on CsA treatment, but the right AW/BW ratio was not significantly different from vehicle-treated α*q52 controls. The right AW/TL ratio dropped slightly but continued to be more than twice that of the wild type. We conclude that the atrial pathology continued on CsA treatment regardless of whether it is primary or a secondary reflection of ventricular dysfunction.
Second, we assessed the development of hypertrophy by analyzing the mRNA expression of hypertrophic markers. Despite treatment with CsA, both ANF and β-MHC were reexpressed in ventricles from α*q52 mice (Fig. 6A). There was considerable variation in ANF and β-MHC mRNA levels. However, ANF mRNA levels were lower in CsA-treated compared with vehicle-treated α*q52 mice (see additional samples in Fig. 6B). Nevertheless, they were always higher than in 2-week-old α*q52 mice, where ANF mRNA was barely detectable (Fig. 6A). The down-regulation of α-MHC mRNA normally seen in α*q52 mice was less apparent after CsA treatment (Fig. 6A). Taken together, these data support the conclusion that CsA blunted some aspects of hypertrophy in α*q52 mice, but did not prevent it.
Figure 6.
Effect of CsA treatment on mRNA expression of hypertrophic markers. Northern blots of 5 μg total RNA extracted from the ventricular apex from CsA- and vehicle-treated α*q52 and wild-type mice. For comparison, RNA extracted from 2-week-old α*q52 and wild-type mice is shown (only in A). The blots were probed with cDNA probes for ANF and GAPDH and oligonucleotide probes for α-MHC and β-MHC. Exposure times were 3 hr (ANF and GAPDH) and 17 hr (α-MHC and β-MHC). Very little ANF mRNA was detectable in 2-week-old α*q52 when the film was overexposed for 13 hr (A).
DISCUSSION
Progression of Cardiac Pathology Despite Transgene Down-Regulation.
We have demonstrated that a transient modest expression of HAα*q is sufficient to induce persistent changes that continue to drive cardiac pathology after the initiating stimulus is gone. In fact, at the time of transition to failure at 2 months or later, the HAα*q protein is no longer detectable and its mRNA is greatly decreased. Since the expression of α-MHC itself is down-regulated in hearts from α*q52 mice, the decrease in HAα*q mRNA is presumably a result of reduced α-MHC promoter activity. The α-MHC promoter activity might depend on the severity of hypertrophy, since the expression of α-MHC mRNA was reported to be unchanged in hypertrophied hearts of mice expressing unactivated αq (6) or PKCβ (10). In several studies using the α-MHC promoter the level of transgene expression over time was not reported (3, 6, 10).
HAα*q leads to increased PLC activity, but there is no linear relationship between the extent of activation of PLC (and presumably its downstream targets) and the development of hypertrophy. Despite a similar level of HAα*q protein in atria and ventricles at 2 weeks, there is a large increase in basal PLC activity in atrial appendages, whereas in ventricles, basal PLC activity was only slightly increased. Possibly, subtle differences in HAα*q expression may cause pronounced differences in PLC activity or the chambers may respond differently to a similar stimulus. It is not clear at present whether the atrial pathology is a consequence of transgene expression itself, or hemodynamic changes resulting from the ventricular abnormalities, or both. That the pathology in both atria and ventricles is very subtle at 2 weeks and progresses with time suggests an interplay of biochemical and hemodynamic factors.
Several factors might be responsible for the progression of the cardiomyopathy after the expression of HAα*q has declined. Possibilities include (i) compensatory changes in the level of endogenous αq/11 or other signaling molecules that sustain persistent differences in gene expression and (ii) compensation for early apoptosis leading to myocyte dropout during the time that the HAα*q is expressed. Activated Gαq has been shown to cause apoptosis in COS-7 or CHO cells (22) and in neonatal rat ventricular cardiomyocytes (23). Further studies will be aimed at defining the relative importance of these and other mechanisms.
Cyclosporin A Blunts, but Does Not Prevent, Myocardial Hypertrophy in α*q52 Mice.
CsA inhibits the phosphatase calcineurin (9). Since HAα*q acts upstream of calcineurin and initiates additional pathways (such as activation of protein kinase C), we expected that CsA might blunt, but not eliminate, the phenotype. This is, indeed, the case. The observation that ANF and β-MHC are still reexpressed in the ventricles at high levels and the continued presence of atrial (and perhaps also ventricular) enlargement in CsA-treated α*q52 mice demonstrate the CsA cannot prevent some aspects of myocardial hypertrophy in these animals. However, it does diminish some manifestations, such as the degree of ANF expression and α-MHC down-regulation, and perhaps ventricular enlargement.
The dose of CsA used in this study was just sublethal and slightly lower than that given by Molkentin et al. (9), but the same as used in (25). It effectively blocked calcineurin in spleen cells. We propose that HAα*q sets in motion pathways in addition to the ones leading to activation of calcineurin that are important in initiating or maintaining the pathology. Defining these additional pathways is a challenge for the future.
CONCLUSIONS
Compensatory changes in response to acute cardiac injury can lead to long-term pathology. We have now shown that transient expression of a single signaling molecule is sufficient to initiate a cascade of changes that lead to hypertrophy, dilatation, and eventually failure. Transgenic mice, such as the ones described here, can serve as informative model systems to identify primary triggers for hypertrophy, to establish the sequence of secondary changes, and to refine our understanding of the genetic basis for continued progression of the pathology after the primary trigger is no longer detectable.
Acknowledgments
We thank Drs. Paul T. Wilson, Henry R. Bourne, Walter J. Koch, and Christine E. Seidman for providing cDNA constructs, Dr. Arlene Sharpe and Lina Du at the Core Transgenic Mouse Facility of Brigham and Women’s Hospital for performing the pronuclear injection and generating the founder animals, and Helen Shing for her expert technical assistance. We are very grateful to Paula McColgan for her excellent secretarial help. This work was supported by National Institutes of Health Grant HL52320 to E.J.N. and an Arthritis Foundation Postdoctoral Fellowship to J.A.
ABBREVIATIONS
- α-MHC and β-MHC
α- and β-myosin heavy chain, respectively
- ANF
atrial natriuretic factor
- CsA
cyclosporin A
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HA
hemagglutinin epitope
- NFAT
nuclear factor of activated T cells
- PLC
phospholipase C
- VW/BW
ventricular weight/body weight
Footnotes
In this study, left and right atrial appendages were used. They are referred to as left and right atria.
References
- 1.Bogoyevitch M A, Sugden P H. Int J Biochem Cell Biol. 1996;28:1–12. doi: 10.1016/1357-2725(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 2.Milano C A, Dolber P C, Rockman H A, Bond R A, Venable M E, Allen L F, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:10109–10113. doi: 10.1073/pnas.91.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhter S A, Milano C A, Shotwell K F, Cho M-C, Rockman H A, Lefkowitz R J, Koch W J. J Biol Chem. 1997;272:21253–21259. doi: 10.1074/jbc.272.34.21253. [DOI] [PubMed] [Google Scholar]
- 4.Hein L, Stevens M E, Barsh G S, Pratt R E, Kobilka B K, Dzau V J. Proc Natl Acad Sci USA. 1997;94:6391–6396. doi: 10.1073/pnas.94.12.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhter S A, Luttrell L M, Rockman H A, Iaccarino G, Lefkowitz R J, Koch W J. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 6.D’Angelo D D, Sakata Y, Lorenz J N, Boivin G P, Walsh R A, Liggett S B, Dorn G W, II. Proc Natl Acad Sci USA. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson P T, Bourne H R. J Biol Chem. 1995;270:9667–9675. doi: 10.1074/jbc.270.16.9667. [DOI] [PubMed] [Google Scholar]
- 8.De Vivo M, Chen J, Codina J, Iyengar R. J Biol Chem. 1992;267:18263–18266. [PubMed] [Google Scholar]
- 9.Molkentin J D, Lu J-R, Antos C L, Markham B, Richardson J, Robbins J, Grant S R, Olson E N. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakasaki H, Koya D, Schoen F J, Jirousek M R, Ways D K, Hoit B D, Walsh R A, King G L. Proc Natl Acad Sci USA. 1997;94:9320–9325. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown S L, Brown J H. Mol Pharmacol. 1983;24:351–356. [PubMed] [Google Scholar]
- 15.Li Y, Sternweis P, Charnecki S, Smith T F, Gilman A G, Neer E J, Kozasa T. J Biol Chem. 1998;273:16265–16272. doi: 10.1074/jbc.273.26.16265. [DOI] [PubMed] [Google Scholar]
- 16.Ho A M, Jain J, Rao A, Hogan P G. J Biol Chem. 1994;269:28181–28186. [PubMed] [Google Scholar]
- 17.Lyons G E, Schiaffino S, Sassoon D, Barton P, Buckingham M. J Cell Biol. 1990;111:2427–2436. doi: 10.1083/jcb.111.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng W A, Grupp I L, Subramaniam A, Robbins J. Circ Res. 1991;69:1742–1750. doi: 10.1161/01.res.68.6.1742. [DOI] [PubMed] [Google Scholar]
- 19.Ojamaa K, Petrie J F, Balkman C, Hong C, Klein I. Proc Natl Acad Sci USA. 1994;91:3468–3472. doi: 10.1073/pnas.91.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chassagne C, Wisnewsky C, Schwartz K. Circ Res. 1993;72:857–864. doi: 10.1161/01.res.72.4.857. [DOI] [PubMed] [Google Scholar]
- 21.Nagai R, Pritzl N, Low R B, Stirewalt W S, Zak R, Alpert N R, Litten R Z. Circ Res. 1987;60:692–699. doi: 10.1161/01.res.60.5.692. [DOI] [PubMed] [Google Scholar]
- 22.Althoefer H, Eversole-Cire P, Simon M I. J Biol Chem. 1997;272:24380–24386. doi: 10.1074/jbc.272.39.24380. [DOI] [PubMed] [Google Scholar]
- 23.Adams J W, Sakata Y, Davis M G, Sah V P, Wang Y, Liggett S B, Chien K R, Brown J H, Dorn G W, II. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valge-Archer V E, de Villiers J, Sinskey A J, Rao A. J Immunol. 1990;145:4355–4364. [PubMed] [Google Scholar]
- 25.Sussman M A, Lim H W, Gude N, Taigen T, Olsen E N, Robbins J, Colbert M C, Gualberto A, Wieczorek D F, Molkentin J D. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]