Abstract
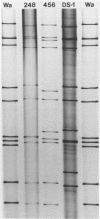
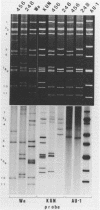
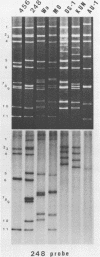
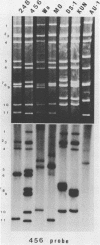
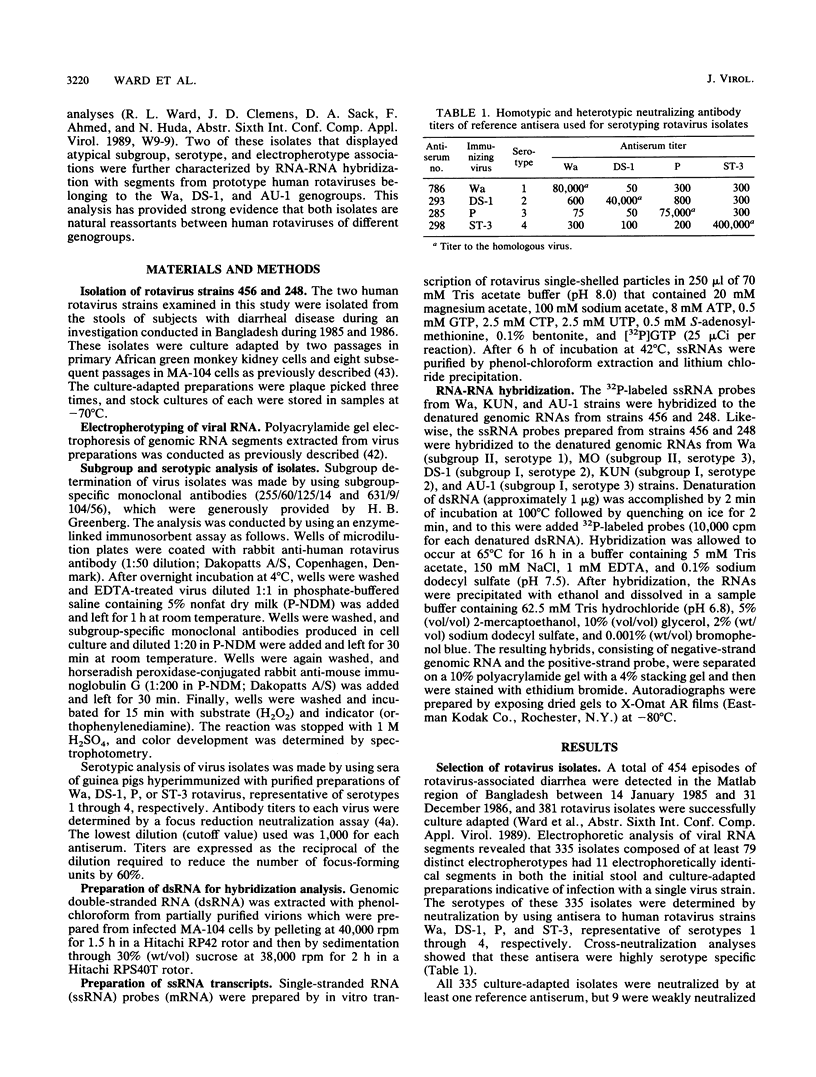
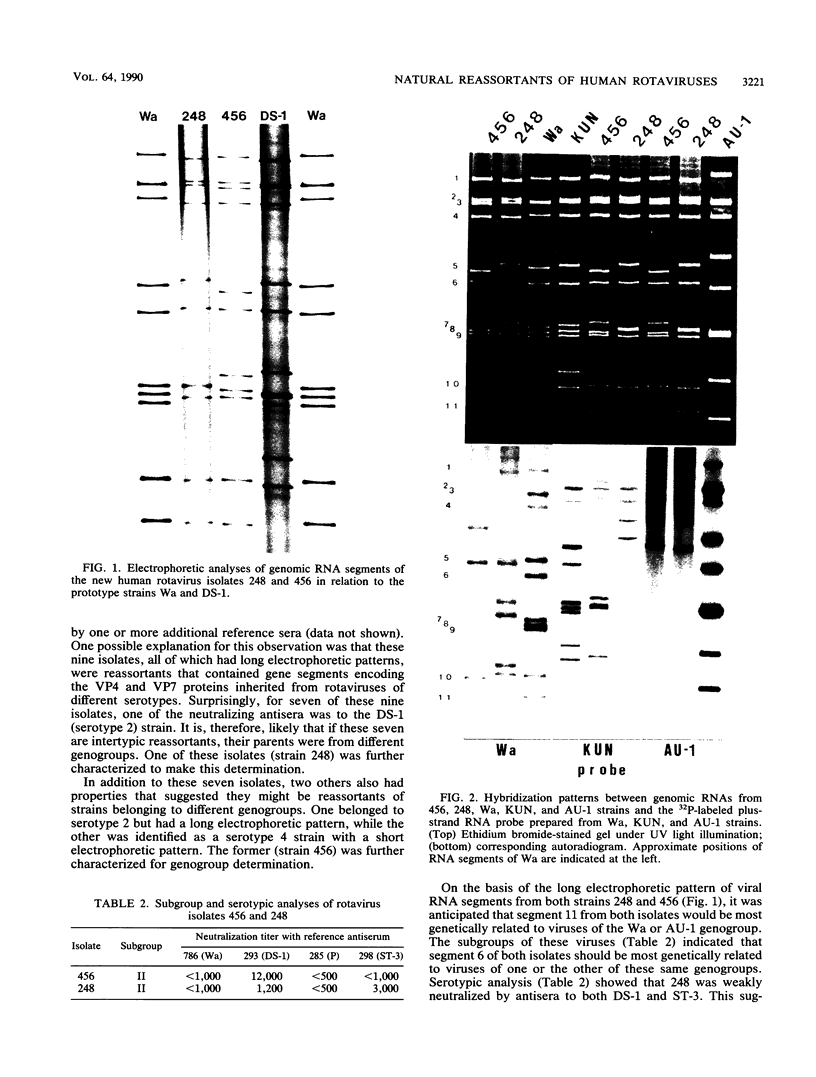
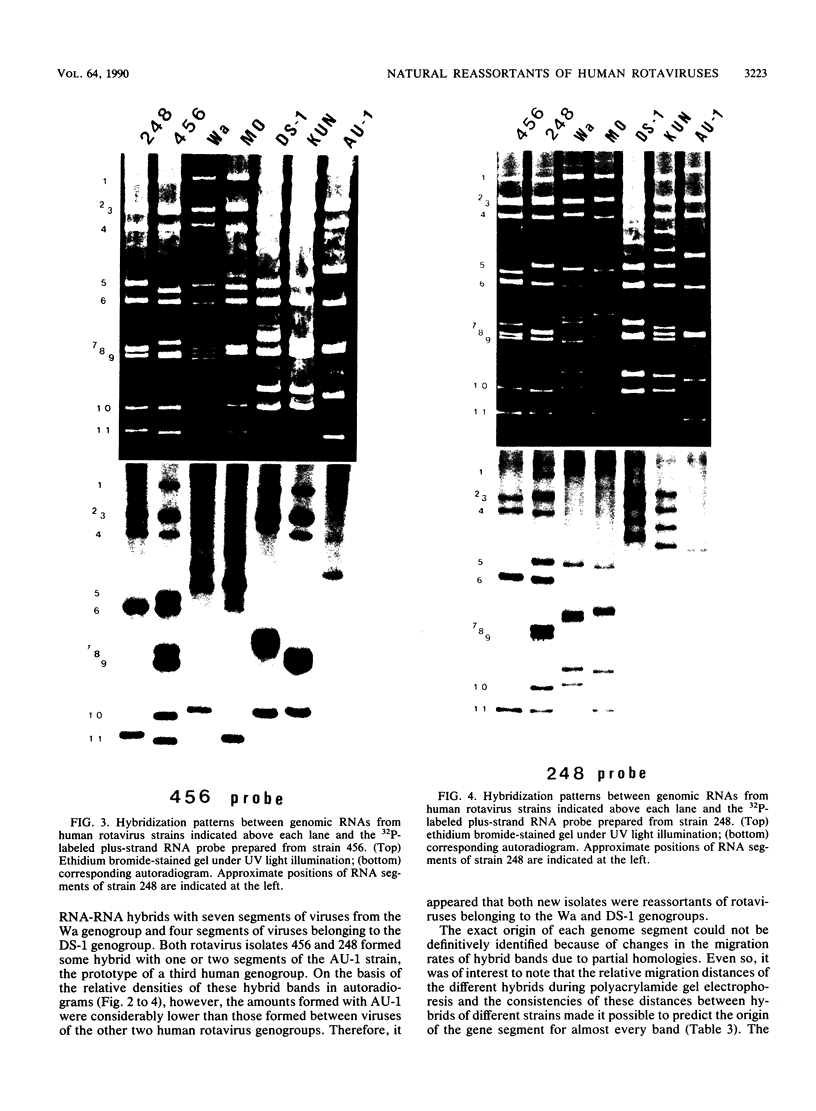
Of 335 rotavirus isolates associated with diarrheal disease in Bangladesh that were culture adapted and subsequently characterized for electropherotype, subgroup, and serotype, 9 had properties that suggested they may be natural reassortants between human rotaviruses belonging to different "genogroups." Two of these were examined in greater detail by RNA-RNA hybridization with prototype strains representative of each of the three proposed human rotavirus genogroups. One subgroup II isolate, 248, with a "long" electrophoretic pattern was neutralized by hyperimmune antisera to both serotype 2 and 4 strains. Consistent with these results, seven RNA segments of this isolate formed hybrids with human strains belonging to the Wa genogroup and four segments hybridized with strains belonging to the DS-1 genogroup. The second isolate examined, 456, belonged to subgroup II and had a long electrophoretic pattern but was found to be a serotype 2 strain. This isolate also appeared to be an intergenogroup reassortant because three of its segments formed hybrids with strains belonging to the Wa genogroup and eight hybridized with viruses of the DS-1 genogroup. On the basis of the relative migration rates of these RNA-RNA hybrids during gel electrophoresis, a suggested origin for each gene segment was proposed which was consistent with the results expected from electrophoretic, subgroup, and serotypic analyses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboudy Y., Shif I., Zilberstein I., Gotlieb-Stematsky T. Use of polyclonal and monoclonal antibodies and analysis of viral RNA in the detection of unusual group A human rotaviruses. J Med Virol. 1988 Jul;25(3):351–359. doi: 10.1002/jmv.1890250312. [DOI] [PubMed] [Google Scholar]
- Ahmed M. U., Taniguchi K., Kobayashi N., Urasawa T., Wakasugi F., Islam M., Shaikh H., Urasawa S. Characterization by enzyme-linked immunosorbent assay using subgroup- and serotype-specific monoclonal antibodies of human rotavirus obtained from diarrheic patients in Bangladesh. J Clin Microbiol. 1989 Jul;27(7):1678–1681. doi: 10.1128/jcm.27.7.1678-1681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. J., Unicomb L. E., Bishop R. F. Cultivation and characterization of human rotaviruses with "super short" RNA patterns. J Clin Microbiol. 1987 Jan;25(1):183–185. doi: 10.1128/jcm.25.1.183-185.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M., Desselberger U., Flewett T. H. Temporal and geographical distributions of human rotavirus serotypes, 1983 to 1988. J Clin Microbiol. 1989 Dec;27(12):2827–2833. doi: 10.1128/jcm.27.12.2827-2833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D. I., Kacica M. A., McNeal M. M., Schiff G. M., Ward R. L. Local and systemic antibody response to rotavirus WC3 vaccine in adult volunteers. Antiviral Res. 1989 Dec;12(5-6):293–300. doi: 10.1016/0166-3542(89)90056-9. [DOI] [PubMed] [Google Scholar]
- Brown D. W., Mathan M. M., Mathew M., Martin R., Beards G. M., Mathan V. I. Rotavirus epidemiology in Vellore, south India: group, subgroup, serotype, and electrophoretype. J Clin Microbiol. 1988 Nov;26(11):2410–2414. doi: 10.1128/jcm.26.11.2410-2414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanock S. J., Wenske E. A., Fields B. N. Human rotaviruses and genome RNA. J Infect Dis. 1983 Jul;148(1):49–50. doi: 10.1093/infdis/148.1.49. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Hoshino Y., Bell L. M., Groff J., Hess G., Bachman P., Offit P. A. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol. 1987 Sep;25(9):1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Hoshino Y., Boeggeman E., Purcell R., Chanock R. M., Kapikian A. Z. Genetic relatedness among animal rotaviruses. Arch Virol. 1986;87(3-4):273–285. doi: 10.1007/BF01315305. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Boeggeman E., White L., Perez M., Purcell R., Hoshino Y., Midthun K., Chanock R. M., Kapikian A. Z. Genetic relatedness among human rotaviruses. J Med Virol. 1985 Oct;17(2):135–143. doi: 10.1002/jmv.1890170206. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez I., White L., Perez M., Kalica A. R., Marquina R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Genetic relatedness among human rotaviruses as determined by RNA hybridization. Infect Immun. 1982 Aug;37(2):648–655. doi: 10.1128/iai.37.2.648-655.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarg-Chenon A., Bricout F., Nicolas J. C. Study of genetic reassortment between two human rotaviruses. Virology. 1984 Dec;139(2):358–365. doi: 10.1016/0042-6822(84)90381-7. [DOI] [PubMed] [Google Scholar]
- Georges-Courbot M. C., Beraud A. M., Beards G. M., Campbell A. D., Gonzalez J. P., Georges A. J., Flewett T. H. Subgroups, serotypes, and electrophoretypes of rotavirus isolated from children in Bangui, Central African Republic. J Clin Microbiol. 1988 Apr;26(4):668–671. doi: 10.1128/jcm.26.4.668-671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. K., Naik T. N. Detection of a large number of subgroup 1 human rotaviruses with a "long" RNA electropherotype. Arch Virol. 1989;105(1-2):119–127. doi: 10.1007/BF01311122. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Espejo R. T., Flores J., Wyatt R. G., Kapikian A. Z., Chanock R. M. Distinctive ribonucleic acid patterns of human rotavirus subgroups 1 and 2. Infect Immun. 1981 Sep;33(3):958–961. doi: 10.1128/iai.33.3.958-961.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Cline W. L., Greenberg H. B., Wyatt R. G., Kalica A. R., Banks C. E., James H. D., Jr, Flores J., Chanock R. M. Antigenic characterization of human and animal rotaviruses by immune adherence hemagglutination assay (IAHA): evidence for distinctness of IAHA and neutralization antigens. Infect Immun. 1981 Aug;33(2):415–425. doi: 10.1128/iai.33.2.415-425.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka S., Nakagomi T., Fukuhara N., Hoshino Y., Suzuki H., Nakagomi O., Kapikian A. Z., Ebina T., Konno T., Ishida N. Serologic characteristics of a human rotavirus isolate, AU-1, which has a "long" RNA pattern and subgroup I specificity. J Med Virol. 1987 Dec;23(4):351–357. doi: 10.1002/jmv.1890230407. [DOI] [PubMed] [Google Scholar]
- Kutsuzawa T., Konno T., Suzuki H., Ebina T., Ishida N. Two distinct electrophoretic migration patterns of RNA segments of human rotaviruses prevalent in Japan in relation to their serotypes. Microbiol Immunol. 1982;26(3):271–273. doi: 10.1111/j.1348-0421.1982.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Mascarenhas J. D., Linhares A. C., Gabbay Y. B., de Freitas R. B., Mendez E., Lopez S., Arias C. F. Naturally occurring serotype 2/subgroup II rotavirus reassortants in northern Brazil. Virus Res. 1989 Nov;14(3):235–240. doi: 10.1016/0168-1702(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A., Hasegawa A., Taniguchi K., Inouye S. Characterization of a human rotavirus strain which is possibly a naturally-occurring reassortant virus. Virus Res. 1988 May;10(2-3):167–175. doi: 10.1016/0168-1702(88)90013-5. [DOI] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Akatani K., Ikegami N. Identification of rotavirus genogroups by RNA-RNA hybridization. Mol Cell Probes. 1989 Sep;3(3):251–261. doi: 10.1016/0890-8508(89)90006-6. [DOI] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Hoshino Y., Flores J., Kapikian A. Z. Genetic analysis of a human rotavirus that belongs to subgroup I but has an RNA pattern typical of subgroup II human rotaviruses. J Clin Microbiol. 1987 Jul;25(7):1159–1164. doi: 10.1128/jcm.25.7.1159-1164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Oyamada H., Suto T. Relative frequency of human rotavirus subgroups 1 and 2 in Japanese children with acute gastroenteritis. J Med Virol. 1985 Sep;17(1):29–34. doi: 10.1002/jmv.1890170105. [DOI] [PubMed] [Google Scholar]
- Nakagomi O., Oyamada H., Kuroki S., Kobayashi Y., Ohshima A., Nakagomi T. Molecular identification of a novel human rotavirus in relation to subgroup and electropherotype of genomic RNA. J Med Virol. 1989 Jul;28(3):163–168. doi: 10.1002/jmv.1890280311. [DOI] [PubMed] [Google Scholar]
- Nakagomi T., Nakagomi O. RNA-RNA hybridization identifies a human rotavirus that is genetically related to feline rotavirus. J Virol. 1989 Mar;63(3):1431–1434. doi: 10.1128/jvi.63.3.1431-1434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Birch C., McLean B., Holmes I. H. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. J Clin Microbiol. 1981 Feb;13(2):272–278. doi: 10.1128/jcm.13.2.272-278.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S. K., Olive D. M., Strannegard O. O., al-Nakib W. Molecular epidemiology of human rotavirus infections based on genome segment variations in viral strains. J Med Virol. 1988 Nov;26(3):249–259. doi: 10.1002/jmv.1890260305. [DOI] [PubMed] [Google Scholar]
- Steele A. D., Alexander J. J. The relative frequency of subgroup I and II rotaviruses in black infants in South Africa. J Med Virol. 1988 Mar;24(3):321–327. doi: 10.1002/jmv.1890240309. [DOI] [PubMed] [Google Scholar]
- Svensson L., Uhnoo I., Grandien M., Wadell G. Molecular epidemiology of rotavirus infections in Uppsala, Sweden, 1981: disappearance of a predominant electropherotype. J Med Virol. 1986 Feb;18(2):101–111. doi: 10.1002/jmv.1890180202. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S., Yasuhara T. Production of subgroup-specific monoclonal antibodies against human rotaviruses and their application to an enzyme-linked immunosorbent assay for subgroup determination. J Med Virol. 1984;14(2):115–125. doi: 10.1002/jmv.1890140205. [DOI] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K. Genetic reassortment between two human rotaviruses having different serotype and subgroup specificities. J Gen Virol. 1986 Aug;67(Pt 8):1551–1559. doi: 10.1099/0022-1317-67-8-1551. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R. Genotypic selection following coinfection of cultured cells with subgroup 1 and subgroup 2 human rotaviruses. J Gen Virol. 1989 Jul;70(Pt 7):1691–1699. doi: 10.1099/0022-1317-70-7-1691. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Hurst P. F. Reassortant formation and selection following coinfection of cultured cells with subgroup 2 human rotaviruses. J Gen Virol. 1988 Jan;69(Pt 1):149–162. doi: 10.1099/0022-1317-69-1-149. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Pierce M. J. Efficiency of human rotavirus propagation in cell culture. J Clin Microbiol. 1984 Jun;19(6):748–753. doi: 10.1128/jcm.19.6.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Schiff G. M., Hoshino Y., Greenberg H. B. Relative concentrations of serum neutralizing antibody to VP3 and VP7 proteins in adults infected with a human rotavirus. J Virol. 1988 May;62(5):1543–1549. doi: 10.1128/jvi.62.5.1543-1549.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]