Abstract
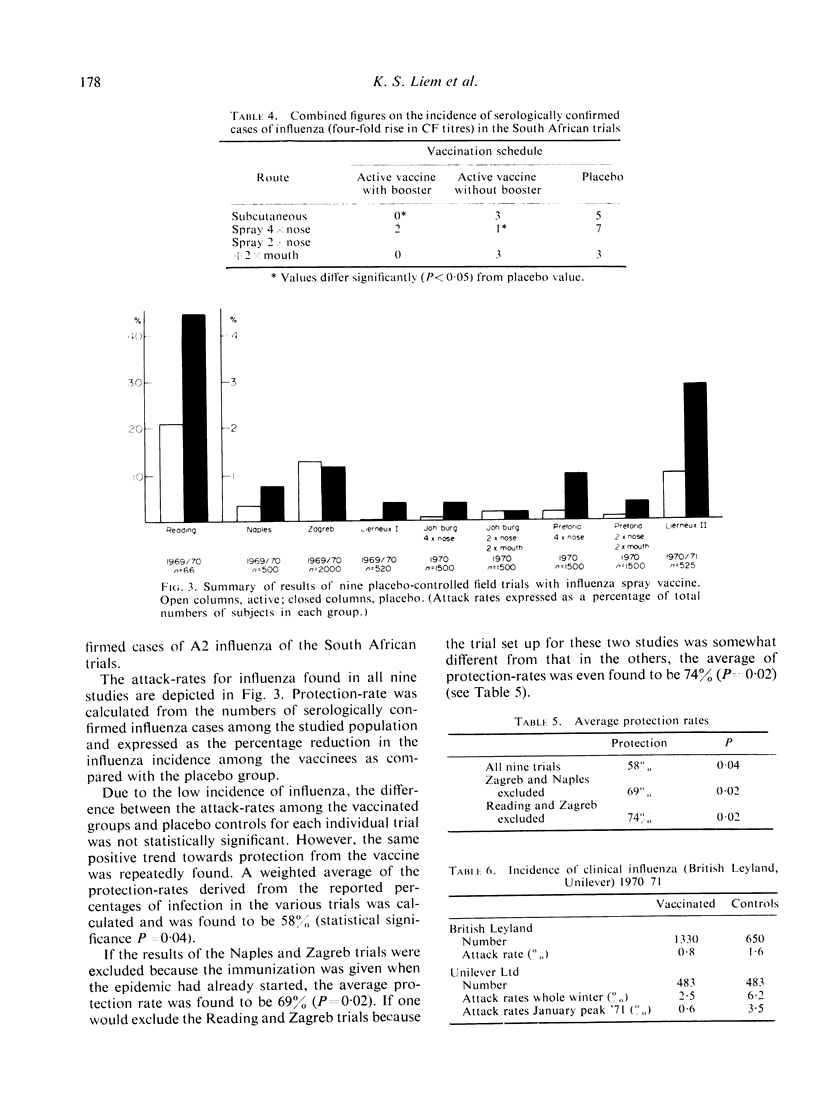
Protection against influenza after killed influenza spray vaccine was assessed in nine double blind, placebocontrolled clinical trials and two field studies, involving a total number of 10,000 subjects.
Monovalent vaccine, containing 300-360 IU A/Hong Kong/1/68 (H3N2) or bivalent vaccine, containing 300-360 IU A/Aichi/2/68 (H3N2) and 200-240 IU B/Mass/3/66 per dose was used.
Administration by the intranasal route appeared to be acceptable as an alternative to the parenteral route. The protective efficacy found was comparable with that attained after parenteral vaccination. It has, however, the advantages of a characteristic lack of side-effects and of the fact that no injection is involved. This will probably enhance the acceptance of influenza immunization by the population and thus lead to a higher vaccination rate in the communities we want to protect against influenza.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard C. W. Demonstration of type-specific influenza antibody in mammalian and avian sera by immunodiffusion. Bull World Health Organ. 1970;42(5):779–785. [PMC free article] [PubMed] [Google Scholar]
- Edmondson W. P., Jr, Rothenberg R., White P. W., Gwaltney J. M., Jr A comparison of subcutaneous, nasal, and combined influenza vaccination. II. Protection against natural challenge. Am J Epidemiol. 1971 Jun;93(6):480–486. doi: 10.1093/oxfordjournals.aje.a121282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis T., Jr THE INACTIVATION OF EPIDEMIC INFLUENZA VIRUS BY NASAL SECRETIONS OF HUMAN INDIVIDUALS. Science. 1940 Feb 23;91(2356):198–199. doi: 10.1126/science.91.2356.198. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Fulk R. V., Togo Y., Hornick R. B., Heiner G. G., Dawkins A. T., Jr, Mann J. J. Influenza antibody in human respiratory secretions after subcutaneous or respiratory immunization with inactivated virus. Nature. 1968 May 11;218(5141):594–595. doi: 10.1038/218594a0. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Hume E. B., Fulk R. V., Togo Y., Huber M., Hornick R. B. Antibody responses in nasal secretions and serum of elderly persons following local or parenteral administration of inactivated influenza virus vaccine. J Immunol. 1969 Mar;102(3):555–562. [PubMed] [Google Scholar]
- PRZESMYCKI F., SAWICKI L., DOBROWOLSKA H. Vaccination against influenza in Poland, 1953/56. Bull World Health Organ. 1959;20(2-3):333–353. [PMC free article] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Buckland R., Rubenstein D., Sharpe D. M. Vaccination against Hong Kong influenza in Britain, 1968-9. A report to the Medical Research Council Committee on Influenza and other Respiratory Virus Vaccines. J Hyg (Lond) 1970 Sep;68(3):359–368. doi: 10.1017/s0022172400042261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Bond J. O., Levitt L. P., Hartwig E. C., Prather E. C., Baratta R. L., Neill J. S., Small P. A., Jr An evaluation of influenza immunization: influence of route of administration and vaccine strain. Bull World Health Organ. 1969;41(3):543–548. [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Coggins W. J. Influenza immunization: field trial on a university campus. J Infect Dis. 1972 Sep;126(3):242–248. doi: 10.1093/infdis/126.3.242. [DOI] [PubMed] [Google Scholar]