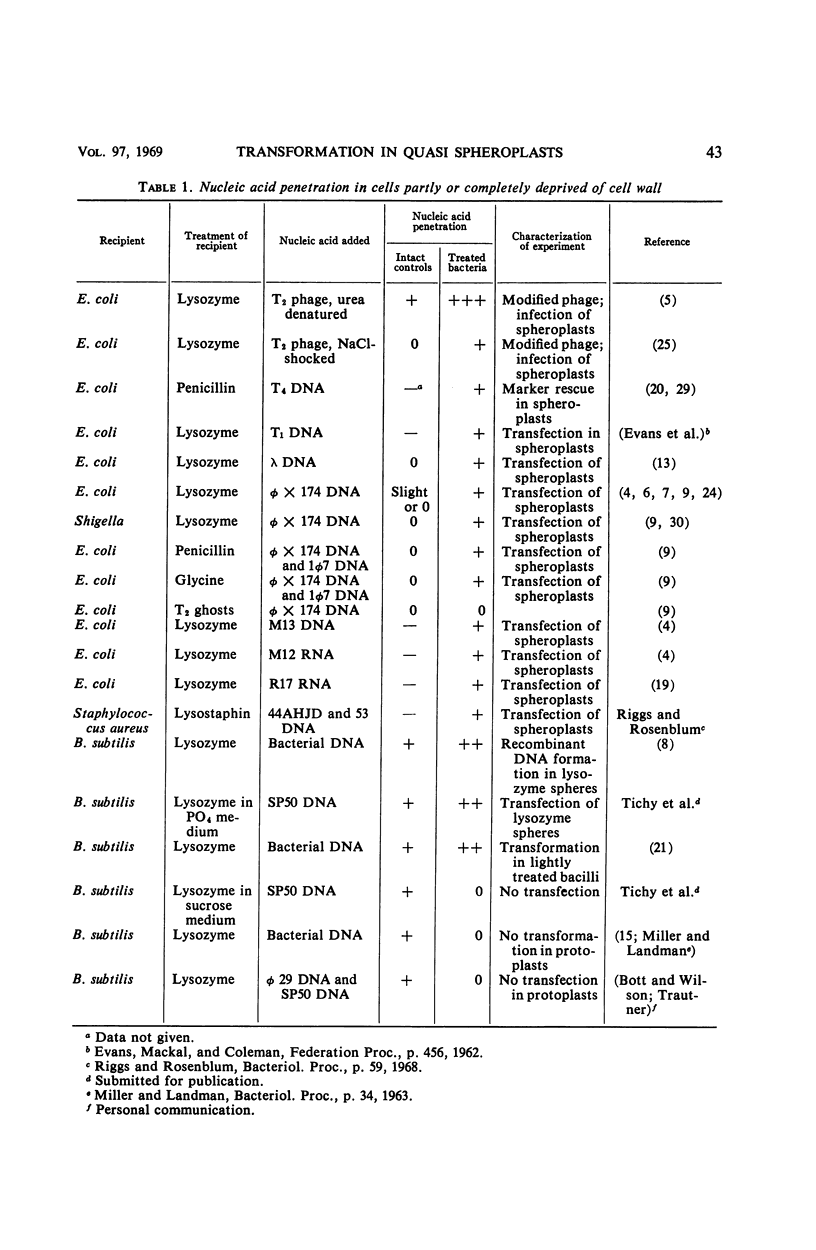
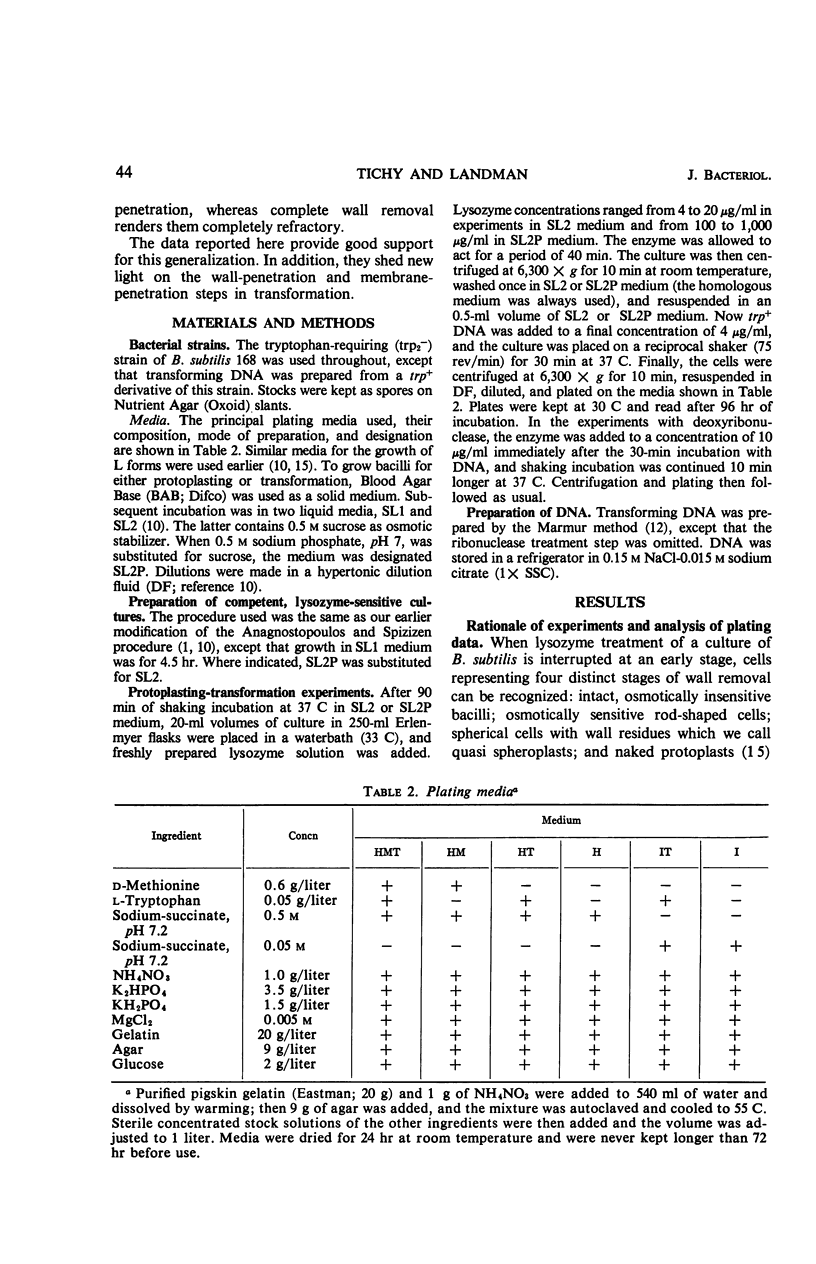
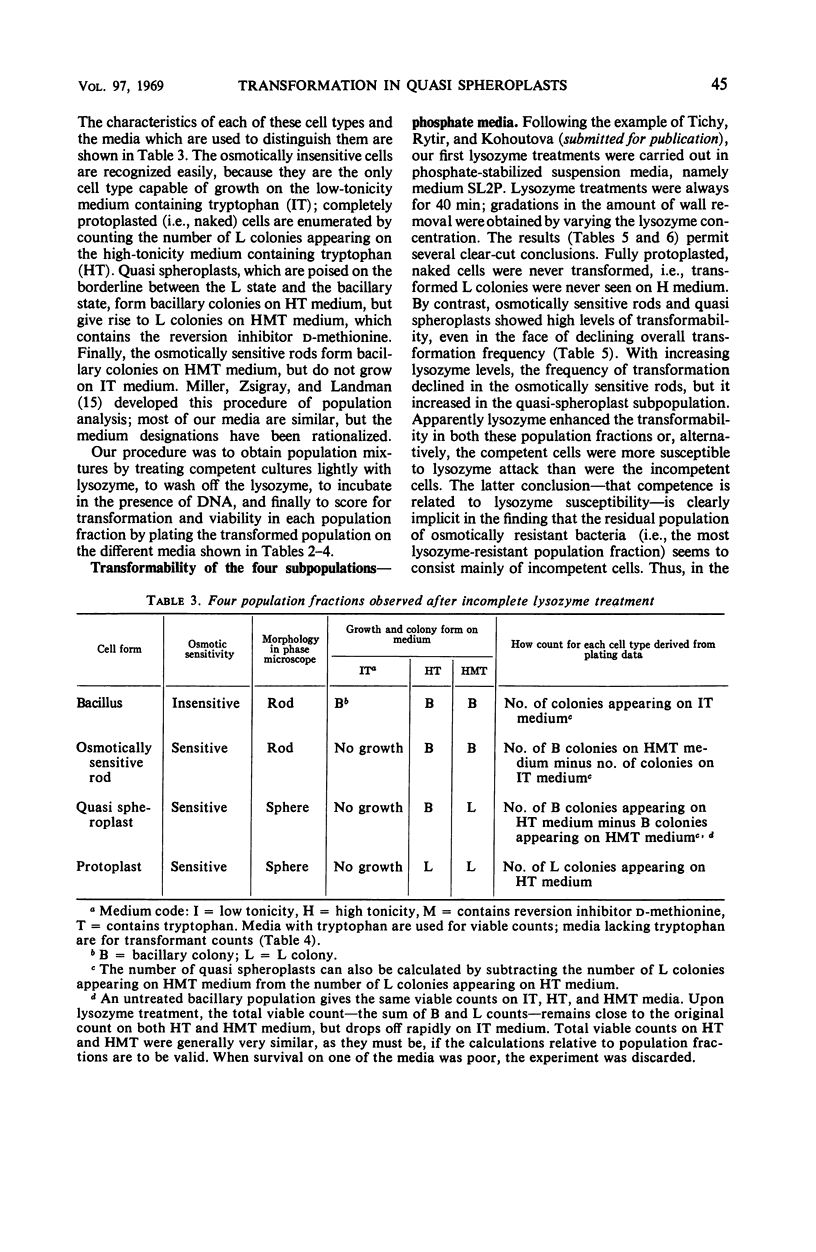
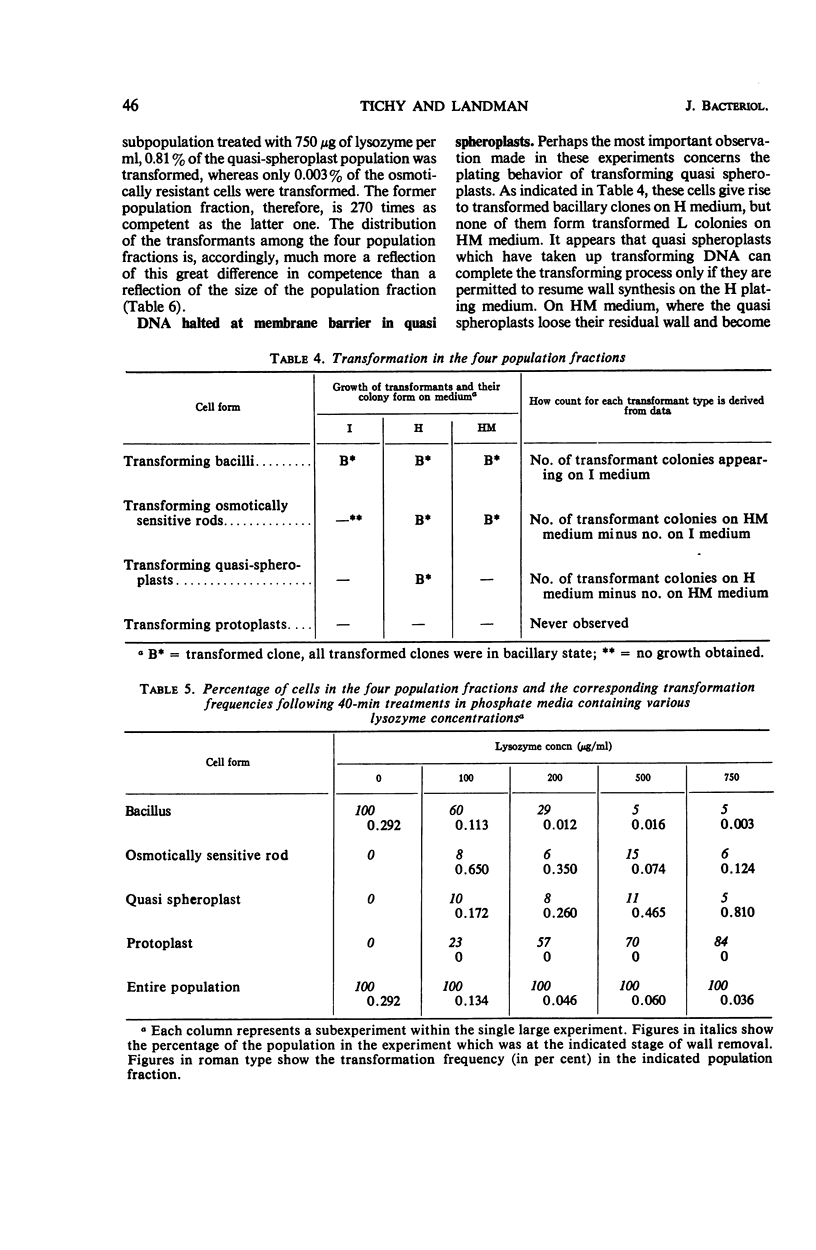
Abstract
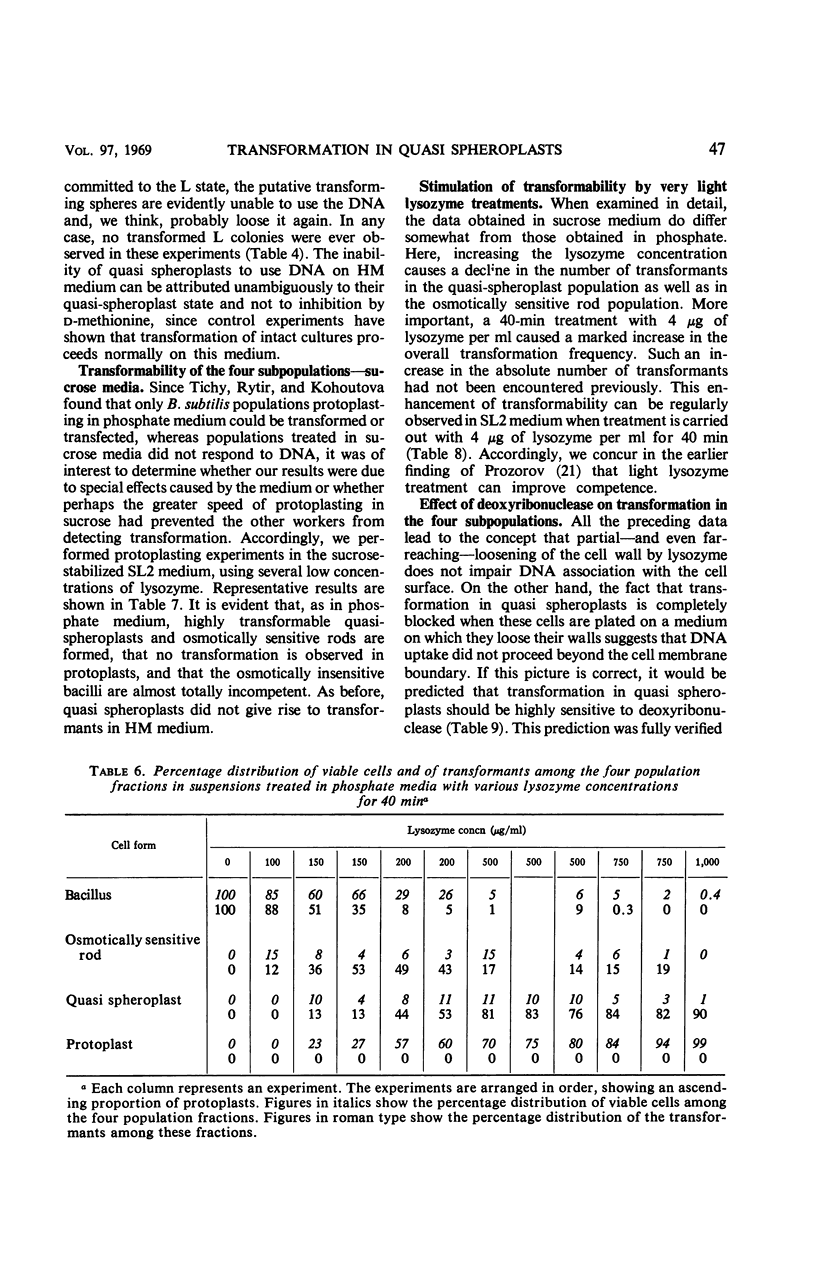
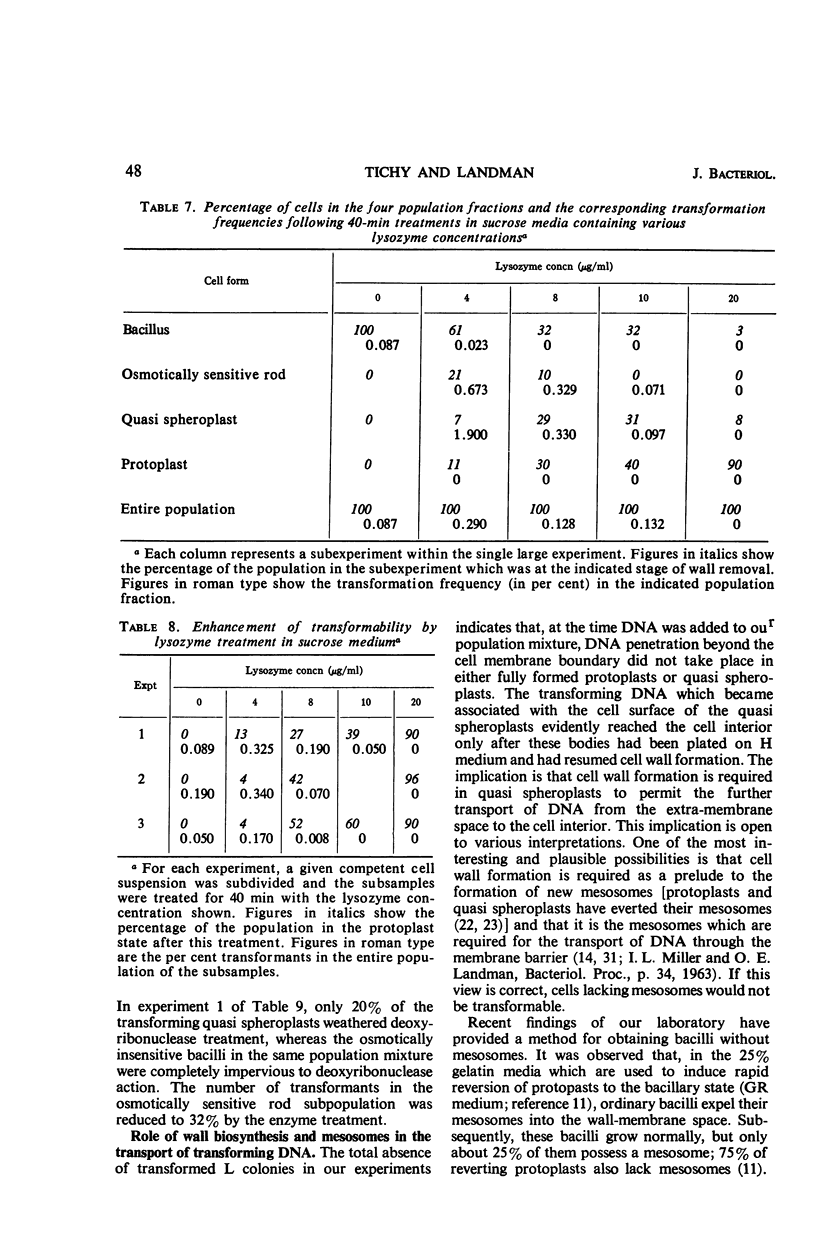
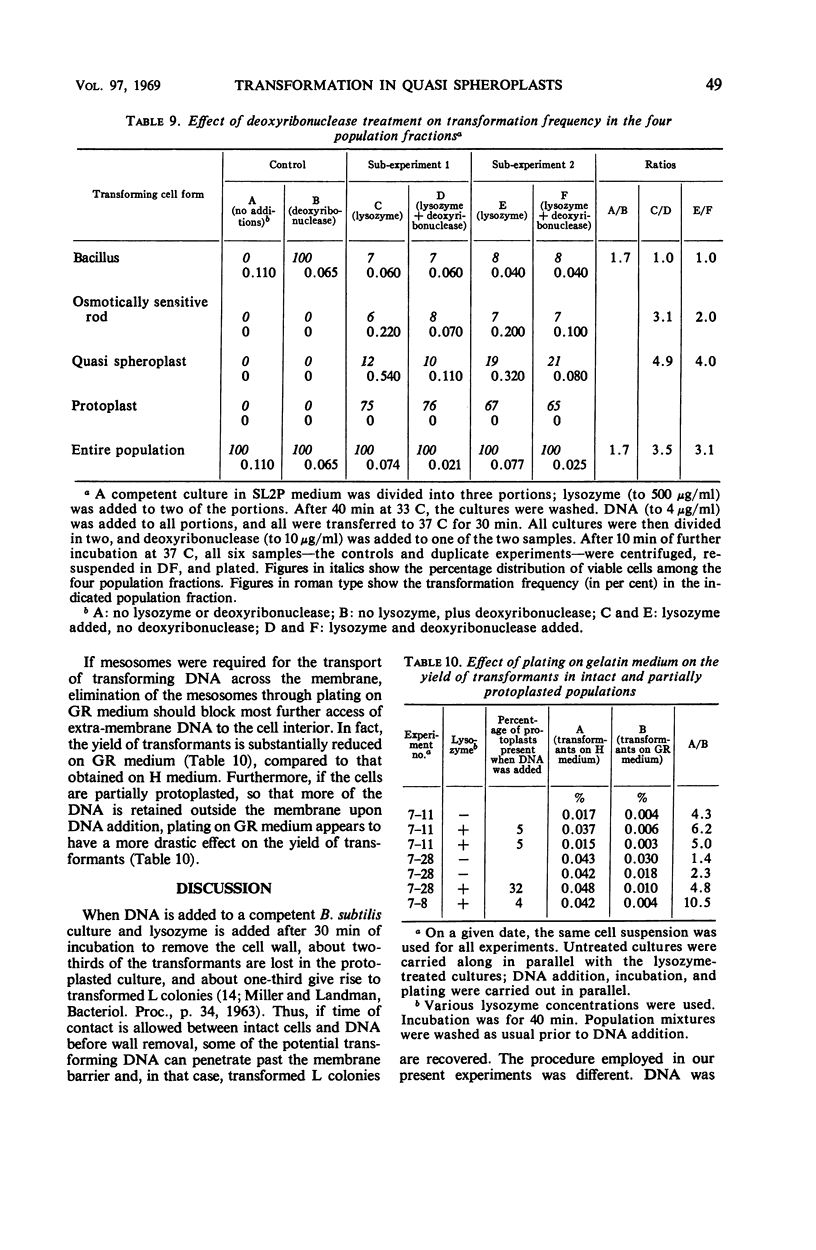
Recently developed differential plating media permit the distinction of four cell types in incompletely protoplasted populations: intact, osmotically insensitive bacilli; osmotically sensitive rods; spheres with adherent wall residues, called quasi spheroplasts; and protoplasts. Such population mixtures were washed free of lysozyme, and then transforming deoxyribonucleic acid (DNA) was added. Transformation was nil in the protoplasts, very low in the residual osmotically insensitive bacilli, and markedly enhanced in both osmotically sensitive rods and quasi spheroplasts. Transformation in the latter two population fractions was reduced, respectively, by about 60% and about 80% by deoxyribonuclease treatment. DNA adhering to the quasi spheroplasts transforms these cells only if they are permitted to resume wall synthesis; when the same cells are plated on a medium where they shed the residual wall and form L colonies, no transformant L colonies are recovered. It is inferred that far-reaching or complete protoplasting blocks all entry of transforming DNA into the cell interior. This may be owing to eversion of mesosomes. Evidence that intact mesosomes may be required for DNA entry is provided by the finding that the recovery of transformants in the intact cell system is sharply reduced on plating media containing 25% gelatin. On such media, cells expel their mesosomes and 75% of them do not re-form any. Our own data and a survey of published results suggest the generalization that partial depolymerization of the cell wall by lysozyme may enhance competence, whereas its complete removal abolishes it.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg A., Ayad S. R., Barker G. R. The nature of a competence-inducing factor in Bacillus subtilis. Biochem Biophys Res Commun. 1967 Sep 27;28(6):1062–1067. doi: 10.1016/0006-291x(67)90090-3. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer L. J., Landman O. E. Transport of donor deoxyribonucleic acid into the cell interior of thymine-starved Bacillus subtilis with chromosomes arrested at the terminus. J Bacteriol. 1969 Jan;97(1):174–181. doi: 10.1128/jb.97.1.174-181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R., Delius H., Janenisch R., Hofschneider P. H. Infectious nucleic acids of Escherichia coli bacteriophages. 10. Preparation and properties of Escherichia coli competent for infectious DNA from bacteriophages phi X 174 and M 13 and RNA from bacteriophage M 12. Eur J Biochem. 1967 Nov;2(4):414–428. doi: 10.1111/j.1432-1033.1967.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Fraser D., Mahler H. R., Shug A. L., Thomas C. A. THE INFECTION OF SUB-CELLULAR ESCHERICHIA COLI, STRAIN B, WITH A DNA PREPARATION FROM T2 BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1957 Nov 15;43(11):939–947. doi: 10.1073/pnas.43.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Hirokawa H., Ikeda Y. Genetic Recombination of Transforming Deoxyribonucleic Acid Molecules with the Recipient Genome and Among Themselves in Protoplasts of Bacillus subtilis. J Bacteriol. 1966 Aug;92(2):455–463. doi: 10.1128/jb.92.2.455-463.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILIASHENKO B. N. SFEROPLASTY BAKTERI I KAK RETSIPIENTY INFEKTSIONNYKH DNK BAKTERIOFAGOV. Mikrobiologiia. 1964 Sep-Oct;33:812–818. [PubMed] [Google Scholar]
- LANDMAN O. E., HALLE S. ENZYMICALLY AND PHYSICALLY INDUCED INHERITANCE CHANGES IN BACILLUS SUBTILIS. J Mol Biol. 1963 Dec;7:721–738. doi: 10.1016/s0022-2836(63)80119-9. [DOI] [PubMed] [Google Scholar]
- Landman O. E., Ryter A., Fréhel C. Gelatin-induced reversion of protoplasts of Bacillus subtilis to the bacillary form: electron-microscopic and physical study. J Bacteriol. 1968 Dec;96(6):2154–2170. doi: 10.1128/jb.96.6.2154-2170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER F., MACKAL R. P., TAO M., EVANS E. A., Jr Infectious deoxyribonucleic acid from gamma bacteriophage. J Biol Chem. 1961 Apr;236:1141–1143. [PubMed] [Google Scholar]
- NAVA G. C., GALIS A., BEISER S. M. Bacterial transformation: an antigen specific for 'competent' pneumococci. Nature. 1963 Mar 2;197:903–904. doi: 10.1038/197903b0. [DOI] [PubMed] [Google Scholar]
- PARANCHYCH W., GRAHAM A. F. Isolation and properties of an RNA-containing bacteriophage. J Cell Comp Physiol. 1962 Dec;60:199–208. doi: 10.1002/jcp.1030600303. [DOI] [PubMed] [Google Scholar]
- PROZOROV A. A. VLIIANIE IAICHNOGO LIZOTSIMA NA PRONITSAEMOST' KLETOK B. SUBTILIS DLIA TRANSFORMIRUIUSHCHE I DNK. Dokl Akad Nauk SSSR. 1965 Jan 11;160:472–474. [PubMed] [Google Scholar]
- Pakula R. Cellular sites for the competence-provoking factor of streptococci. J Bacteriol. 1967 Jul;94(1):75–79. doi: 10.1128/jb.94.1.75-79.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakula R. Production of competence-provoking factor and development of competence of a transformable streptococcus in serum-free media. Can J Microbiol. 1965 Oct;11(5):811–822. doi: 10.1139/m65-110. [DOI] [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Frehel C., Ferrandes B. Comportement des mésosomes lors de l'attaque de Bacillus subtilis par le lysozyme en milieu hyper- ou hypotonique. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 23;265(17):1259–1262. [PubMed] [Google Scholar]
- SEKIGUCHI M., TAKETO A., TAKAGI Y. An infective deoxyribonucleic acid from bacteriophage phi-X174. Biochim Biophys Acta. 1960 Dec 4;45:199–200. doi: 10.1016/0006-3002(60)91443-8. [DOI] [PubMed] [Google Scholar]
- Spizizen J. INFECTION OF PROTOPLASTS BY DISRUPTED T2 VIRUS. Proc Natl Acad Sci U S A. 1957 Aug 15;43(8):694–701. doi: 10.1073/pnas.43.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Mosser J. L. On the nature of the pneumococcal activator substance. Proc Natl Acad Sci U S A. 1966 Jan;55(1):58–66. doi: 10.1073/pnas.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Vermeulen C. A., Venema G. Evidence for the involvement of membranous bodies in the processes leading to genetic transformation in Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):1111–1121. doi: 10.1128/jb.92.4.1111-1121.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. II. AUTOLYTIC ENZYME ACTIVITY OF CELL WALLS. J Biol Chem. 1963 Sep;238:3126–3130. [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J., CRAWFORD I. P. BIOCHEMICAL ASPECTS OF COMPETENCE IN THE BACILLUS SUBTILIS TRANSFORMATION SYSTEM. I. CHEMICAL COMPOSITION OF CELL WALLS. J Biol Chem. 1963 Sep;238:3119–3125. [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]


