Abstract
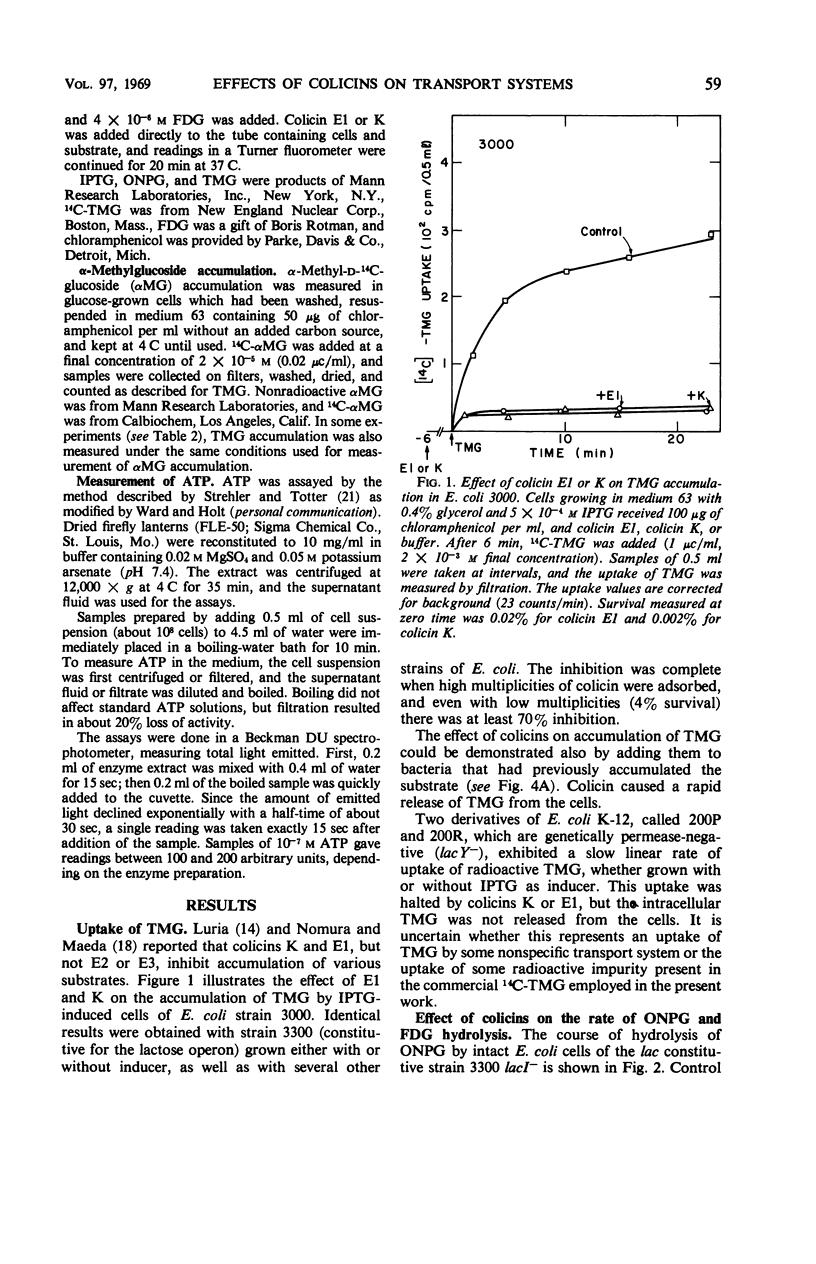
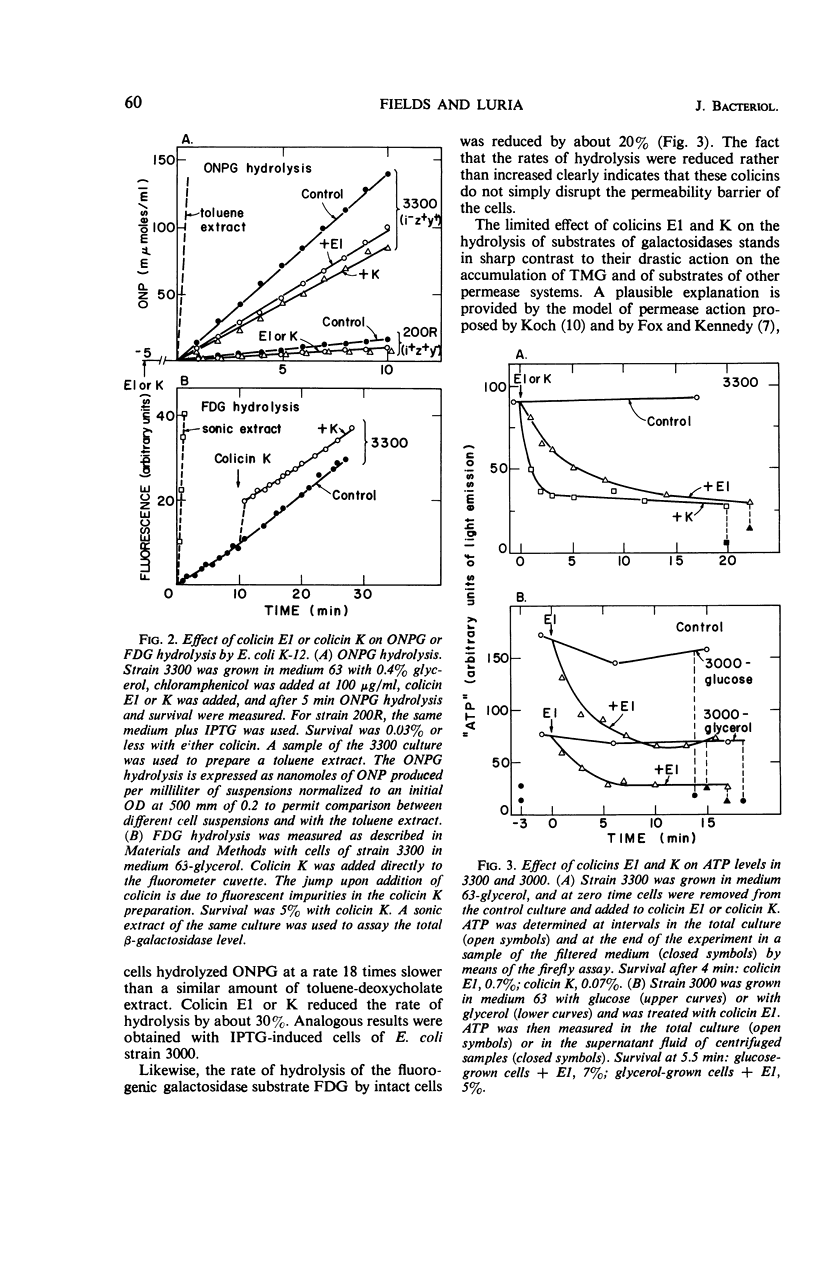
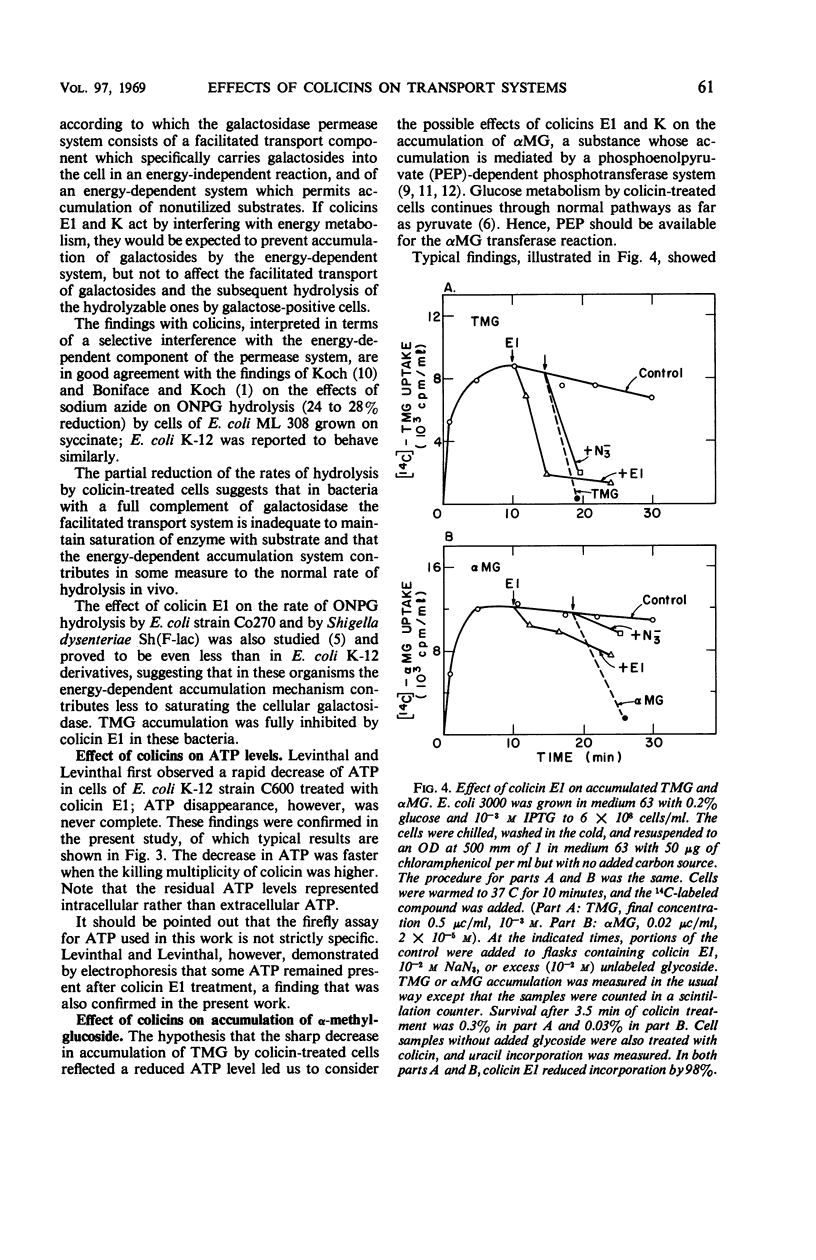
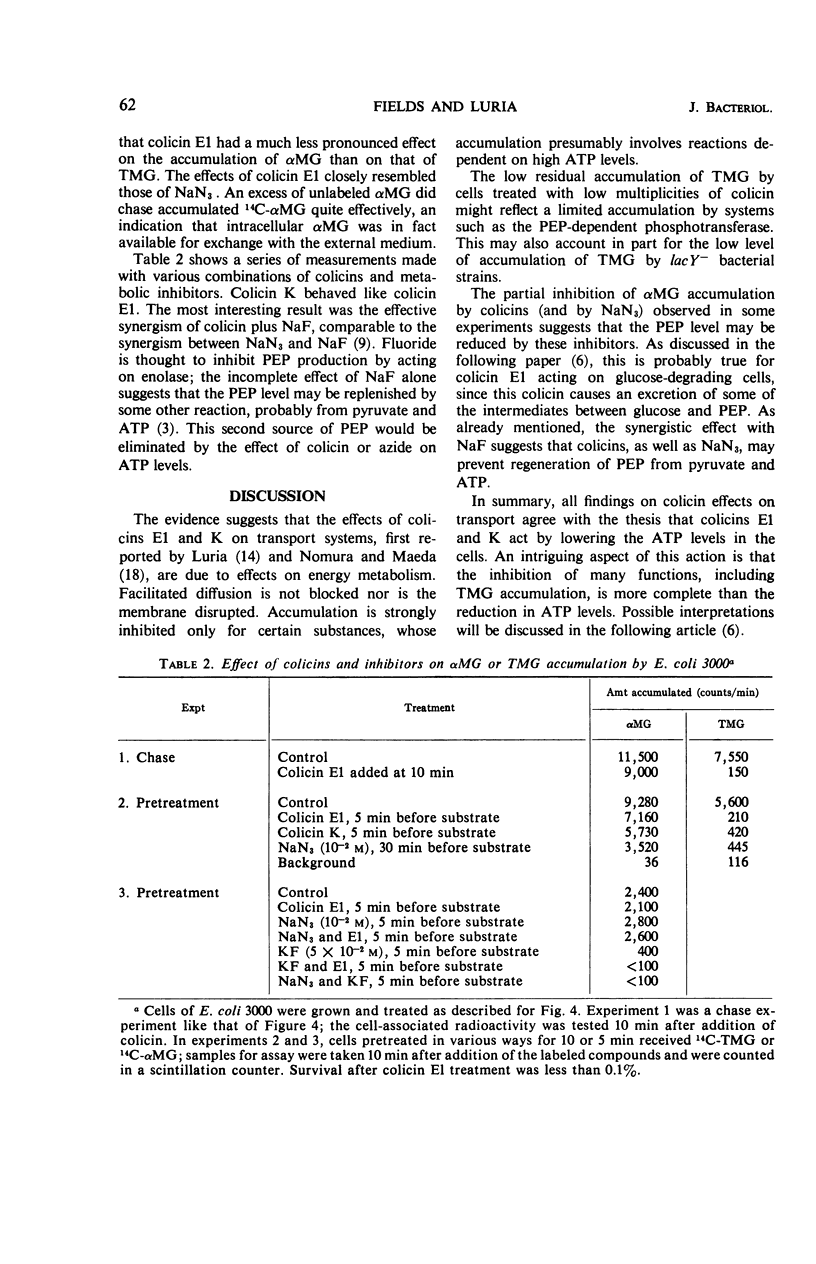
The effect of colicins E1 and K on active transport of β-d-galactosides and of α-methyl-d-glucoside (αMG) by Escherichia coli was studied. These colicins strongly inhibited the accumulation of thio-methyl-galactoside (TMG) by bacteria and caused rapid exit of previously accumulated TMG. The inhibition effect was limited to the accumulation phase of galactoside transport; the rate of hydrolysis of o-nitrophenyl galactoside, which is dependent on transport of the substrate by the lactose-permease system, was only slightly affected. The accumulation of αMG was highly resistant to inhibition by these colicins under conditions which caused complete suppression of TMG accumulation. These effects of the colicins on transport resemble qualitatively those of sodium azide. The findings were interpreted by assuming that colicins E1 and K inhibit the energy-dependent steps in the accumulation of TMG but do not affect facilitated diffusion of galactosides mediated by the specific transport mechanism. The continued accumulation of αMG was attributed to the fact that this compound is stored by E. coli cells as a phosphorylated compound by a phosphoenolpyruvate-dependent transport system rather than by an adenosine triphosphate-linked accumulation mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boniface J., Koch A. L. The interaction between permeases as a tool to find their relationship on the membrane. Biochim Biophys Acta. 1967 Sep 9;135(4):756–770. doi: 10.1016/0005-2736(67)90107-1. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. Net formation of phosphoenolpyruvate from pyruvate by Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):618–620. doi: 10.1016/0304-4165(65)90374-0. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Fields K. L. Comparison of the action of colicins E1 and K on Escherichia coli with the effects of abortive infection by virulent bacteriophages. J Bacteriol. 1969 Jan;97(1):78–82. doi: 10.1128/jb.97.1.78-82.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on cellular metabolism. J Bacteriol. 1969 Jan;97(1):64–77. doi: 10.1128/jb.97.1.64-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., SIMINOVITCH L., WOLLMAN E. Sur la biosynthèse d'une colicine et sur son mode d'action. Ann Inst Pasteur (Paris) 1952 Sep;83(3):295–315. [PubMed] [Google Scholar]
- KOCH A. L. THE ROLE OF PERMEASE IN TRANSPORT. Biochim Biophys Acta. 1964 Jan 27;79:177–200. doi: 10.1016/0926-6577(64)90050-6. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Scarborough G. A. Mechanism of hydrolysis of O-nitrophenyl-beta-galactoside in Staphylococcus aureus and its significance for theories of sugar transport. Proc Natl Acad Sci U S A. 1967 Jul;58(1):225–228. doi: 10.1073/pnas.58.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W., Kundig F. D., Anderson B., Roseman S. Restoration of active transport of glycosides in Escherichia coli by a component of a phosphotransferase system. J Biol Chem. 1966 Jul 10;241(13):3243–3246. [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- REVEL H. R., LURIA S. E., ROTMAN B. Biosynthesis of B-D-galactosidase controlled by phage-carried genes. I. Induced beta-D-galactosidase biosynthesis after transduction of gene z-plus by phage. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1956–1967. doi: 10.1073/pnas.47.12.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTMAN B. Measurement of activity of single molecules of beta-D-galactosidase. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1981–1991. doi: 10.1073/pnas.47.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREHLER B. L., TOTTER J. R. Firefly luminescence in the study of energy transfer mechanisms. I. Substrate and enzyme determination. Arch Biochem Biophys. 1952 Sep;40(1):28–41. doi: 10.1016/0003-9861(52)90070-2. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]



