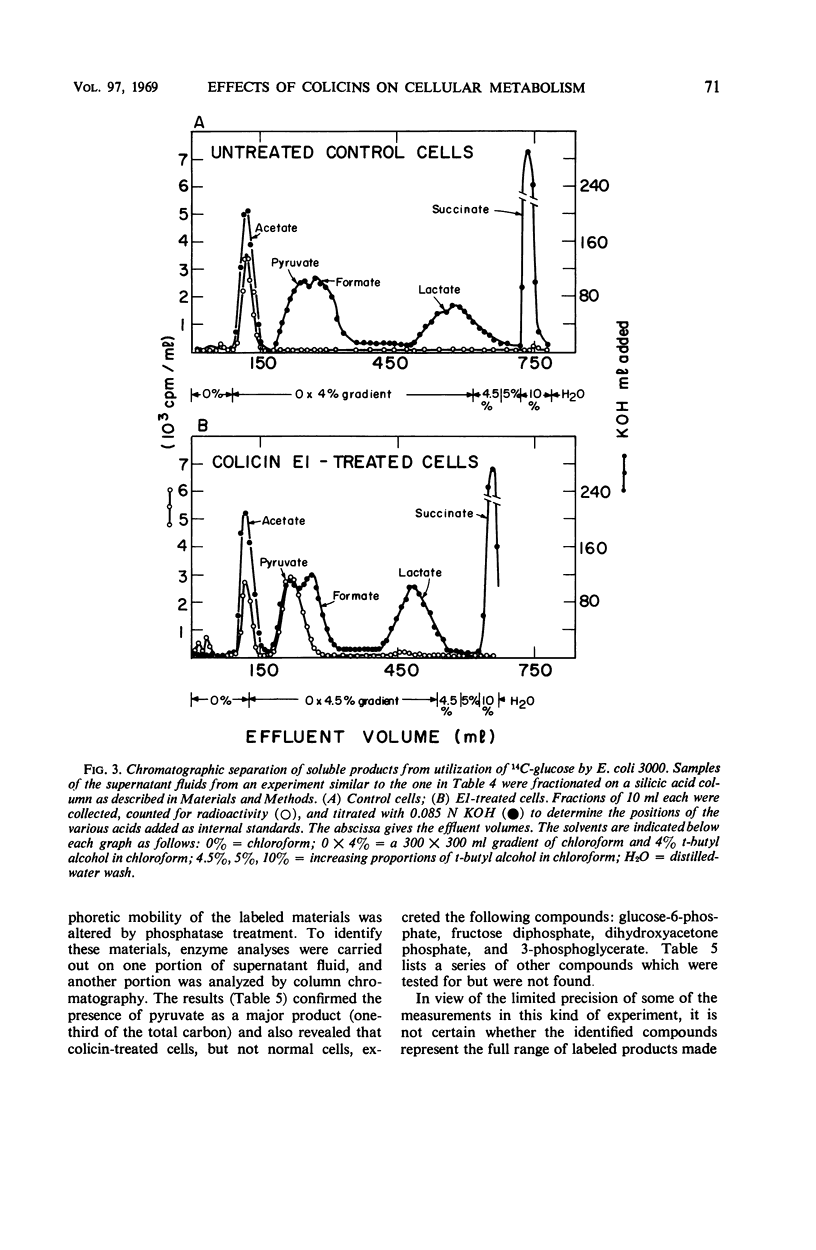
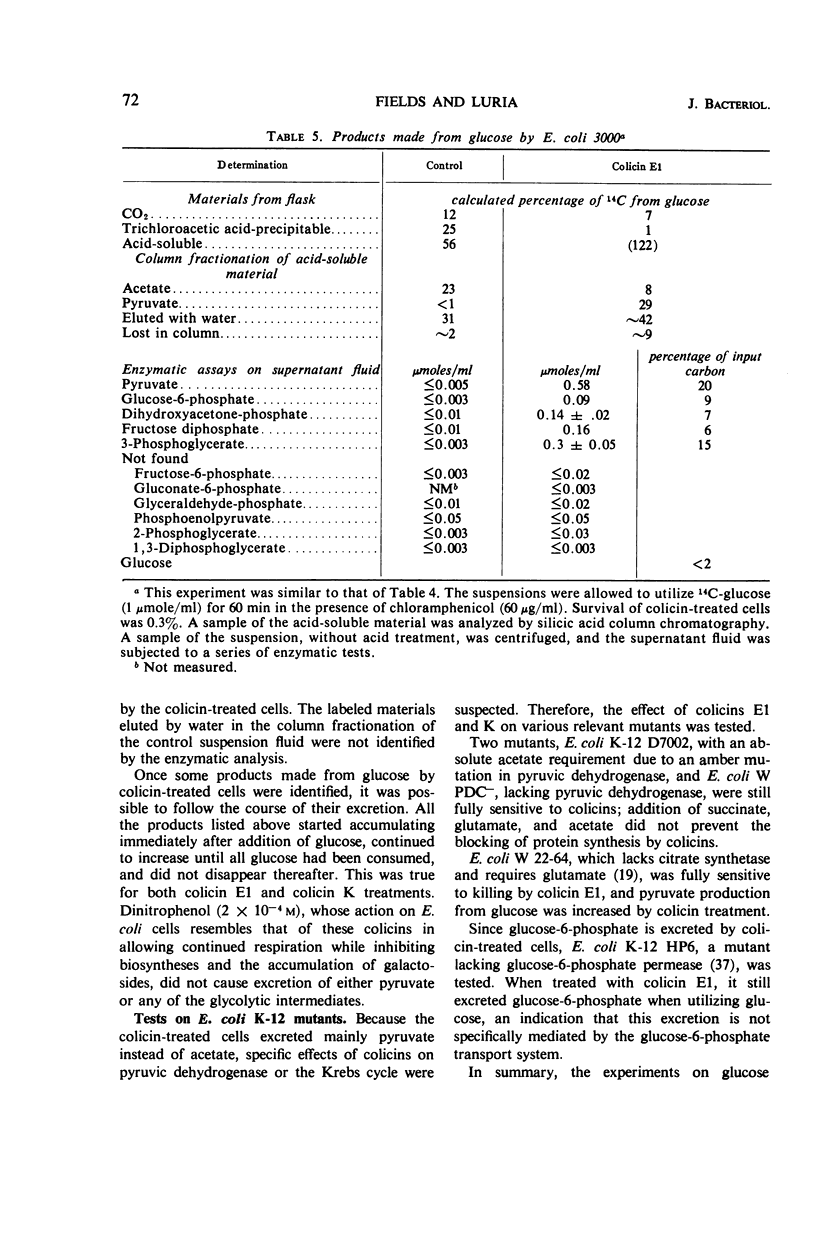
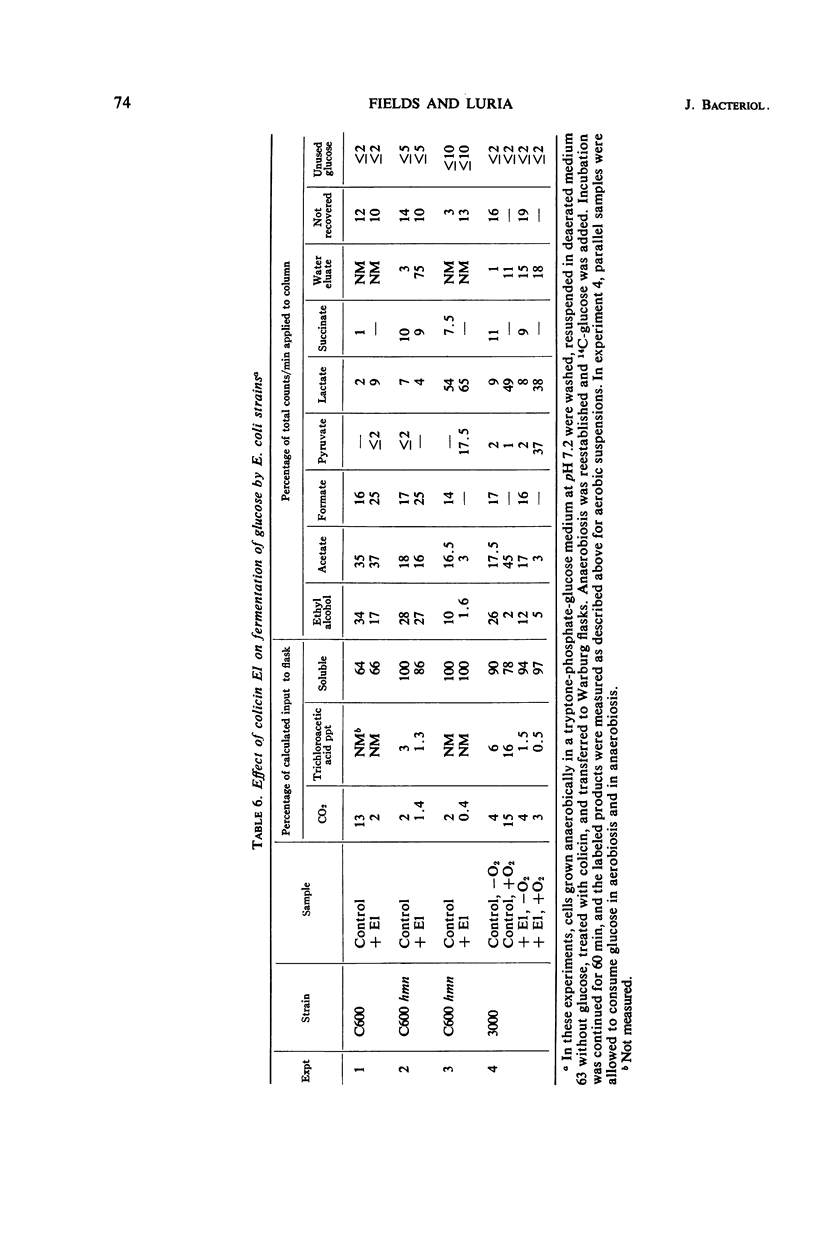
Abstract
Colicins E1 and K inhibited a whole series of energy-dependent reactions in Escherichia coli cells, including motility, biosynthesis of nucleic acids, proteins and polysaccharides, and the conversion of ornithine to citrulline. Respiration was only partially affected, and substrates such as glucose continued to be catabolized through the normal pathways, albeit with reduced CO2 production. The soluble products of aerobic glucose catabolism by colicin-treated cells were analyzed. Pyruvate replaced acetate as the major excreted product, and the following intermediates of glycolysis were excreted in significant amounts: glucose-6-phosphate, fructose-1,6-diphosphate, dihydroxyacetone phosphate, and 3-phosphoglycerate. Anaerobically growing cells manifested a somewhat enhanced tolerance to the colicins. This protection by anaerobiosis appeared to depend on the exclusion of oxygen more than on the extent of fermentative catabolism versus catabolism of the respiratory type. These results are interpreted in terms of possible functions of colicin in lowering the adenosine triphosphate (ATP) content of the cells and in terms of the role of lowered ATP levels in inhibiting many of the energy-requiring reactions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Adler J., Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol. 1967 Feb;46(2):175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. Biological feedback control at the molecular level. Science. 1965 Nov 12;150(3698):851–857. doi: 10.1126/science.150.3698.851. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E., Fall L. Adenosine triphosphate conservation in biosynthetic regulation. Escherichia coli phosphoribosylpyrophosphate synthase. J Biol Chem. 1967 Jul 10;242(13):3241–3242. [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BELJANSKI M. Sur la formation d'enzymes respiratoires chez un mutant d'Escherichia coli streptomycino-résistant et auxotrophe pour l'hémine. Ann Inst Pasteur (Paris) 1957 Mar;92(3):396–412. [PubMed] [Google Scholar]
- BUHLER D. R. A simple scintillation counting technique for assaying C1402 in a Warburg flask. Anal Biochem. 1962 Nov;4:413–417. doi: 10.1016/0003-2697(62)90143-4. [DOI] [PubMed] [Google Scholar]
- Cousin D., Belaïch J. P. Sur une mutatio thermosensible d'Escherichia coli affectant une fonction énergétique. C R Acad Sci Hebd Seances Acad Sci D. 1966 Sep 19;263(12):886–888. [PubMed] [Google Scholar]
- Cousin D. Mutants thermosensibles d'Escherichia coli K12. II. Etude d'une mutation létale affectant une réaction génératrice d'énercie. Ann Inst Pasteur (Paris) 1967 Sep;113(3):309–325. [PubMed] [Google Scholar]
- DAVIS B. D., GILVARG C. The role of the tricarboxylic acid cycle in acetate oxidation in Escherichia coli. J Biol Chem. 1956 Sep;222(1):307–319. [PubMed] [Google Scholar]
- Dobrogosz W. J. Altered end-product patterns and catabolite repression in Escherichia coli. J Bacteriol. 1966 Jun;91(6):2263–2269. doi: 10.1128/jb.91.6.2263-2269.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C. C., Houck B. N., Brand L. Purification of Escherichia coli phosphofructokinase. Biochem Biophys Res Commun. 1967 May 5;27(3):287–293. doi: 10.1016/s0006-291x(67)80094-9. [DOI] [PubMed] [Google Scholar]
- JACOB F., SIMINOVITCH L., WOLLMAN E. Sur la biosynthèse d'une colicine et sur son mode d'action. Ann Inst Pasteur (Paris) 1952 Sep;83(3):295–315. [PubMed] [Google Scholar]
- KORNBERG H. L., PHIZACKERLEY P. J., SADLER J. R. The metabolism of C2 compounds in micro-organisms. 5. Biosynthesis of cell materials from acetate in Escherichia coli. Biochem J. 1960 Dec;77:438–445. doi: 10.1042/bj0770438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohiyama M., Cousin D., Ryter A., Jacob F. Mutants thermosensibles d'Escherichia coli K 12. I. Isolement et caractérisation rapide. Ann Inst Pasteur (Paris) 1966 Apr;110(4):465–486. [PubMed] [Google Scholar]
- Kovác L., Kuzela S. Effect of uncoupling agents and azide on the synthesis of beta-galactosidase in aerobically and anaerobically grown Escherichia coli. Biochim Biophys Acta. 1966 Oct 31;127(2):355–365. [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- Preiss J., Shen L., Greenberg E., Gentner N. Biosynthesis of bacterial glycogen. IV. Activation and inhibition of the adenosine diphosphate glucose pyrophosphorylase of Escherichia coli B. Biochemistry. 1966 Jun;5(6):1833–1845. doi: 10.1021/bi00870a008. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Fraenkel D. G., Lin E. C. The enzymatic lesion of strain MM-6, a pleiotropic carbohydrate-negative mutant of Escherichia coli. Biochem Biophys Res Commun. 1967 Apr 7;27(1):63–67. doi: 10.1016/s0006-291x(67)80040-8. [DOI] [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. Fractionation of phosphates by paper ionophoresis and chromatography. Biochem J. 1955 Jun;60(2):264–270. doi: 10.1042/bj0600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C. H., STERN I., GILMOUR C. M., KLUNGSOYR S., REED D. J., BIALY J. J., CHRISTENSEN B. E., CHELDELIN V. H. Comparative study of glucose catabolism by the radiorespirometric method. J Bacteriol. 1958 Aug;76(2):207–216. doi: 10.1128/jb.76.2.207-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]



