Abstract
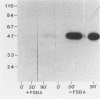
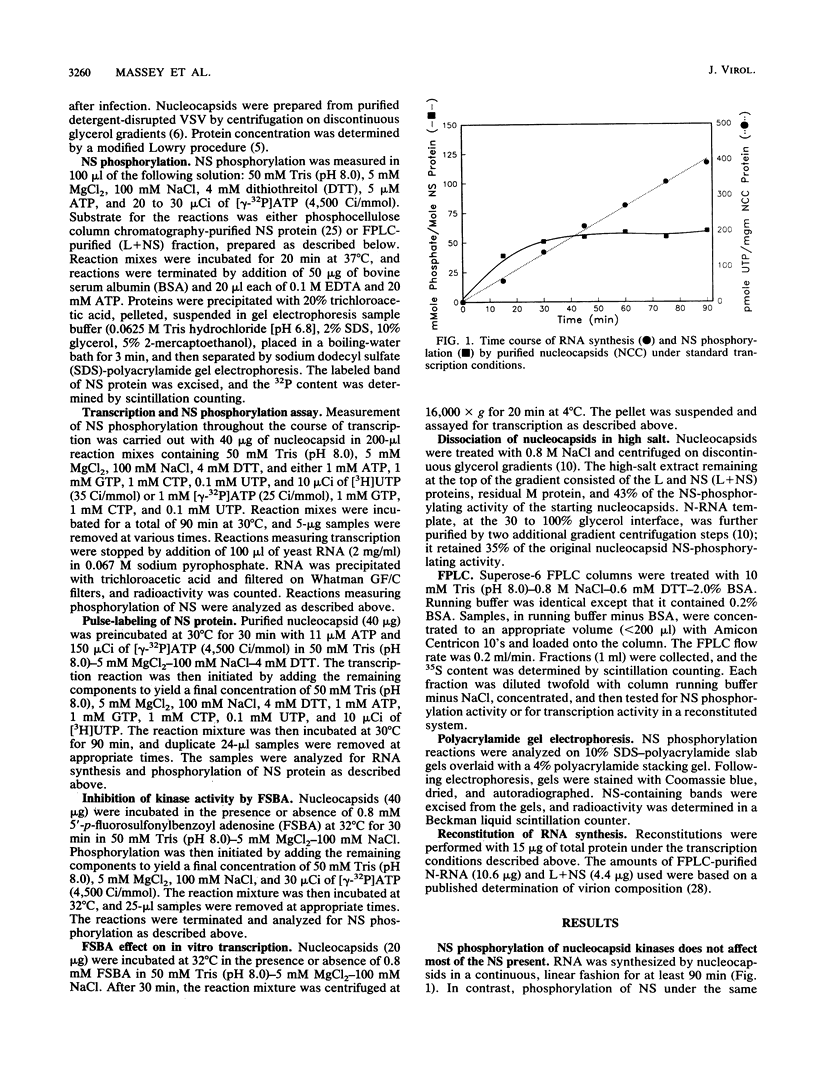
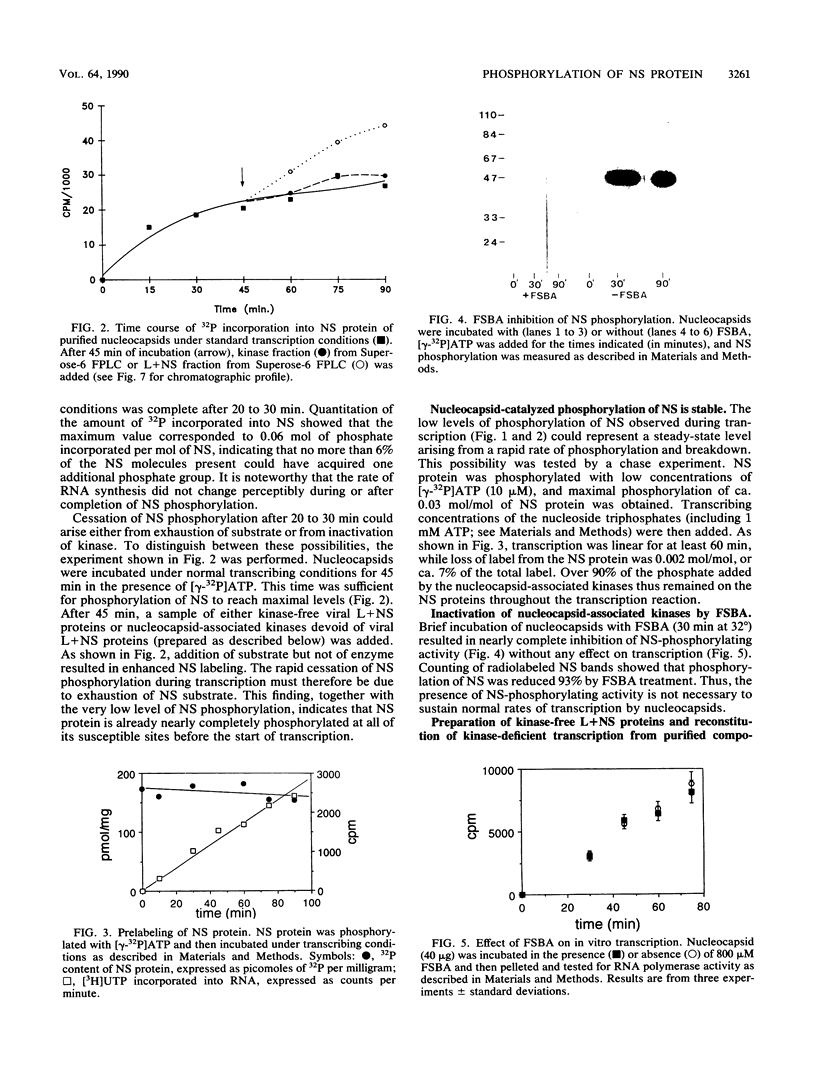
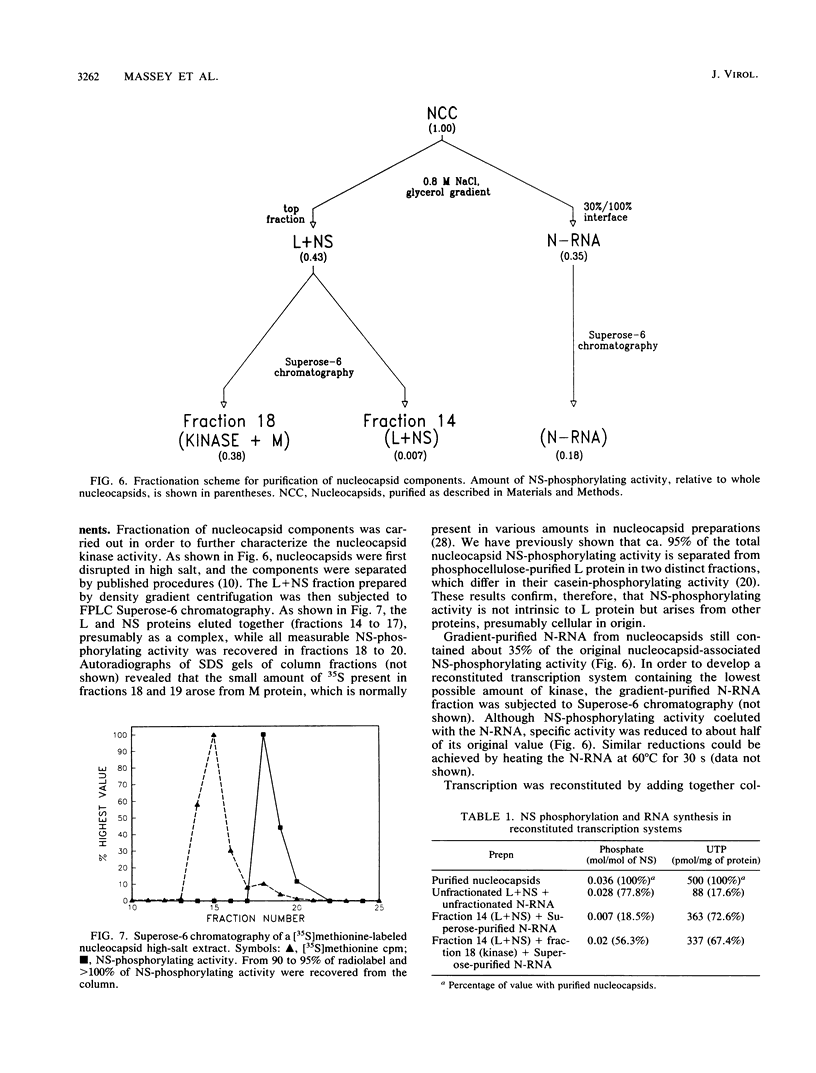
The relationship between NS protein phosphorylation and RNA polymerase activities was determined in nucleocapsids purified from vesicular stomatitis virus grown in BHK cells. Phosphate incorporation into endogenous NS protein under transcription conditions reached a maximum value of 0.06 mol/mol of NS within 20 to 30 min, while RNA synthesis remained linear for 90 min. Phosphate incorporation into NS increased further upon addition of kinase-free NS protein but not upon addition of nucleocapsid kinase (prepared as described below), indicating that cessation of NS phosphorylation under transcribing conditions was due to substrate exhaustion. When NS was phosphorylated with 32P, less than 8% of the radiolabel was lost during subsequent transcription, indicating that this phosphate did not turn over. Treatment of nucleocapsids with 5'-p-fluorosulfonylbenzoyl adenosine resulted in greater than 90% inhibition of NS phosphorylation but had no effect on RNA polymerase activity. Fast protein liquid (Superose-6) chromatography of a nucleocapsid (L + NS) fraction resulted in complete separation of the viral (L + NS) protein from NS-phosphorylating activity. The addition of this kinase-free (L + NS) fraction to a kinase-deficient N-RNA fraction reconstituted an active RNA polymerase containing less than 20% of the original NS-phosphorylating activity. These results demonstrate that NS-phosphorylating activity is unnecessary during vesicular stomatitis virus RNA synthesis and indicate that all of the protein kinase(s) present in purified nucleocapsids is probably of cellular rather than viral origin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. The transcription complex of vesicular stomatitis virus. Cell. 1987 Feb 13;48(3):363–364. doi: 10.1016/0092-8674(87)90184-x. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckes J. D., Childers L. C., Perrault J. Phosphorylation of vesicular stomatitis virus M protein: evidence for a second virion-associated protein serine kinase activity. Virology. 1989 Mar;169(1):161–171. doi: 10.1016/0042-6822(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Bell J. C., Brown E. G., Takayesu D., Prevec L. Protein kinase activity associated with immunoprecipitates of the vesicular stomatitis virus phosphoprotein NS. Virology. 1984 Jan 30;132(2):229–238. doi: 10.1016/0042-6822(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Guerina N. G., Guo H. Y., Huang A. S. Host-dependent phosphorylation and kinase activity associated with vesicular stomatitis virus. J Biol Chem. 1982 Mar 25;257(6):3313–3319. [PubMed] [Google Scholar]
- Colman R. F. Affinity labeling of purine nucleotide sites in proteins. Annu Rev Biochem. 1983;52:67–91. doi: 10.1146/annurev.bi.52.070183.000435. [DOI] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Specific interactions of vesicular stomatitis virus L and NS proteins with heterologous genome ribonucleoprotein template lead to mRNA synthesis in vitro. J Virol. 1984 Sep;51(3):628–634. doi: 10.1128/jvi.51.3.628-634.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. S., Banerjee A. K. Vesicular stomatitis virus NS proteins: structural similarity without extensive sequence homology. J Virol. 1985 Jul;55(1):60–66. doi: 10.1128/jvi.55.1.60-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. A., Marnell L. L., Summers D. F. The major ribonucleoprotein-associated protein kinase of vesicular stomatitis virus is a host cell protein. J Biol Chem. 1983 Dec 25;258(24):15283–15290. [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. Constitutively phosphorylated residues in the NS protein of vesicular stomatitis virus. J Biol Chem. 1985 Jul 25;260(15):8990–8995. [PubMed] [Google Scholar]
- Hsu C. H., Morgan E. M., Kingsbury D. W. Site-specific phosphorylation regulates the transcriptive activity of vesicular stomatitis virus NS protein. J Virol. 1982 Jul;43(1):104–112. doi: 10.1128/jvi.43.1.104-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi T., Yokoyama M., Ohtsuki K. Characterization of a polypeptide-dependent membrane protein kinase that specifically phosphorylates NS protein of vesicular stomatitis virus in vitro. Tohoku J Exp Med. 1988 May;155(1):41–56. doi: 10.1620/tjem.155.41. [DOI] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsford L., Emerson S. U. Transcriptional activities of different phosphorylated species of NS protein purified from vesicular stomatitis virions and cytoplasm of infected cells. J Virol. 1980 Mar;33(3):1097–1105. doi: 10.1128/jvi.33.3.1097-1105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey D. M., Lenard J. Inactivation of the RNA polymerase of vesicular stomatitis virus by N-ethylmaleimide and protection by nucleoside triphosphates. Evidence for a second ATP binding site on L protein. J Biol Chem. 1987 Jun 25;262(18):8734–8737. [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Phosphoprotein NS of vesicular stomatitis virus: phosphorylated states and transcriptional activities of intracellular and virion forms. Virology. 1986 Oct 30;154(2):259–270. doi: 10.1016/0042-6822(86)90452-6. [DOI] [PubMed] [Google Scholar]
- Miller D. K., Lenard J. Inhibition of vesicular stomatitis virus infection by spike glycoprotein. Evidence for an intracellular, G protein-requiring step. J Cell Biol. 1980 Feb;84(2):430–437. doi: 10.1083/jcb.84.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinacore M. S., Lucas-Lenard J. The effect of the vesicular stomatitis virus-associated protein kinase on viral mRNA transcription in vitro. Virology. 1982 Sep;121(2):404–413. doi: 10.1016/0042-6822(82)90178-7. [DOI] [PubMed] [Google Scholar]
- Sánchez A., De B. P., Banerjee A. K. In vitro phosphorylation of NS protein by the L protein of vesicular stomatitis virus. J Gen Virol. 1985 May;66(Pt 5):1025–1036. doi: 10.1099/0022-1317-66-5-1025. [DOI] [PubMed] [Google Scholar]
- Thomas D., Newcomb W. W., Brown J. C., Wall J. S., Hainfeld J. F., Trus B. L., Steven A. C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985 May;54(2):598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Sakuma S., Tanaka S. A possible biological function of the protein kinase associated with vaccinia and vesicular stomatitis virions. FEBS Lett. 1974 May 1;41(2):331–334. doi: 10.1016/0014-5793(74)81241-x. [DOI] [PubMed] [Google Scholar]
- Witt D. J., Summers D. F. Relationship between virion-associated kinase-effected phosphorylation and transcription activity of vesicular stomatitis virus. Virology. 1980 Nov;107(1):34–49. doi: 10.1016/0042-6822(80)90270-6. [DOI] [PubMed] [Google Scholar]