Abstract
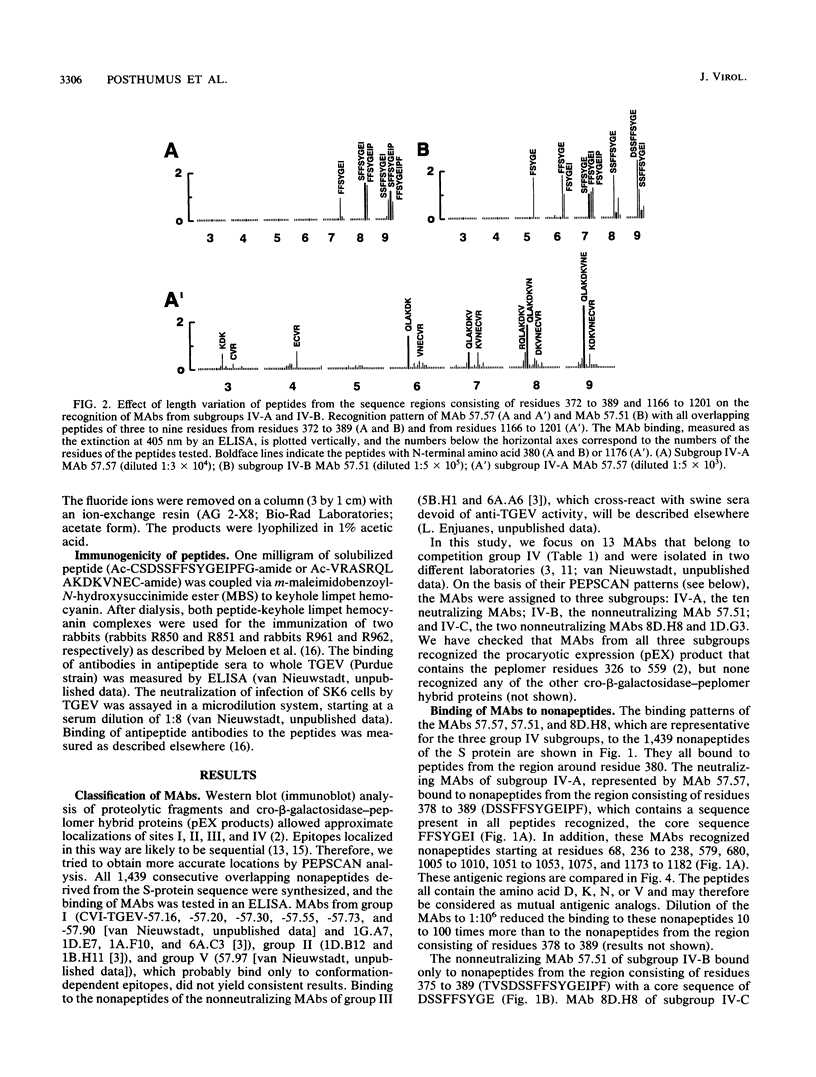
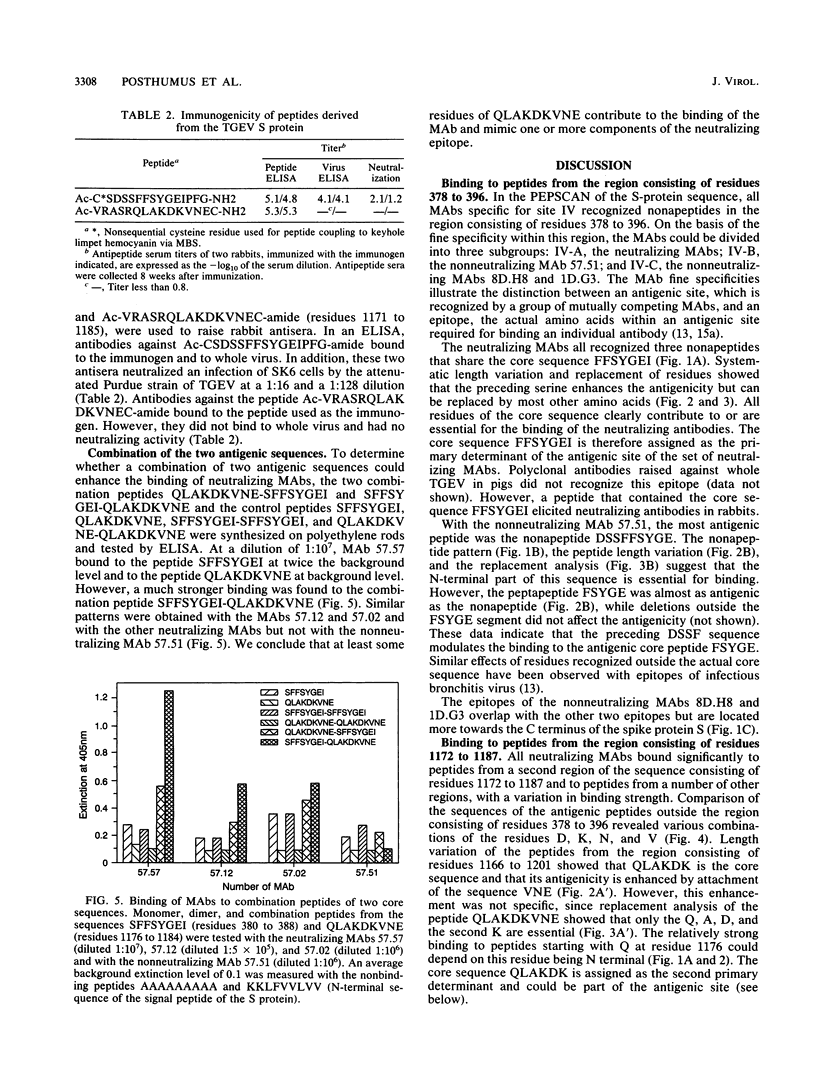
The amino acid sequences recognized by monoclonal antibodies (MAbs) specific for the antigenic site IV of the spike protein S of transmissible gastroenteritis virus were analyzed by PEPSCAN. All MAbs of group IV recognized peptides from the S region consisting of residues 378 to 390. In addition, the neutralizing MAbs (subgroup IV-A) also bound to peptides from the region consisting of residues 1173 to 1184 and to several other peptides with a related amino acid composition. The contribution of the individual residues of both sequences to the binding of a MAb was determined by varying the length of the peptide and by a consecutive deletion or replacement of parental residues by the 19 other amino acids. The sequence consisting of residues 326 to 558, tested as part of a cro-beta-galactosidase hybrid protein, was antigenic, but the sequence consisting of residues 1150 to 1239 was not. Furthermore, antibodies raised in rabbits against the peptide SDSSFFSYGEIPFGN (residues 377 to 391), but not those raised against the peptide VRASRQLAKDKVNEC (residues 1171 to 1185), recognized the virus and had neutralizing activity. We infer that the epitope of the neutralizing MAbs is composite and consists of the linear sequence SFFSYGEI (residues 380 to 387) with contributions of A, D, K, N, Q, or V residues from other parts of the S molecule. The complex epitope was simulated by synthesizing peptides in which the sequences consisting of residues 380 to 387 and 1176 to 1184 were combined. MAbs of subgroup IV-A recognized the combination peptides two to six times better than the individual sequences. These results may offer prospects for the development of an experimental vaccine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature. 1989 Feb 23;337(6209):709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- Correa I., Gebauer F., Bullido M. J., Suñ C., Baay M. F., Zwaagstra K. A., Posthumus W. P., Lenstra J. A., Enjuanes L. Localization of antigenic sites of the E2 glycoprotein of transmissible gastroenteritis coronavirus. J Gen Virol. 1990 Feb;71(Pt 2):271–279. doi: 10.1099/0022-1317-71-2-271. [DOI] [PubMed] [Google Scholar]
- Correa I., Jiménez G., Suñ C., Bullido M. J., Enjuanes L. Antigenic structure of the E2 glycoprotein from transmissible gastroenteritis coronavirus. Virus Res. 1988 Apr;10(1):77–93. doi: 10.1016/0168-1702(88)90059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., Laude H. Antigenic structure of transmissible gastroenteritis virus. II. Domains in the peplomer glycoprotein. J Gen Virol. 1986 Jul;67(Pt 7):1405–1418. doi: 10.1099/0022-1317-67-7-1405. [DOI] [PubMed] [Google Scholar]
- Fournier A., Wang C. T., Felix A. M. Applications of BOP reagent in solid phase synthesis. Advantages of BOP reagent for difficult couplings exemplified by a synthesis of [Ala 15]-GRF(1-29)-NH2. Int J Pept Protein Res. 1988 Jan;31(1):86–97. doi: 10.1111/j.1399-3011.1988.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Stewart F., Elleman C. J. Identification of epitopes of immunological importance on the peplomer of porcine transmissible gastroenteritis virus. Adv Exp Med Biol. 1987;218:509–515. doi: 10.1007/978-1-4684-1280-2_66. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Barteling S. J., Meloen R. H. Small peptides induce antibodies with a sequence and structural requirement for binding antigen comparable to antibodies raised against the native protein. Proc Natl Acad Sci U S A. 1985 Jan;82(1):178–182. doi: 10.1073/pnas.82.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle J. M., Filman D. J. The antigenic structure of poliovirus. Philos Trans R Soc Lond B Biol Sci. 1989 Jun 12;323(1217):467–478. doi: 10.1098/rstb.1989.0024. [DOI] [PubMed] [Google Scholar]
- Jacobs L., de Groot R., van der Zeijst B. A., Horzinek M. C., Spaan W. The nucleotide sequence of the peplomer gene of porcine transmissible gastroenteritis virus (TGEV): comparison with the sequence of the peplomer protein of feline infectious peritonitis virus (FIPV). Virus Res. 1987 Nov;8(4):363–371. doi: 10.1016/0168-1702(87)90008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G., Correa I., Melgosa M. P., Bullido M. J., Enjuanes L. Critical epitopes in transmissible gastroenteritis virus neutralization. J Virol. 1986 Oct;60(1):131–139. doi: 10.1128/jvi.60.1.131-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Kusters J. G., Jager E. J., Lenstra J. A., Koch G., Posthumus W. P., Meloen R. H., van der Zeijst B. A. Analysis of an immunodominant region of infectious bronchitis virus. J Immunol. 1989 Oct 15;143(8):2692–2698. [PubMed] [Google Scholar]
- Laude H., Chapsal J. M., Gelfi J., Labiau S., Grosclaude J. Antigenic structure of transmissible gastroenteritis virus. I. Properties of monoclonal antibodies directed against virion proteins. J Gen Virol. 1986 Jan;67(Pt 1):119–130. doi: 10.1099/0022-1317-67-1-119. [DOI] [PubMed] [Google Scholar]
- Lenstra J. A., Kusters J. G., Koch G., van der Zeijst B. A. Antigenicity of the peplomer protein of infectious bronchitis virus. Mol Immunol. 1989 Jan;26(1):7–15. doi: 10.1016/0161-5890(89)90014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra J. A., Kusters J. G., van der Zeijst B. A. Mapping of viral epitopes with prokaryotic expression products. Arch Virol. 1990;110(1-2):1–24. doi: 10.1007/BF01310699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloen R. H., Puyk W. C., Meijer D. J., Lankhof H., Posthumus W. P., Schaaper W. M. Antigenicity and immunogenicity of synthetic peptides of foot-and-mouth disease virus. J Gen Virol. 1987 Feb;68(Pt 2):305–314. doi: 10.1099/0022-1317-68-2-305. [DOI] [PubMed] [Google Scholar]
- Rasschaert D., Laude H. The predicted primary structure of the peplomer protein E2 of the porcine coronavirus transmissible gastroenteritis virus. J Gen Virol. 1987 Jul;68(Pt 7):1883–1890. doi: 10.1099/0022-1317-68-7-1883. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Carson D. A., Valbracht J., Houghten R., Vaughan J. H. Human immune responses to synthetic peptides from the Epstein-Barr nuclear antigen. J Immunol. 1985 Jan;134(1):211–216. [PubMed] [Google Scholar]
- Siddell S., Wege H., Ter Meulen V. The biology of coronaviruses. J Gen Virol. 1983 Apr;64(Pt 4):761–776. doi: 10.1099/0022-1317-64-4-761. [DOI] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



