Abstract
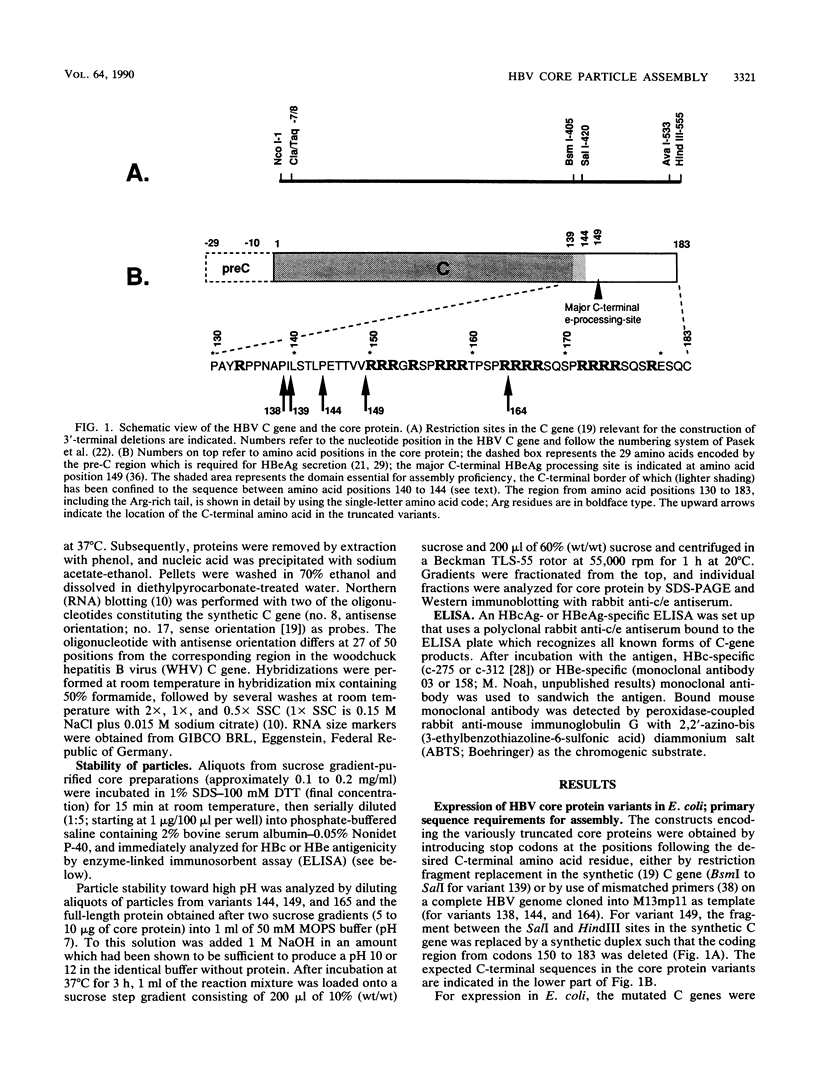
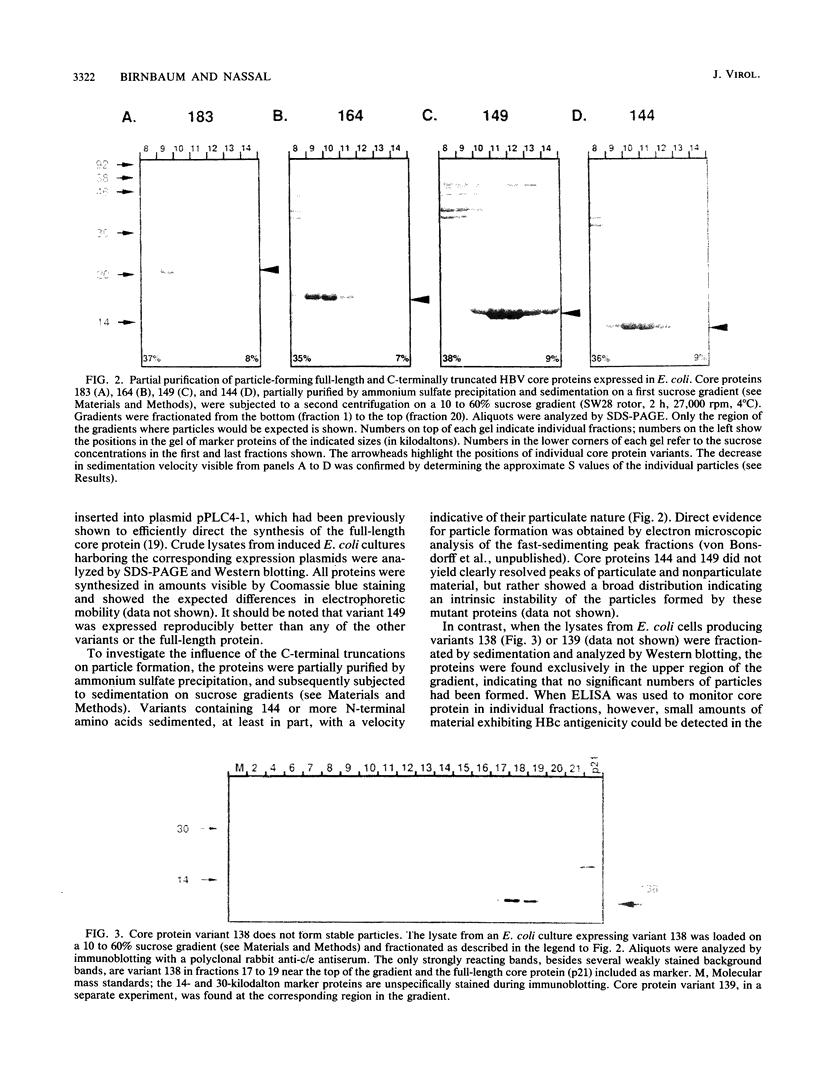
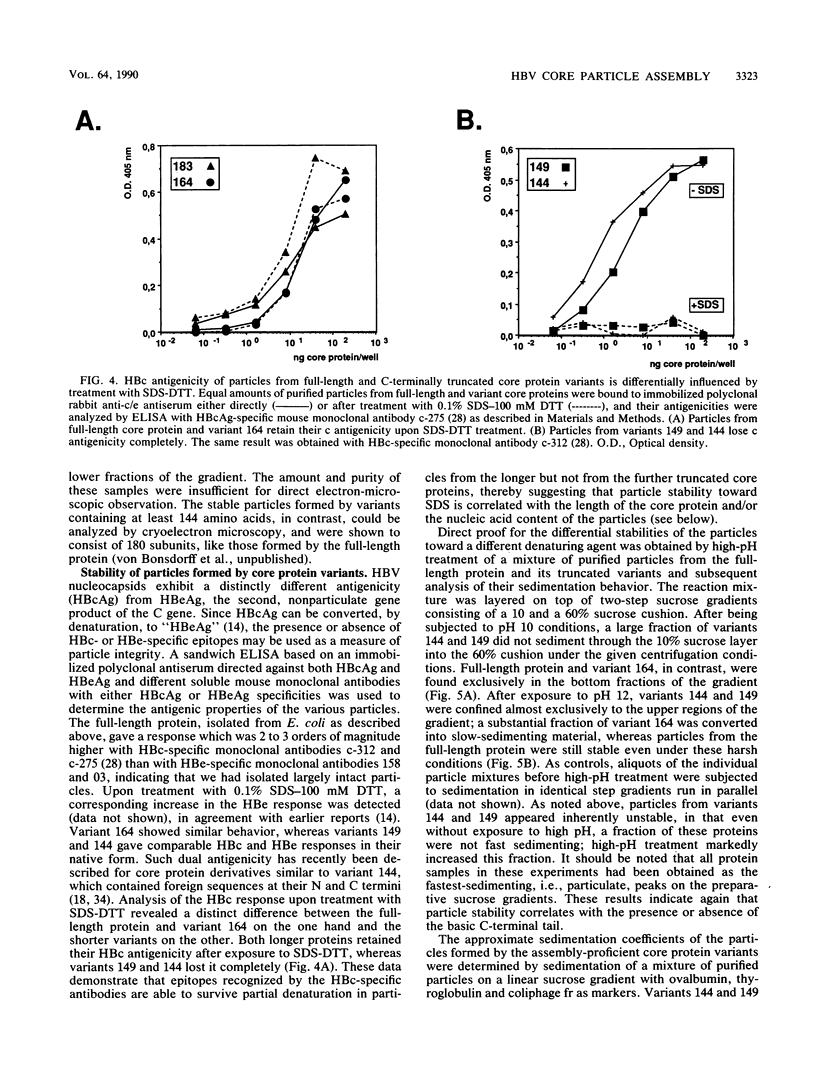
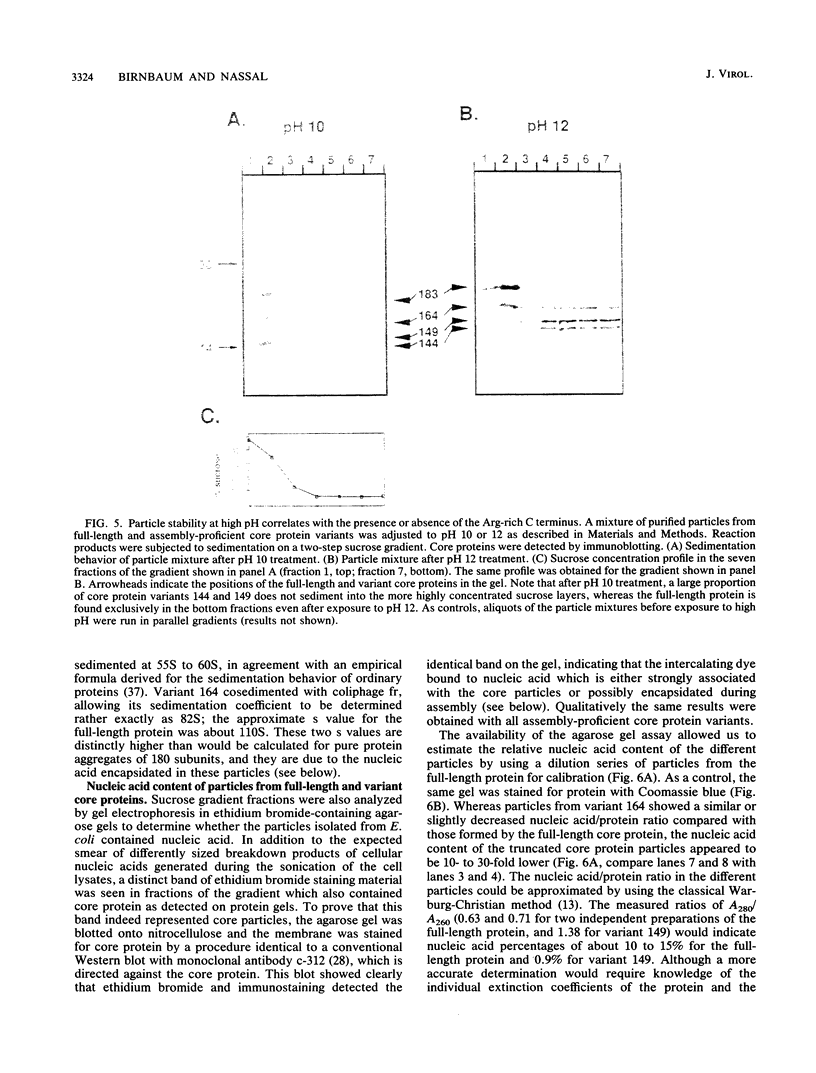
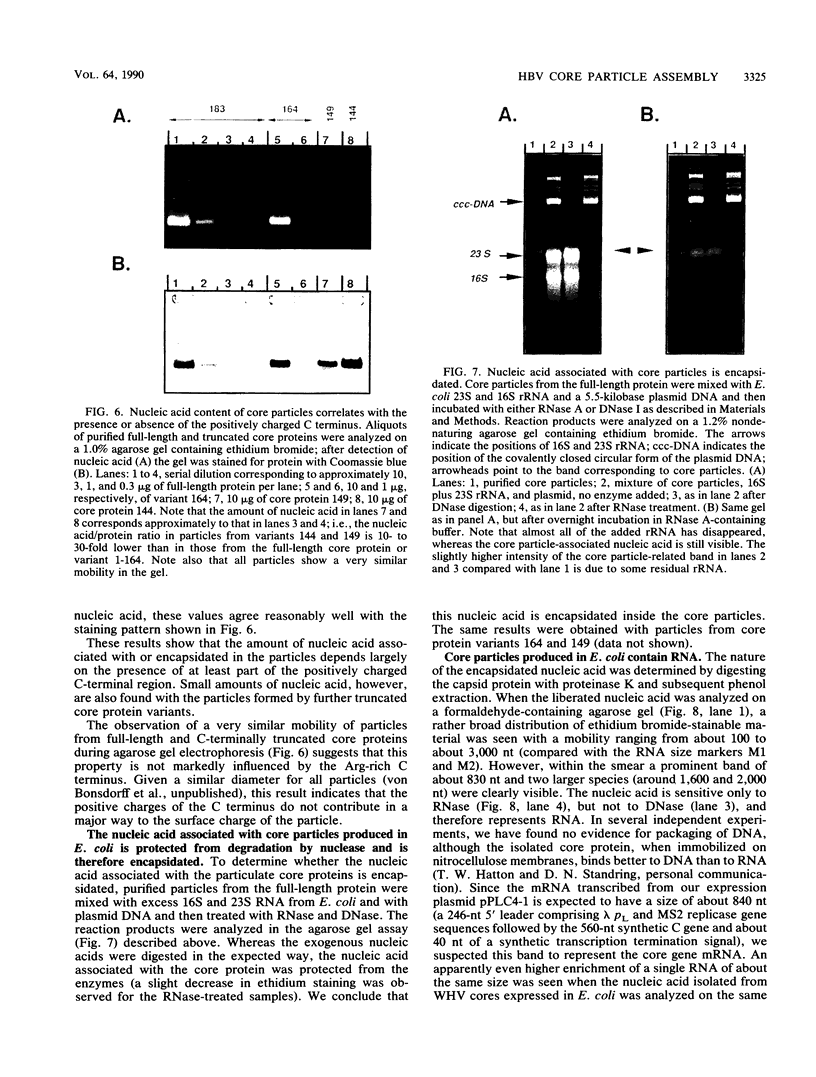
As a step toward understanding the assembly of the hepatitis B virus (HBV) nucleocapsid at a molecular level, we sought to define the primary sequence requirements for assembly of the HBV core protein. This protein can self assemble upon expression in Escherichia coli. Applying this system to a series of C-terminally truncated core protein variants, we mapped the C-terminal limit for assembly to the region between amino acid residues 139 and 144. The size of this domain agrees well with the minimum length of RNA virus capsid proteins that fold into an eight-stranded beta-barrel structure. The entire Arg-rich C-terminal domain of the HBV core protein is not necessary for assembly. However, the nucleic acid content of particles formed by assembly-competent core protein variants correlates with the presence or absence of this region, as does particle stability. The nucleic acid found in the particles is RNA, between about 100 to some 3,000 nucleotides in length. In particles formed by the full-length protein, the core protein mRNA appears to be enriched over other, cellular RNAs. These data indicate that protein-protein interactions provided by the core protein domain from the N terminus to the region around amino acid 144 are the major factor in HBV capsid assembly, which proceeds without the need for substantial amounts of nucleic acid. The presence of the basic C terminus, however, greatly enhances encapsidation of nucleic acid and appears to make an important contribution to capsid stability via protein-nucleic acid interactions. The observation of low but detectable levels of nucleic acid in particles formed by core protein variants lacking the Arg-rich C terminus suggests the presence of a second nucleic acid-binding motif in the first 144 amino acids of the core protein. Based on these findings, the potential importance of the C-terminal core protein region during assembly in vivo into authentic, replication-competent nucleocapsids is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Fuller S. D. A model for the hepatitis B virus core protein: prediction of antigenic sites and relationship to RNA virus capsid proteins. EMBO J. 1988 Mar;7(3):819–824. doi: 10.1002/j.1460-2075.1988.tb02880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V., Gerlich W. H. Formation of transmembraneous hepatitis B e-antigen by cotranslational in vitro processing of the viral precore protein. Virology. 1988 Apr;163(2):268–275. doi: 10.1016/0042-6822(88)90266-8. [DOI] [PubMed] [Google Scholar]
- Chen Z. G., Stauffacher C., Li Y., Schmidt T., Bomu W., Kamer G., Shanks M., Lomonossoff G., Johnson J. E. Protein-RNA interactions in an icosahedral virus at 3.0 A resolution. Science. 1989 Jul 14;245(4914):154–159. doi: 10.1126/science.2749253. [DOI] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gallina A., Bonelli F., Zentilin L., Rindi G., Muttini M., Milanesi G. A recombinant hepatitis B core antigen polypeptide with the protamine-like domain deleted self-assembles into capsid particles but fails to bind nucleic acids. J Virol. 1989 Nov;63(11):4645–4652. doi: 10.1128/jvi.63.11.4645-4652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Garcia P. D., Ou J. H., Rutter W. J., Walter P. Targeting of the hepatitis B virus precore protein to the endoplasmic reticulum membrane: after signal peptide cleavage translocation can be aborted and the product released into the cytoplasm. J Cell Biol. 1988 Apr;106(4):1093–1104. doi: 10.1083/jcb.106.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich W. H., Goldmann U., Müller R., Stibbe W., Wolff W. Specificity and localization of the hepatitis B virus-associated protein kinase. J Virol. 1982 Jun;42(3):761–766. doi: 10.1128/jvi.42.3.761-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. M., Ford E. C., Purcell R. H., Gerin J. L. Demonstration of subpopulations of Dane particles. J Virol. 1976 Mar;17(3):885–893. doi: 10.1128/jvi.17.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacKay P., Lees J., Murray K. The conversion of hepatitis B core antigen synthesized in E coli into e antigen. J Med Virol. 1981;8(4):237–243. doi: 10.1002/jmv.1890080404. [DOI] [PubMed] [Google Scholar]
- Magnius L. O., Espmark J. A. New specificities in Australia antigen positive sera distinct from the Le Bouvier determinants. J Immunol. 1972 Nov;109(5):1017–1021. [PubMed] [Google Scholar]
- Matsuda K., Satoh S., Ohori H. DNA-binding activity of hepatitis B e antigen polypeptide lacking the protaminelike sequence of nucleocapsid protein of human hepatitis B virus. J Virol. 1988 Sep;62(9):3517–3521. doi: 10.1128/jvi.62.9.3517-3521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Stahl S., Wingfield P., Thornton G. B., Hughes J. L., Jones J. E. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988 Nov 15;141(10):3617–3624. [PubMed] [Google Scholar]
- Nassal M., Mogi T., Karnik S. S., Khorana H. G. Structure-function studies on bacteriorhodopsin. III. Total synthesis of a gene for bacterio-opsin and its expression in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9264–9270. [PubMed] [Google Scholar]
- Nassal M. Total chemical synthesis of a gene for hepatitis B virus core protein and its functional characterization. Gene. 1988 Jun 30;66(2):279–294. doi: 10.1016/0378-1119(88)90364-2. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Pillot J. HBc and HBe antigenicity and DNA-binding activity of major core protein P22 in hepatitis B virus core particles isolated from the cytoplasm of human liver cells. J Virol. 1985 Feb;53(2):543–551. doi: 10.1128/jvi.53.2.543-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Johnson J. E. Icosahedral RNA virus structure. Annu Rev Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y., Yamada G., Mizuno M., Nishihara T., Kinoyama S., Kobayashi T., Takahashi T., Nagashima H. Full and empty particles of hepatitis B virus in hepatocytes from patients with HBsAg-positive chronic active hepatitis. Lab Invest. 1983 Jun;48(6):678–682. [PubMed] [Google Scholar]
- Salfeld J., Pfaff E., Noah M., Schaller H. Antigenic determinants and functional domains in core antigen and e antigen from hepatitis B virus. J Virol. 1989 Feb;63(2):798–808. doi: 10.1128/jvi.63.2.798-808.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Salfeld J., Schaller H. The duck hepatitis B virus pre-C region encodes a signal sequence which is essential for synthesis and secretion of processed core proteins but not for virus formation. J Virol. 1987 Dec;61(12):3701–3709. doi: 10.1128/jvi.61.12.3701-3709.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Schaller H. The secretory core protein of human hepatitis B virus is expressed on the cell surface. J Virol. 1989 Dec;63(12):5399–5404. doi: 10.1128/jvi.63.12.5399-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P. Use of gel electrophoresis to characterize multimolecular aggregates. Methods Enzymol. 1986;130:116–132. doi: 10.1016/0076-6879(86)30010-7. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Stockley P. G., Harrison S. C. Structure and assembly of turnip crinkle virus. II. Mechanism of reassembly in vitro. J Mol Biol. 1986 Oct 20;191(4):639–658. doi: 10.1016/0022-2836(86)90451-1. [DOI] [PubMed] [Google Scholar]
- Stahl S. J., Murray K. Immunogenicity of peptide fusions to hepatitis B virus core antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6283–6287. doi: 10.1073/pnas.86.16.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. SPKK, a new nucleic acid-binding unit of protein found in histone. EMBO J. 1989 Mar;8(3):797–804. doi: 10.1002/j.1460-2075.1989.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Machida A., Funatsu G., Nomura M., Usuda S., Aoyagi S., Tachibana K., Miyamoto H., Imai M., Nakamura T. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol. 1983 Jun;130(6):2903–2907. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]