Abstract
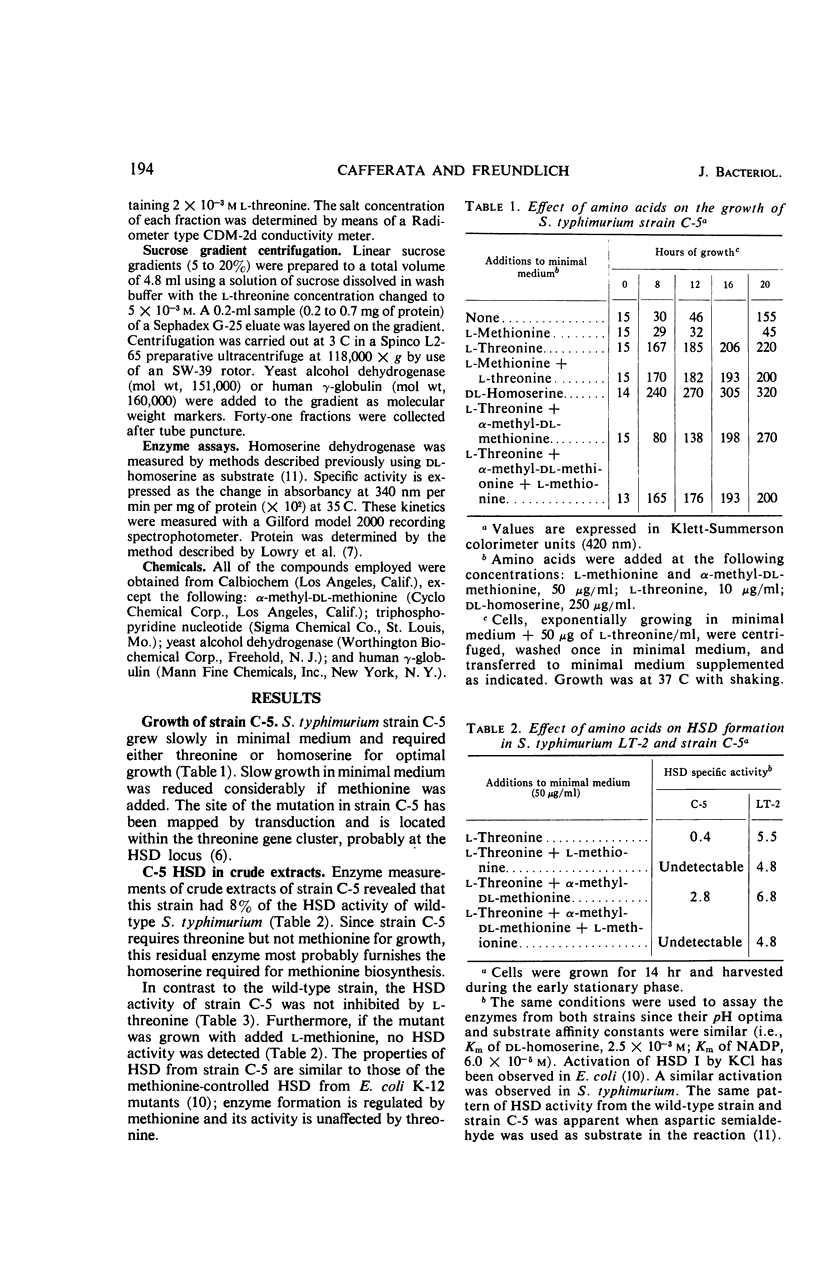
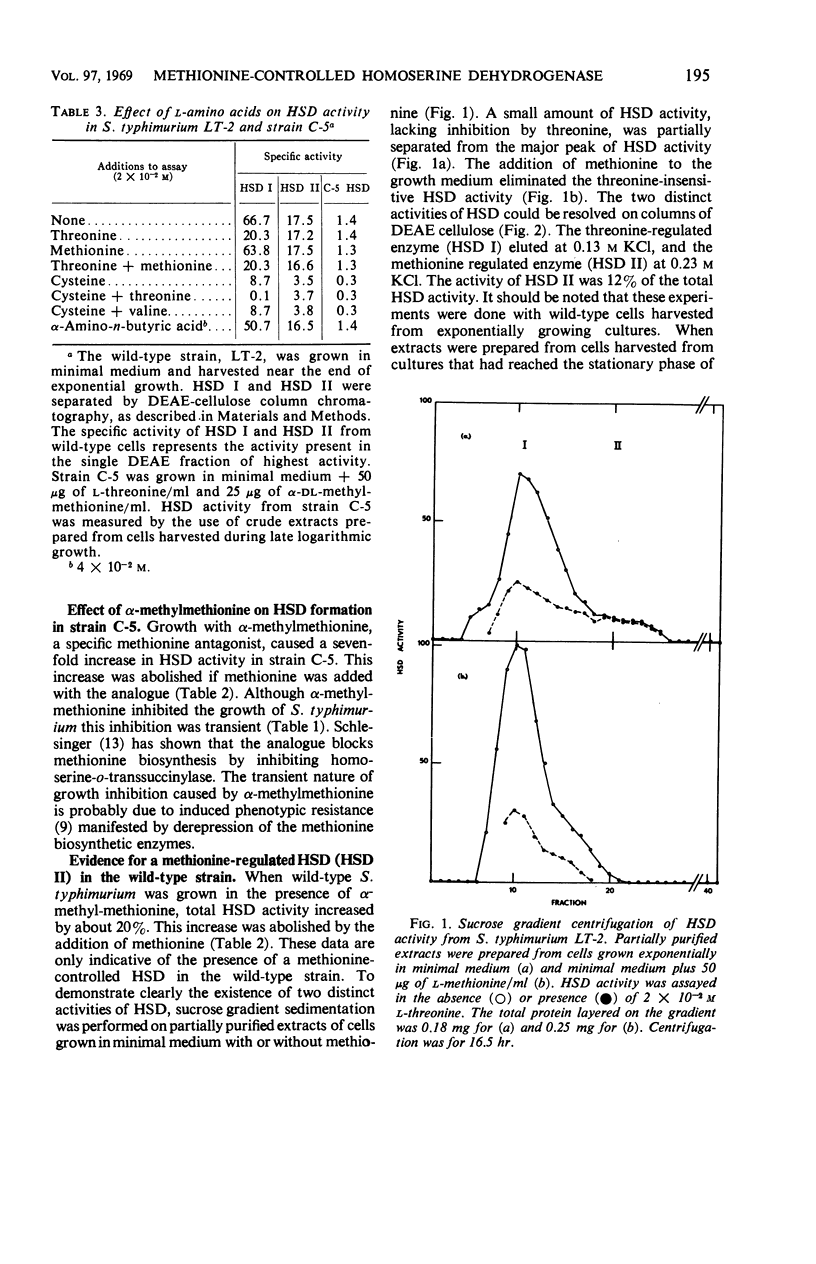
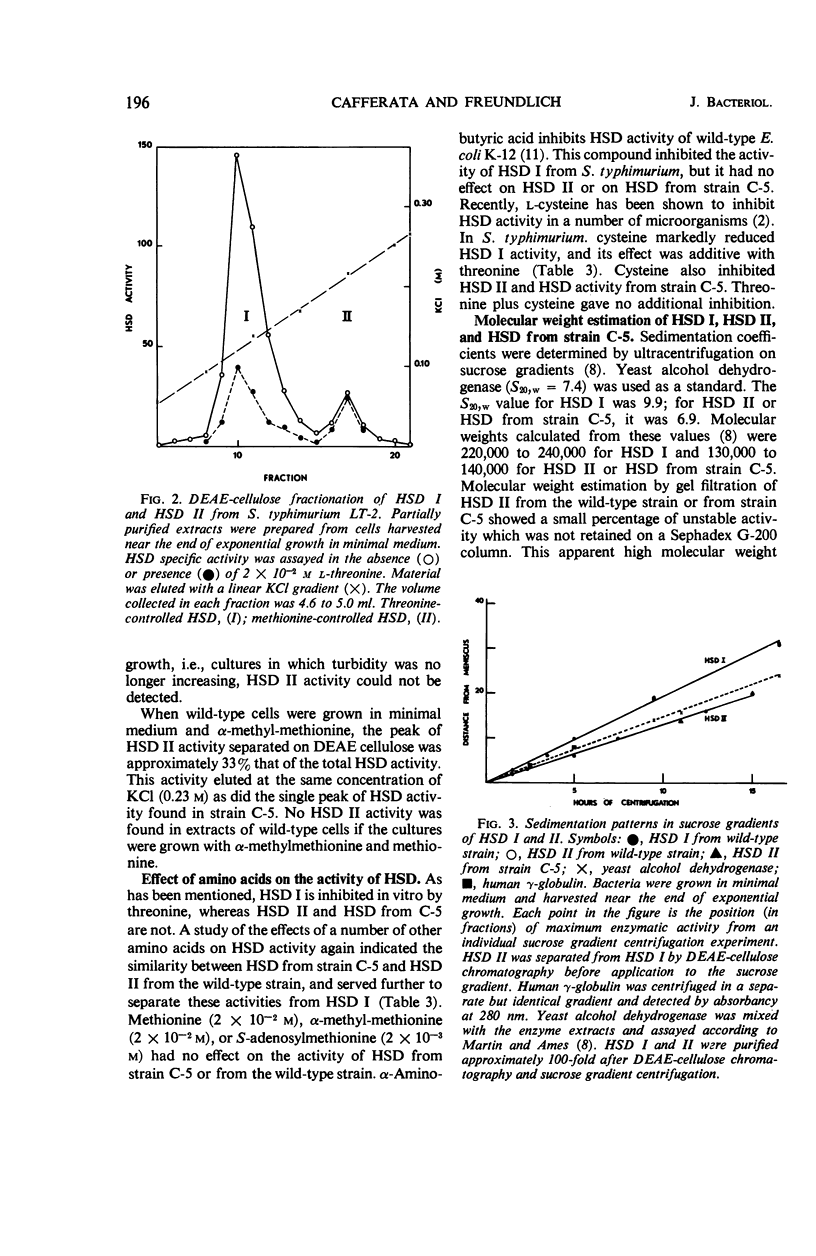
Evidence is presented for the existence of a second homoserine dehydrogenase in Salmonella typhimurium. The formation, but not the activity, of this enzyme is controlled by methionine. Two distinct homoserine dehydrogenases were separated from wild-type cells by diethylaminoethyl (cellulose) column chromatography. Sucrose gradient ultracentrifugation gave molecular weight estimates for the threonine-regulated enzyme (HSD I) of 220,000 to 240,000 and for the methionine controlled enzyme (HSD II) of 130,000 to 140,000. Approximately 12% of the total HSD activity in wild-type cells was accounted for by HSD II. A threonine-requiring strain of S. typhimurium was found to lack HSD I but not HSD II. Under certain conditions, this mutant grew rapidly in minimal medium. Rapid growth in minimal medium was correlated with the appearance of an enzyme with similar characteristics to HSD I. The possible origins of this HSD I-like enzyme are presented.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham G. N., Maul S. B., Shive W. Multiple interactions of threonine with an aspartokinase-homoserine dehydrogenase complex. Biochem Biophys Res Commun. 1968 Jan 25;30(2):159–165. doi: 10.1016/0006-291x(68)90464-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M. Multivalent repression in the biosynthesis of threonine in Salmonella typhimurium and Escherichia coli. Biochem Biophys Res Commun. 1963 Feb 6;10:277–282. doi: 10.1016/0006-291x(63)90430-3. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of prophyrin and bacteriochlorophyll by Rhodoseudomonas spheroides. 3. The effect of threonine on the biosynthesis of homoserine and methionine. Biochem J. 1962 Sep;84:483–490. doi: 10.1042/bj0840483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville E V, Demerec M. Threonine, Isoleucine, and Isoleucine-Valine Mutants of Salmonella Typhimurium. Genetics. 1960 Oct;45(10):1359–1374. doi: 10.1093/genetics/45.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MOYED H. S. Interference with feedback control of enzyme activity. Cold Spring Harb Symp Quant Biol. 1961;26:323–329. doi: 10.1101/sqb.1961.026.01.039. [DOI] [PubMed] [Google Scholar]
- PATTE J. C., LE BRAS G., LOVINY T., COHEN G. N. [Retro-inhibition and repression of the homoserine dehydrogenase of Escherichia coli]. Biochim Biophys Acta. 1963 Jan 8;67:16–30. doi: 10.1016/0006-3002(63)91793-1. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- STADTMAN E. R. Symptosium on multiple forms of enzymes and control mechanisms. II. Enzyme multiplicity and function in the regulation of divergent metabolic pathways. Bacteriol Rev. 1963 Jun;27:170–181. doi: 10.1128/br.27.2.170-181.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S. Inhibition of growth of Escherichia coli and of homoserine O-transsuccinylase by alpha-methylmethionine. J Bacteriol. 1967 Aug;94(2):327–332. doi: 10.1128/jb.94.2.327-332.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



