Abstract
Demonstration of thymic homing dependent on Gαi proteins is one of the keys to determine whether thymic entrance of blood-borne progenitors is a highly selective process. The present study provides compelling evidence of an indispensable role for GαI proteins in this process. Absence of either Gαi2 or Gαi3 significantly abrogated thymic homing, with an effect of Gαi3 being greater than that of Gαi2. Pertussis toxin treatment that blocks both Gαi2 and Gαi3 almost completely blocked thymic seeding in the thymus. Null mutation of Gαi3 also hindered bone marrow cell development and thus reduced production of pre-thymic progenitors. In contrast, Gαi2 exhibited a more prominent role than Gαi3 in guidance of CD4−CD8− double negative (DN) 1 cell migration and early thymic differentiation. The Gαi-deficiency-induced defects might be compensated for in part via augmented function of thymic stromal cells so that a nearly normal output of mature T cells could be maintained in these Gαi-deficient mice. These studies underscore the importance of Gαi in regulating thymic homing and pre-thymic and early thymocyte differentiation.
Introduction
The thymus is the primary site for generating self-tolerant, major histocompatibility (MHC) complex-restricted, and immunocompetent T cells. The process begins with ingress of blood-borne progenitors to the thymus through post-capillary venules near the corticomeduallary junction (CMJ) (Donskoy and Goldschneider 1992;Lind et al. 2001). These T cell lineage progenitors migrate outward and then inward, covering several thousand μm within the thymus, during which they undergo a series of developmental events and become mature T cells, capable of emigration to the periphery. This long journey is directed by the cellular action of heterotrimeric Gαi-protein coupled receptors (GPCRs) in response to chemokines or bioactive lysophospholipids (Lind et al. 2001;Petrie 2003;Annunziato et al. 2001;Norment and Bevan 2000;Kurobe et al. 2006).
Our earliest appreciation of the role of heterotrimeric Gαi proteins in thymocyte emigration stems from the study of lck-PTX-Tg mice that express the pertussis toxin (PTX) catalytic S1 subunit under the control of an lck promoter (Chaffin and Perlmutter 1991). PTX irreversibly blocks GPCRs’ signaling by preventing coupling of Gαi proteins to the receptors (Kaslow and Burns 1992). The transgenic mice harbor 2~4 fold more CD4 and CD8 single positive (SP) thymocytes in the thymus than control mice do and have few or no T cells in the peripheral lymphoid organs, owing to a defect in thymic emigration, presumably in association with uncoupling of the S1P1 receptor (Chaffin and Perlmutter 1991;Matloubian et al. 2004). Unfortunately, thymic homing and early thymocyte development could not be evaluated in these animals, since lck expression occurs down stream of CD4CD8 double negative (DN) cell differentiation. PTX-sensitive GPCRs, including chemokine receptors CXCR4, CCR7 and CCR9, have consistently been found to play decisive roles in early thymocyte development (Kwan and Killeen 2004;Suzuki et al. 1999;Wurbel et al. 2000;Plotkin et al. 2003;Misslitz et al. 2004), but direct evidence for Gαi involvement in early thymocyte differentiation is still lacking. It also remains elusive whether or not Gαi-proteins or any particular GPCR is required for thymic homing.
Among the PTX-sensitive heterotrimeric G proteins, T lymphocytes and thymocytes express primarily Gαi2 and Gαi3 proteins (Beals et al. 1987;Kim et al. 1988). Unlike lck-PTX-Tg mice, Gαi2- and Gαi3-knockout mice display a nearly normal thymic output, which is somewhat unexpected, given the essential roles of GPCRs in guiding thymocyte migration (Zhang et al. 2005). We show here that deletion of either Gαi or Gαi3 blocked thymic homing and PTX treatment almost completely abrogated thymic recruitment of blood-borne progenitors, arguing strongly that thymic homing is a Gαi-regulated process. Moreover, lack of either Gαi2 or Gαi3 hinders the migration of DN1 cells and impedes early thymic differentiation, with the effect of Gαi2 being stronger than that of Gαi3. In contrast, Gαi3 has a more pronounced impact in thymic homing and development of pre-thymic progenitors in the bone marrow. These studies underscore the importance of Gαi proteins in regulating thymic homing and early thymic development and provide key information in drawing mechanistic similarities between thymic recruitment and lymphocyte homing to peripheral lymphoid tissues.
Materials and Methods
Mice
Gαi2-knockout (KO), Gαi3-KO, and wild type (WT) control mice on the mixed 129Sv/C57BL/6 background were generated by gene targeting and backcrossed with C57BL/6 (B6) (CD45.2) mice for seven generations as described (Rudolph et al. 1995;Jiang et al. 2001). B6/SJL mice (CD45.1) were obtained from Taconic Farms (Germantown, NJ) and used as recipients for bone marrow transplantation at 5~6 weeks of age. The mice were housed in conventional cages at the animal facilities of the Massachusetts General Hospital in accordance with institutional guidelines.
Flow cytometry analysis
Single-cell suspensions were prepared from thymi, spleens and lymph nodes by mincing the tissues against a 40 μm cell strainer (BD Bioscience) as described (Thompson et al. 2007). The cells were stained with anti-CD4 and anti-CD8 antibodies (Abs) for CD4+ and CD8+ T cell analysis. To analyze DN subsets, single-cell suspensions of thymocytes were treated with a cocktail of lineage marker-specific Abs recognizing CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), Mac-1 (M1/70), B220 (TIB 146), Gr-1 (RB6-8C5), and erythroid (TER-119), followed by three consecutive depletions of Ab-bound cells with BioMag goat anti-rat IgG per the manufacturer’s instruction. The lineage-depleted cells were reacted with FITC-conjugated Abs against CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), Mac-1 (M1/70), B220 (TIB 146), Gr-1 (RB6-8C5), and erythroid (TER-119) to label residual lineage-positive cells, and anti-CD44 and anti-CD25 Abs to distinguish DN subsets. DN1, DN2, and DN3 cells were determined in gated lineage-negative (lin-) cells in the basis of CD44 and CD25 expression on a FACSCalibur cytometer equipped with Cellquest software (BD Bioscience) (Godfrey et al. 1993). A lymphoid forward scatter/side scatter (FCS/SSC) gating was used to exclude other cell lineages, within which greater than 98% cells were CD45.2+. Additional Abs used included anti-CD45.2 (104), anti-CD45.1 (A20), anti-stem cell Ag-1 (Sca-1) (E13-161.7), anti-c-kit (2B8), anti-CXCR4, anti-CCR7, and anti-CCR9 Abs. All Abs were directly conjugated to FITC, PE (phycoerythrin), cy-Chrome (cy5.5), allphycocyanin, PE-Cy7 or biotin, and purchased from BD Pharmingen, with the exceptions of chemokine receptor Abs (BioLegend). Biotinylated Abs were revealed with fluorochrome-conjugated streptavidin. Chemokine receptor expressions on DN subsets were analyzed using a BD FACSAria flow cytometer equipped with BD FACSDiva™ software (BD Bioscience).
Transwell migration assay
Migration assays were performed in a 48-well micro-chemotaxis chamber (NeuroProbe, Gαithersburg, MD). Chemokines CXCL12/SDF-1 (100ng/mL), CCL19 (1μg/mL), CCL21 (1μg/mL) and CCL25 (50ng/mL) were purchased from PeproTech (Rocky Hill) and added to the lower chamber in triplicate in complete RPMI medium (10% fetal calf serum, 2 mM L-glutamine, 100U/ml penicillin, 100μg/ml streptomycin, and 50 μM β-mercaptoethanol). The chemokine concentrations used were found to induce maximal chemotaxis of WT cells in a number of preliminary studies. Transwell membranes with 5-μm pores were carefully placed on top, and 50μl of cell suspension containing 1×104 lin- cells prepared from indicated mice were added to the upper chambers. Migration was carried out for 4 hours at 37°C with 5% CO2. Migrated cells were collected in the lower chambers, pooled from every six wells, and counted for DN1 cell subset analysis. The cells were stained with FITC-conjugated Abs specific for lineage markers, PE-anti-CD44, and biotin-anti-CD25 plus cy-chrome-conjugated streptavidin, and percentages of DN1 subset (CD44highCD25−) were determined in gated lin- cell population on a FACSCalibur cytometer as described. Migration of the DN1 subset was compared with or without an indicated chemokine using a chemotactic index (CI), where CI = (a total number of cells migrating to a chemokine × % DN1/the number of cells migrating to the medium control × %DN1 − 1) x 100%.
Fetal thymus organ culture (FTOC)
Thymic lobes were isolated from E15.5 timed pregnant Gαi2-KO, Gαi3-KO and WT mice and cultured on membrane filters (Millipore) in DMEM medium supplemented with 10% FBS, 2 mM L-glutamine, 50μM 2-mercaptoethanol, 1mM sodium pyruvate, 100U/ml penicillin, 100μg/ml streptomycin, and 1.35 mM 2-deoxyguanosine (Sigma) at 37°C with 5% CO2 for 5 days as described (Jenkinson et al. 1992). The lobes were then washed and cocultured each with 105 DN cells isolated from thymuses of indicated mice in a Terasaki well for 48 hours to allow them to enter the lobes. The lobes were washed, moved to membrane filters, and cultured for another week in the same medium for thymocyte differentiation. The lobes were then collected and minced against a 40 μm cell strainer to prepare a single-cell suspension. Percentages of DP and SP thymocytes were determined by flow cytometry analysis after staining with anti-CD4 and anti-CD8 Abs and compared in the presence or absence of either Gαi protein.
Competitive repopulation of the thymus
Donor bone marrow cells were isolated from tibias and femurs of indicated knockout mice and age- and sex-matched wild type mice and treated with ACK lysis buffer to remove red blood cells. To prevent potential graft versus-host reaction, bone marrow cells were treated with Abs specific for CD3, B220 (clone TIB 146) and Gr-1 (RB6-8C5) followed by three consecutive depletions of Ab-bound cells with BioMag goat anti-rat IgG per the manufacturer’s instruction (Polysciences, Inc.). Sex-matched B6/SJL mice (CD45.1) at 5~6 weeks of age were used as recipients and irradiated with 750 rad 2 hrs before bone marrow transplantation. To the recipient mice, bone marrow cells at a dose of 1 x 107 cells per mouse were intravenously administered on 1:1 ratio of CD45.2+ Gαi2-KO, Gαi3-KO or WT bone marrow cells to CD45.1+ cells from B6/SJL mice. Thymi, spleens and lymph nodes were harvested four weeks later for analysis of DN subsets or CD4+ and CD8+ T cells by flow cytometry in gated CD45.1+ or CD45.2+ cells.
Short-term thymic homing
Bone marrow cells from 5 wk-old WT, Gαi2−/− or Gαi3−/−mice were labeled for 10 min at room temperature with 10 μM green dye CFSE (5-carboxyfluorescein diacetate succinimidyl ester) (Molecular Probes). In some samples, WT bone marrow cells were treated with 100 μg/ml PTX for 2 hrs prior CFSE-labeling. As internal references, WT bone marrow cells were also labeled with 10 μM red fluorescent dye tetramethylrhodamine isothiocyanate (TRITC) for 10 min in RPMI medium containing 10% fetal calf serum at 37°C with 5% CO2. Ten million cells of each dye-labeled sample in indicated combinations were mixed and injected intravenously into 4.5-week-old normal mice or irradiated 5-week-old WT mice.
To assay thymic homing of purified progenitors, bone marrow cells isolated from WT and Gαi-deficient mice were treated with a cocktail of lineage-specific Abs followed by three consecutive depletions of Ab-bound cells with BioMag goat anti-rat IgG as above. The resulting cells were stained with FITC-conjugated Abs specific for various lineage-markers, PE-anti-c-kit, allophycocyanin-Cy7-anti-Sca-1, and Biotin-anti-CD44 plus cy5.5-streptavidin. The lin− Sca1+c-kit+CD44+ (LSK) progenitors were sorted on a FACSAria cell sorter. The sorted LSK cells from WT and Gαi-deficient mice were labeled with different fluorescence dyes, mixed on 1:1 ratio, and injected into 4.5-week-old normal mice at 105 cells/mouse as above.
The mice were sacrificed two or seven days after the transfer and perfused with 1 x Hanks balanced salt solution lacking Mg2+ and Ca2+. Blood displaced by perfusion was collected, spun, and treated with ACK lysis buffer to remove red blood cells. Thymi were isolated and single-cell suspensions were prepared as above. The cell samples were scanned by FACSAria and analyzed using BD FACSDivatm software. Flow cytometric data were expressed as absolute numbers of CFSE- or TRICT-positive cells detected in 500,000 peripheral blood lymphocytes or 2 million thymocytes counted (Rossi et al. 2005).
Histological analysis
Thymuses were fixed with 10% formalin, embedded in paraffin, and sectioned at 4 microns. Sections were stained with hematoxylin (H) and eosin (E) by standard methods and subjected to histological examination.
Statistical analysis
The Student’s two-tailed t test was used to analyze significance of experimental groups and relevant controls.
Results
Aberrant early thymocyte development in Gαi-deficient mice
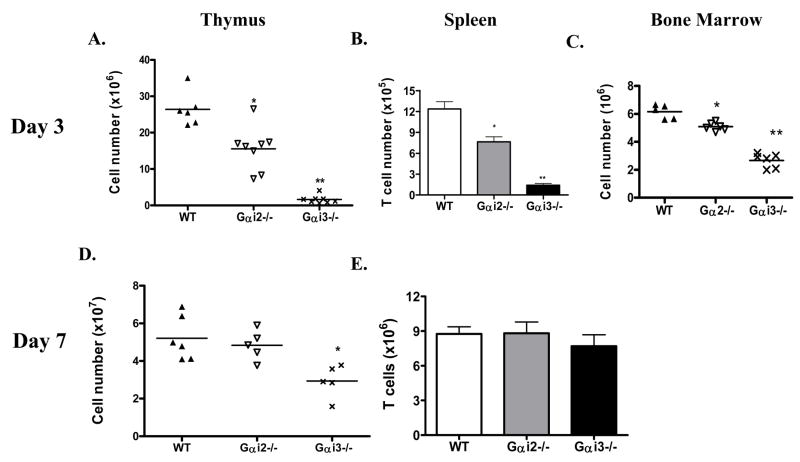
A severe reduction in thymic cellularity was found in newborn Gαi3−/− mice and to a lesser degree, in Gαi2−/− mice, despite the absence of gross abnormalities in T cell distribution in the periphery in their adulthood (Rudolph et al. 1995;Huang et al. 2003;Zhang et al. 2005). Absolute numbers of thymocytes were diminished by 12- or 2-fold, respectively, in 3-d (day)-old Gαi3- and Gαi2-deficient mice, in comparison with age-matched control mice (Fig. 1A and Table 1). This difference became less prominent with age: there was no significant difference in the absolute number of thymocytes between Gαi2−/− and control WT mice and only a 2-fold decrease in Gαi3−/− mice at one week of age (figure 1D), which was no longer seen after 3 weeks of age. The drastic decreases in thymic cellularity in the neonatal period of Gαi3-deficient mice had an obvious impact in T cell supply to the periphery, resulting in a 6-fold reduction of T cells in the spleens (Fig. 1B). In agreement with a smaller reduction in thymic cellularity of Gαi2−/− mice than that of Gαi3−/− mice, the absolute number of T cells in the spleen was also relatively lower in Gαi2−/− mice (figure 1B). As the numbers of thymocytes were gradually normalized with age, T cell number in the spleen was also restored to a normal level in both knockouts at 7 days of age (figure 1E). It is not clear at present whether or not this is attributed to both an increased thymic output and homeostatic proliferation in the periphery. Strikingly, despite severely reduced thymic cellularity, T cell development in these animals proceeded normally to a large degree even in newborn mice (Table 1). There was no significant difference in the percentages of CD4+ and CD8+ SP T cells in these three strains of mice at this age, except for a slight increase in the proportions of CD4 and CD8 SP thymocytes in Gαi3−/− mice as a result of decreased proportions of DP thymocytes.
Fig. 1.
Abnormal thymocyte development in neonatal Gαi-deficient mice. Thymi (A), spleen (B) and bone marrow (C) were isolated from wild type (WT) and Gαi2−/− and Gαi3−/− mice at 3 days of age. Single cell suspensions were prepared and counted. T cell numbers in the spleen were obtained in the basis of percentages of CD3+ cells identified by flow cytometry. Each symbol in A, C and D relates to data obtained from a single animal and the data in B and E are the means ± standard deviation (SD) of 6 (WT), 8 (Gαi2−/−) and 7 (Gαi3−/−) mice. *, p<0.01 and **, p<0.001 in the presence or absence of Gαi2 or Gαi3.
Table 1.
Thymocyte numbers and subsets in 3-d-old mice
| WT | Gαi2−/− | Gαi3−/− | ||||
|---|---|---|---|---|---|---|
| % | Cell number
(106) |
% | Cell number
(106) |
% | Cell number
(106) |
|
| Total cells | 26.40±4.62 | 15.57±2.11* | 2.13±1.34** | |||
| CD4+CD8+ | 80.40±1.97 | 21.22±3.68 | 80.08±1.53 | 12.47±0.24 | 58.90±14.7 | 1.25±0.31 |
| CD4+ | 12.67±1.77 | 3.37±0.88 | 13.21±0.92 | 2.05±0.14 | 16.69±4.03 | 0.36±0.09 |
| CD8+ | 3.31±0.48 | 0.86±0.12 | 3.39±0.41 | 0.52±0.05 | 9.94±6.62 | 0.21±0.14 |
|
| ||||||
| % | Cell number
(104) |
% | Cell number
(104) |
% | Cell number
(104) |
|
|
| ||||||
| DN1 | 5.99±1.19 | 3.43±0.68 | 31.06±4.41** | 7.33±1.04* | 15.15±1.8** | 0.58±0.07** |
| DN2 | 14.92±2.56 | 8.53±1.46 | 4.54±0.61** | 1.27±0.14** | 7.18±1.26** | 0.27±0.1** |
| DN3 | 52.58±2.16 | 30.08±1.23 | 40.01±3.99 | 9.44±0.94** | 55.41±5.17 | 2.14±0.19** |
| DN4 | 11.31±1.46 | 6.47±0.84 | 10.73±2.03 | 2.53±0.48 | 12.29±4.00 | 0.47±0.15 |
p<0.01; and
p<0.001 in the presence or absence of either Gαi protein.
A defect in early thymic development can significantly reduce thymic cellularity as has been shown in CCR7-deficient mice and plt/plt mice (Misslitz et al. 2004;Liu et al. 2005). This, in line with coupling Gαi proteins to the CXCR4 and CCR7 receptors, two important chemokine receptors involved in early thymic differentiation, promoted us to evaluate early thymic differentiation in these knockouts. It was found that the proportions of DN1 cells were increased by 5-fold in Gαi2−/− mice (p <0.001), and 2-fold in Gαi3−/− mice (p <0.001) at 3 days of age (Table 1). The increment in the proportion of DN1 cells was accompanied by proportional decreases in the percentages of DN2 cells (p<0.001). The results suggest a partial arrest at DN1 developmental stage, which may account partially for reduced thymic cellularity in these two knockouts, as robust proliferation of thymocytes takes place during the transition of the DN-to-DP thymocytes. With gradual normalization of thymic cellularity as the mice were maturing, the difference in the proportions of DN1 and DN2 cells in the presence or absence of either Gαi dwindled away at 7 days of age (data not shown). Remarkably, in spite of the DN1 blockage, the absolute number of DN1 cells was actually diminished by six-fold in Gαi3−/− mice when compared to that of WT DN1 cells (Table 1), which may only partially result from a severe reduction in the number of bone marrow cells in the mice (figure 1C).
Decreased ability of Gαi-deficient progenitors to repopulate the thymus
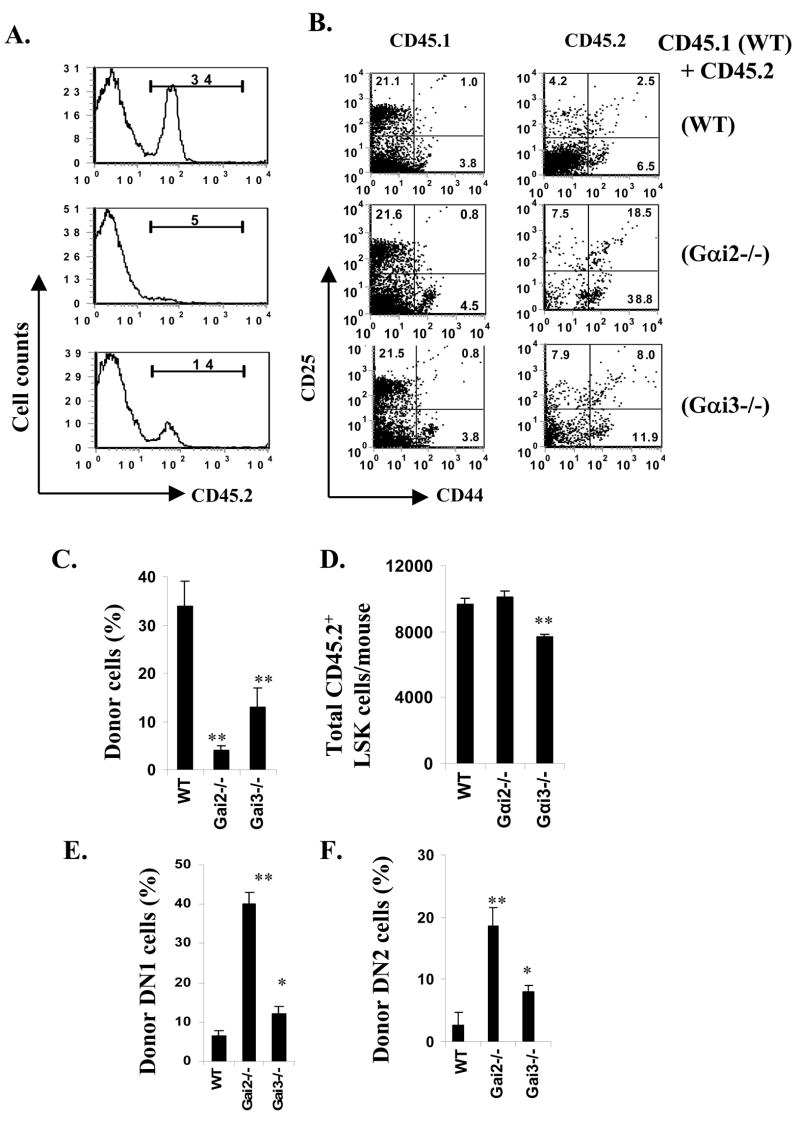
Thymic hypocellularity seen only in neonatal Gαi2- and Gαi3-KO mice but not in adult mice questioned the role of Gαi in early thymic differentiation, which contradicts well-described function of Gαi protein-coupled receptors in this process. To address this, competitive repopulation of thymi by bone marrow cells was carried out by mixing equal numbers of CD45.2 WT, Gαi2−/− or Gαi3−/− bone marrow cells with CD45.1 WT bone marrow cells and transferring into irradiated B6/SJL (CD45.1) WT mice. Analysis of thymic chimeras, four weeks after transplant, revealed that reconstitution of WT cells was sevenfold or twofold more efficient than reconstitution by Gαi2- or Gαi3-deficient cells, respectively (p<0.001, figure 2A and C). When early thymic differentiation was analyzed in the lin-negative population, DN1 cells were found to accumulate at levels 6-fold or 2-fold higher, respectively, in the absence of Gαi2 or Gαi3 than its presence (figure 2B and E). Distinguished from Gαi-deficient mice, however, these chimeras showed an increase, rather than reduction, in proportions of DN2 cells in the absence of either Gαi protein (figure 2F). Perhaps, altered production of cytokines and chemokines and largely vacated hemopoietic niches in the thymus after lethal irradiation resulted in migration of DN1 cells independently of Gαi2 or Gαi3, and DN2 development proportional to the number of accumulated DN1 cells in these chimeras (figure 2F vs. 2E) as implicated by previous studies (Zubkova et al. 2005). The increased proportion of DN1 cells confirms thymocyte differentiation arrest at the DN1 stage in the absence of either Gαi2 or Gαi3. Furthermore, these chimeras displayed significantly fewer pre-thymic progenitors marked as a Lin−Sca-1+c-Kit+ (LSK) cell subset in the bone marrow in the absence of Gαi3 (figure 2D), although the mice received equal numbers of bone marrow cells that were comprised of similar proportions of LSK cells in the presence or absence of Gαi3. The results implicated function of Gαi3 on efficient retention of pre-thymic progenitors in the bone marrow. This may be another factor for a greater reduction of the total number of DN1 thymocytes in newborn Gαi3−/− mice (Table 1).
Fig. 2.
Requirement of Gαi protein in early thymic differentiation. Bone barrow chimeras were generated by transfer of equal numbers of CD45.2 WT (upper), Gαi2−/−(middle) or Gαi3−/− (low) bone marrow cells with CD45.1 WT bone marrow cells into irradiated B6/SJL (CD45.1) mice, respectively (A and B). At four weeks after the transfer, donor cells were analyzed by flow cytometry using anti-CD45.2 Ab (A and C). DN subsets in these chimeras were analyzed in thymocytes in gated CD45.1 or CD45.2 population (B, E and F). The total numbers of pre-thymic progenitors marked as lin−Sca-1+c-Kit+ (LSK) cells per mouse were calculated by the number of LSK cells in 1 million bone marrow cells counted by a FACSAria flow cytometer as detailed in short-term thymic homing assays (D). Representative flow cytometric profiles are shown in A and B, where the numbers in each gate indicate percentages of positive cells. The data represent the mean percentages ± SD (C, E and F) or mean counts ± SD (D) of 4 mice in each group.
A defect in migration of these cells out of the CMJ is likely to account for the increased percentages of DN1 cells, as this vectorial migration is controlled by the CXCR4 and CCR7 receptors, both of which are coupled to either Gαi2 and/or Gαi3 in mature T cells (Thompson et al. 2007). To corroborate this in early developing thymocytes, lin-negative thymocytes were isolated and their chemotactic responses were assayed in the presence of increasing gradients of chemokines SDF-1, CCL19, CCL21, and CCL25. Similar to what has been described in mature T cells, migration of DN1 cells to SDF-1 was impaired in the absence of either Gαi2 or Gαi3 (figure 3A) (Thompson et al. 2007). These Gαi-deficient DN1 cells responded to CCL21 or CCL19 to a nearly normal degree, but they displayed weakened chemotactic responses to CCL25, a ligand for the CCR9 receptor (Figure 3A). The diminished chemotactic responses, in particular to SDF-1, was not attributed to decreased expression of the specific receptor, since similar levels of CCR7, CCR9 and CXCR4 expression were observed in these cells in the presence or absence of Gαi2 or Gαi3 (data not shown). The results explain DN1 developmental arrest seen in the absence of Gαi2 or Gαi3, in light of an importance of the CXCR4 receptor in early thymic development (Misslitz et al.2004;Plotkin et al. 2003).
Fig. 3.
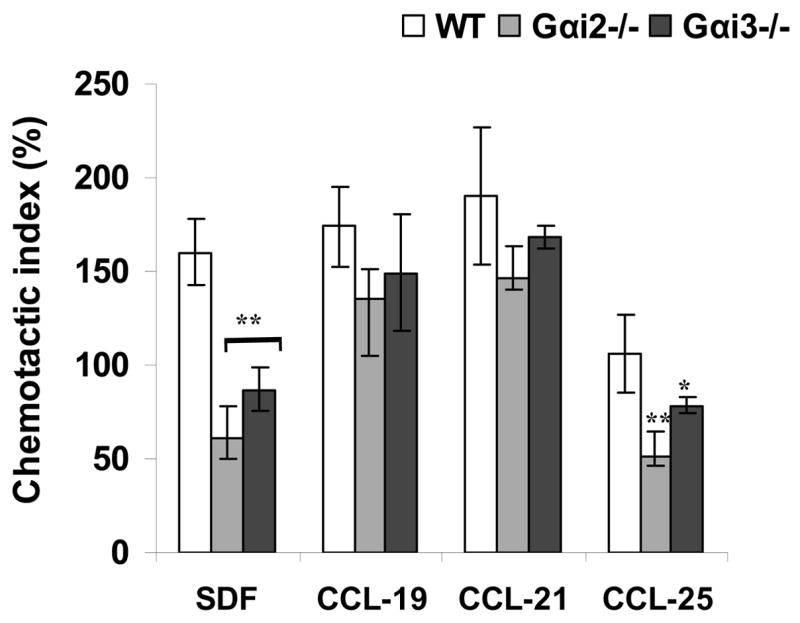
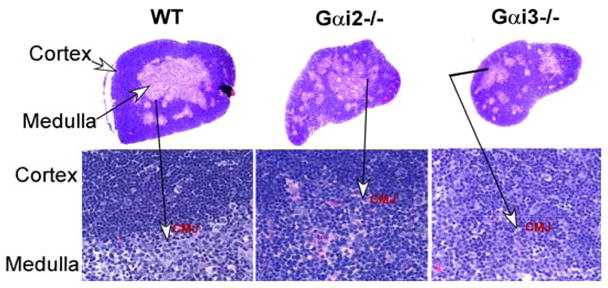
Altered chemotactic responses of DN1 cells and thymic architecture in the absence of either Gαi2 or Gαi3. A. A defect in chemotactic responses of Gαi-deficient DN1 cells to SDF-1. Lin-negative cells were isolated from indicated mice and assayed for 4 hrs at 37C with 5% CO2 in a 48-well chemotaxis for their responses to various chemokines. DN1 (CD44highCD25−) cells were analyzed by flow cytometry in gated lin-cells. Data are presented as the mean chemotactic index ± SD as defined in materials and methods. Cumulative data from at least five independent experiments with each in duplicate are shown. *, p< 0.05 and ** p<0.01 in the presence vs. absence of Gαi2 or Gαi3. B. Altered thymus architecture in Gαi-deficient mice. Shown are hematoxylin and eosin staining of thymic sections prepared from wild-type (WT), Gαi2−/− and Gαi3−/−mice animals at 3 days of age. Note: reduction in the cell density in Gαi3−/− mice and altered thymic architecture in Gαi2- and Gαi3-deficient mice. Magnification, 5X in the upper panel and the cortico-meduallary junction (CMJ) areas are indicated by arrows and enlarged (100 X) at the lower panel. Representative results of five mice in each group.
It has been shown that reduced thymic cellularity caused by DN1 developmental arrest is frequently accompanied by altered thymic architecture (Misslitz et al. 2004;Plotkin et al. 2003), due to the fact that thymic stromal cells and early developing thymocytes must communicate with each other to regulate the development of appropriate microenvironments for further thymocyte development (Savino et al. 2002;Lind et al. 2001;Anderson et al. 1996). This was also true for Gαi2- and Gαi3-KO mice (figure 3B). Null mutation of Gαi2 or Gαi3 gave rise to a relatively small thymus with medullary zones that were randomly distributing throughout the thymus, in contrast to large confluent medullas in control thymus. While the overall area of the cortex might be comparable between Gαi3-KO and WT mouse thymi, it was significantly reduced in Gαi2−/− mice. Conversely, the overall medullary area was decreased in Gαi3−/− thymus, but it was similar in Gαi2−/− thymus, when compared with WT thymus. Moreover, in contrast to densely packed lymphoid cells in WT and Gαi2-deficient cortex, cell density was diminished in the cortex of Gαi3-deficient thymuses, consistent with thymic hypocellularity in the animals. On higher magnification, the corticomedullary junction in Gαi3−/− and Gαi2−/− mice was not as distinct as that of WT mice (Figure 3B, lower panel).
Altered function of thymic stromal cells in the absence of Gαi proteins
As mentioned earlier, Gαi2- and Gαi3-deficient mice exhibit a nearly normal thymic output, despite impairment in early thymic differentiation. To investigate whether altered function of Gαi-deficient thymic stromal cells plays any role for that, fetal thymic organ culture (FTOC) was set up using WT thymic stromal cells cultivated with lin- progenitor cells isolated from Gαi2−/− or Gαi3−/− mice or vice versa. We found little difference in the total numbers of T cells when Gαi2- or Gαi3-deficient lin- progenitor cells were differentiated in WT thymic lobes, compared to differentiation of WT lin- progenitor cells under similar conditions (Fig 4 A&C). Likewise, the total number of T cells did not differ significantly if WT and Gαi3-deficient progenitors were cocultured with thymic stromal cells prepared from Gαi3−/− mice (figure 4B), or WT and Gαi2-deficient progenitors were cocultured with thymic lobes from Gαi2−/− mice (Fig 4D). These observations suggest that severe reduction in thymic cellularity in Gαi2- and Gαi3-knockouts is unlikely to be caused by decreased proliferation or increased apoptosis of thymocytes.
Fig. 4.
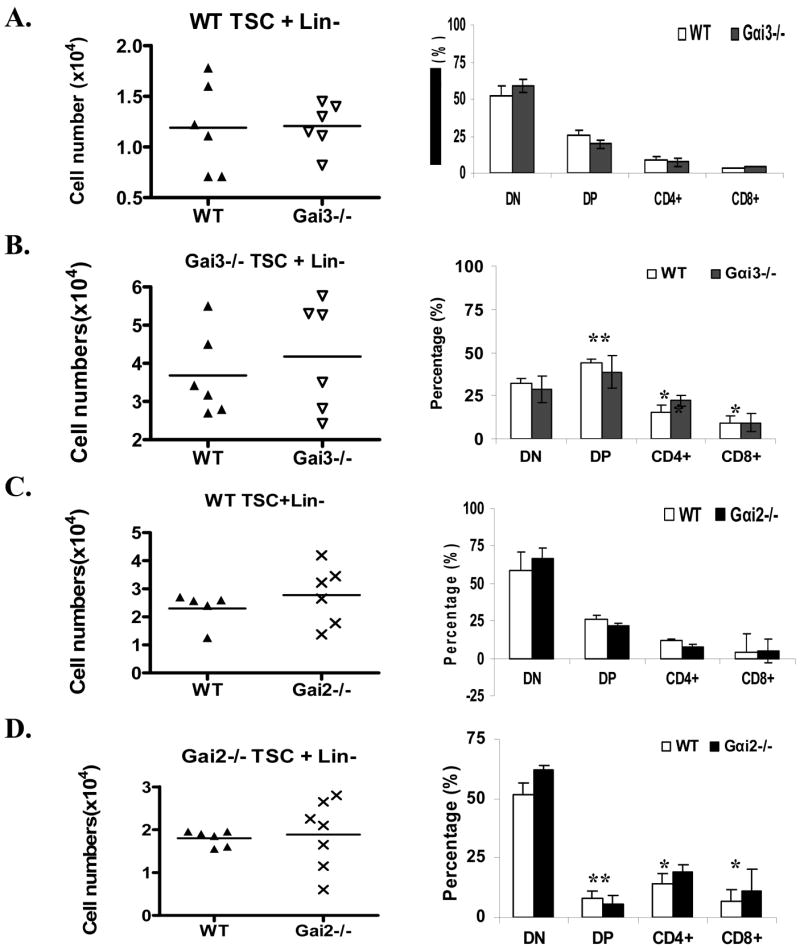
Augmented thymic stromal function in the absence of either Gαi2 or Gαi3. Fetal thymic organ cultures (FTOC) were set up, including WT thymic stromal cells (TSC) cocultured with either WT or Gαi3−/− lin- progenitors (A), Gαi3−/− TSC with WT or Gαi3−/− lin- progenitors (B), WT TSC with WT or Gαi2−/− lin- progenitors (C), and Gαi2−/− STC with WT or Gαi2−/− lin- progenitors (D). Between the two lobes from each fetus, one was co-cultured with WT lin- progenitors and the other with Gαi-deficient lin-progenitors for comparison in each panel. Thymocytes in single-cell suspensions were counted (left panels) and stained with FITC-CD4 and PE-CD8 Abs, followed by flow cytometric analysis (right panels). Each symbol relates to data obtained from a single lobe. *, p< 0.05 and ** p<0.01 when Gαi2−/− or Gαi3−/− TSC were compared with WT TSC for their ability in support of the differentiation of DP and CD4+ and CD8+ thymocytes.
Interestingly, Gαi3-deficient thymic stromal cells appear to facilitate DP cell differentiation. Percentages of DP cells developed in Gαi3-deficient thymic lobes were increased to 45%, from 25% obtained with WT thymic stromal cells, irrespective of Gαi3 expression in lin- progenitor cells (Fig 4 right panel, B vs. A and C). The increased differentiation of DP thymocytes concurred with a proportional decrease in DN cell population when compared to the DN cells recovered in the presence of WT thymic stromal cells (figure 4B vs. A). Conceivably, enhanced differentiation of DP thymocytes from DN thymocytes would compensate for early thymocyte developmental arrest and a defect in production of pre-thymic progenitors, giving rise to a nearly normal thymic output in Gαi3−/− mice, although further investigation is required to demonstrate this. In contrast to Gαi3−/− thymic stromal cells, Gαi2−/− thymic stromal cells favored the differentiation of mature SP cells. CD4+ and CD8+ cell subsets were significantly higher in the presence of Gαi2-deficient thymic lobes than WT thymic lobes, regardless of whether or not progenitor cells expressed Gαi2 (19% CD4+ and 9% CD8+ T cells with Gαi2−/− stromal cells vs. 7% CD4+ and 5% CD8+T cells with WT stromal cells) (Fig 4D vs. C, right panel). A twofold decrease in the proportion of DP T cells was attributed to increased transition of these cells into SP cells, because the decrease was not concomitant with an increase in the proportion of DN cells in the culture (Figure 4D) (Zhang et al. 2005).
Thymic homing depends on Gαi proteins
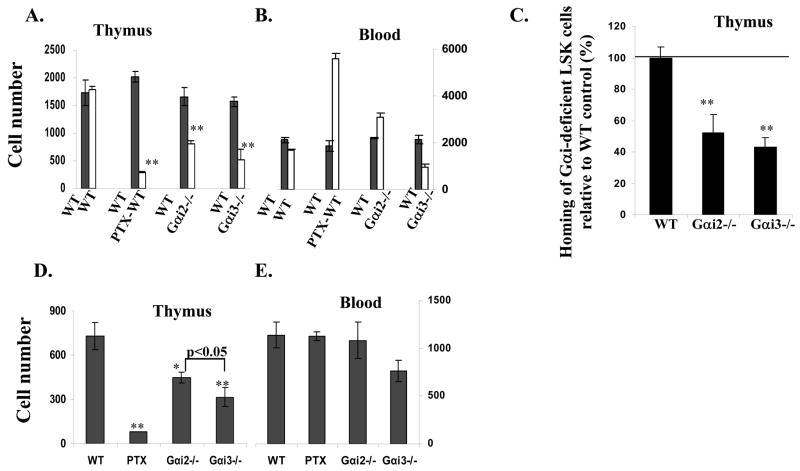
We next addressed whether Gαi proteins affected thymic entrance of blood-borne pre-T progenitors by short-term thymic homing assays. The assay directly tracked blood-to-thymic trafficking of intravenously injected, fluorescently labeled bone marrow cells separating from the intrathymic migration as well as progenitor mobilization from bone marrow to blood (Rossi et al. 2005). This is particularly important, because of the disparity of Gαi2 and Gαi3 in development of bone marrow cells (figure 1C). To this end, Gαi2−/− or Gαi3−/− bone marrow cells were labeled with the vital green fluorescence dye CFSE, and WT cells were labeled with the vital red fluorescence dye TRITC. Mixtures of these labeled cells on 1:1 ratio were transferred into unmanipulated WT adult mice at 4.5 weeks of age, an age at which the thymus has been reported to be most receptive to settling by blood-borne progenitors (Foss et al. 2001;Porritt et al. 2003;Schwarz et al. 2007). Flow cytometric scanning of donor cells within the thymus, 48 hr after the transfer, revealed that thymic seeding of blood-borne pre-T progenitors to unmanipulated WT adult mice was decreased by more than half in the absence of either Gαi compared to its presence (p<0.01, figure 5A), with Gαi3 influence of this process greater than Gαi2, in particularly, at one week after bone marrow cell infusion (figure 5C). To exclude that the reduced thymic recruitment was due to altered numbers of progenitors in bone marrows of Gαi-deficient mice, equal numbers of bone marrow progenitors sorted from WT or Gαi-deficient mice were labeled with different fluorescence dyes, mixed on 1:1 ratio, and assayed as above in 4.5-week-old normal mice. Similar diminishment in thymic seeding of sorted progenitors as unfractionated bone marrows was observed in Gαi2−/− or Gαi3−/− progenitors when compared to WT counterparts (figure 5C).
Fig. 5.
Thymic homing depends on Gαi proteins. Wild type (WT) bone marrow cells were labeled with TRITC and mixed with an equal number of CFSE-labeled bone marrow cells of indicated phenotypes (A, B, D, and E). Or, LSK progenitors were sorted from bone marrows of WT, Gαi2−/− and Gαi3−/− mice, fluorescently labeled, and mixed as above (C). The mixtures were injected intravenously into 4.5-week-old WT mice. Thymi (A, C and D) and blood (B and E) were analyzed two days (A, B, and C) or seven days (D and E) later. The data are the means ± SD of the absolute numbers of labeled cells per thymus (A and D) or in a million nucleated blood cells (B and E) of 5 mice in each group. Homing of Gαi2- or Gαi3-deficient LSK cells relatively to WT LSK cells (100%) in the same mouse was shown (C). *, p< 0.05 and ** p<0.01 in the presence vs. absence of Gαi2 or Gαi3.
Importantly, thymic recruitment was reduced to background levels when bone marrow cells were treated with PTX for 2 hr prior to injection (figure 5, A&C), arguing strongly that thymic homing is dependent on Gαi protein-mediated signaling, similar to thymic emigration (Chaffin and Perlmutter 1991). A possibility that the drastic reduction in PTX-treated cells homing to the thymus resulted from cytotoxic effects of the inhibitor could be ruled out by an increased or comparable number of viable PTX-treated cells in the blood of the animals analyzed in parallel (Figure 5B and D). The increased number of PTX-treated cells in the blood was due to their inefficient homing to bone marrow as a consequence of a blockage in Gαi activity.
It has been questioned whether the cells entering the thymus during a short-term homing assay are all relevant T lineage progenitors (Schwarz et al. 2007). To address this, we further analyzed the differentiation of injected, fluorescently labeled bone marrow progenitors within the thymus one week after the injection. In these experiments, we evaluated CFSE-labeled cells within the thymus, because TRIC red dye faded with time and was hardly detectable after one week. Moreover, studies of progenitor differentiation kinetics in the steady-state thymus have identified a maximal number and proportion of DN1 cells around 7~9 days following thymic settling (Porritt, Gordon, and Petrie 2003), after which DN1 cells decline in number and differentiate into DN2 cells. Thus, at 7 days after adoptive transfer, a significant number of CFSE-labeled WT cells were found within the thymus (figure 5D). These cells were phenotypically early T lineage progenitors, with ~65% expressing a high level of c-kit (data not shown). In agreement with short-term thymic homing assays, deletion of Gαi2 or Gαi3 prevented 40% or 60% blood-borne progenitors, respectively, from entering the thymus compared with WT bone marrow cells (p<0.01) (figure 5D). PTX treatment of bone marrow cells blocked thymic settling by more than 90%, despite similar numbers of donor cells in the blood. These data provide strong evidence that thymic homing is regulated by a Gαi-dependent process.
Discussion
T cells are continuously produced in the thymus throughout adult life, but this organ does not harbor long-term self-renewing progenitors. Therefore, the thymus must periodically recruit hematopoietic progenitors from bone marrow via the bloodstream. However, the mechanism by which T progenitors enter the thymus from the blood is poorly defined and the T-lineage progenitor that directly traffics to the thymus from the blood has not been identified to date. The present study, along with several other investigations, suggests that thymic recruitment is very likely analogous to the homing of lymphocytes to other organs in the periphery, requiring selectin-mediated tethering and rolling on endothelial cells, followed by strong adhesion through integrins and transmigration triggered by Gαi-protein coupled receptor signaling (Wu et al. 1993;Scimone et al. 2006;Rossi et al. 2005). CD44 may be one of the adhesion molecules involved in thymic recruitment as demonstrated by a blockage of this process with a specific Ab for CD44(Wu, Kincade, and Shortman 1993). The molecule is expressed on bone marrow hematopoietic progenitors and circulating lineage-negative Sca-1hic-Kithi (LSK). Likewise, the members of the β2 and α4 subfamilies like αLβ2 and α4β1 integrins have been shown to take part in thymic settling of bone marrow progenitors(Scimone et al. 2006). Besides adhesion molecules, null mutation of platelet selectin (P-selectin) or its glycoprotein ligand-1 (PSGL-1) impeded thymus recruitment (Rossi et al. 2005). P-selectin is expressed by endothelial cells lining the thymic vasculature when thymic niches are vacant, whereas its ligand PSGL-1 is found on bone marrow LSK progenitors and blood LSK cells. Blood progenitors may thus begin with their way to the thymus by interaction of P-selectin on the endothelial cells and PSGL-1 on the blood LSK progenitors(Rossi et al. 2005). As has been well documented in the multiple-step adhesion cascade, after initiating the homing process by adhesion molecule-mediated tethering and rolling on the endothelial cell layer, blood lymphocytes come to a stop only after their cell-surface integrins are activated into a high-affinity conformation by signaling through a Gαi-coupled receptor (Bargatze and Butcher 1993;Campbell et al. 1998;Warnock et al. 1998). The Gαi-mediated signaling directs precisely where extravasation takes place. Our investigation shows that lack of either Gαi2 or Gαi3 significantly inhibits the trafficking of pro-thymic progenitors to the thymus and treatment of unfractionated bone marrow cells with PTX almost completely ablates this event. The indispensable role of Gαi proteins in thymic homing strongly supports mechanistic similarities between thymic and lymphocyte homing.
Our data show a Gαi protein-mediated mechanism in thymic homing, but the Gαi protein-coupled receptor(s) involved in the process remains to be yet determined. Identification of the receptor may be a challenge, given the exceedingly small number of progenitors in the blood and possibly transient expression of the receptor. Blood-borne CCR9+ progenitors were shown to preferentially home to the thymus over CCR9-deficient progenitors, and anti-CCR9 antibody could partially block thymic homing (Schwarz et al. 2007;Scimone et al. 2006). An importance of the CCR9 receptor in thymic recruitment is however conflicting with unaltered thymic seeding of CCR7/CCR9-double knockout blood-borne progenitors in the thymus (Liu et al. 2006). There is also no evidence so far indicating that SDF-1, a ligand of the CXCR4 receptor is important for thymic settling. Our data suggest that the GPCR involved in thymic homing is coupled to both Gαi2 and Gαi3, as is the case with the S1P1 receptor. The investigation may help to hunt for the specific GPCRs involved in thymic homing and facilitate identification of blood-borne T progenitors that will have enormous implications for ageing, immunodeficiency, bone marrow transplantation, and thymic transplantation.
GPCRs are important at almost every key stage of thymocyte development, ranging from development of pre-thymic progenitors to thymic homing to egress, from intrathymic migration inward to outward, and from early thymocyte differentiations to T cell maturation (Norment and Bevan 2000). These GPCRs, including CCR7, CXCR4, CCR9, and the S1P1 receptor, are all known to be coupled to PTX-sensitive heterotrimeric Gαi proteins (Hamm 2001;Matloubian et al. 2004;Plotkin et al. 2003;Misslitz et al 2004;Uehara et al. 2002). Remarkably, deletion of either Gαi2 or Gαi3 has little impact on thymic output and thymocyte development in adult mice, in spite of a defect in early thymic differentiation and thymic homing in newborn mice, suggesting redundant and distinct roles for Gαi2 and Gαi3 in the signaling of these GPCRs. In accordance with this, lack of either Gαi2 or Gαi3 doesn’t significantly influence the function of the CCR7 receptor or the S1P1 receptor, and has only a weak effect on the signaling of the CCR9 receptor. The CXCR4 receptor appeared to depend on both Gαi2 and Gαi3 for a full chemotactic response. Thus, the defect in the early thymic differentiation may be mainly attributed to altered function of the CXCR4 receptor in Gαi2- and Gαi3-deficient mice. Unfortunately, deficiency in both Gαi2 and Gαi3 causes a severe growth retardation and lethality around embryonic day 10 (E10.5) (Gohla et al. 2007), which makes it difficult to determine potentially non-redundant function for Gαi2 and Gαi3 proteins in thymic homing conclusively without using PTX. Moreover, although impairment in thymic homing in Gαi2−/− and Gαi3−/− progenitors was clearly demonstrated in a competitive environment or in short-time homing assays, it appeared to affect thymocyte development minimally in adult Gαi-deficient mice, resembling that seen with P-Selectin-deficient mice (Rossi et al. 2005). Perhaps, a reduced rate of thymic homing can be efficiently compensated for by the expansion of the limited number of progenitors capable of reaching the thymus, consistent with the notion that early progenitor expansion is regulated by the availability of thymic progenitor niches. Alternatively, constitutive or more frequent thymic homing may be driven by unoccupied stromal niches in Gαi-deficient thymus, in contrast to periodical thymic recruitment in WT mice (Porritt, Gordon, and Petrie 2003).
Thymocytes migrate outward and then inward, covering thousands of microns of the thymic territory during T cell differentiation. This long journey may be necessary to regulate the rate of T cell generation and to allow adequate output buffering in case any of the steps goes awry under various physiological and pathologic conditions throughout life. Gαi proteins may be the most important proteins in this rate-limited control in T cell differentiation in light of their well-described roles in guidance of cell migration.
Acknowledgments
We thank members in Dr. Wu’s group for stimulating discussion, Dr. Lutz Birnbaumer for Gαi2- and Gαi3-knockouts, and Dr. Robert Edwards for critical reading and reviewing of the manuscript. This work is supported by the National Institutes of Health grants AI050822 and AI070785, Research Scholar Grant RSG-01-178-01-MGO from the American Cancer Society, and Senior Research Award1657 from the Crohn’s & Colitis Foundation of America (to M.X.W.) and by the Intramural Research Program of the NIH, NIEHS (to L.B).
Abbreviations used in this paper
- CMJ
Corticomeduallary junction
- DN cells
CD4−CD8−cells
- DP
CD4+CD8+ cells
- Gαi
G protein inhibitory subunit α
- KO
knockout
- lin-cells
lineage-negative cells
- mAb
monoclonal antibody
- PTX
Pertussis toxin
- SP cells
CD4+ or CD8+ cells
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Anderson G, Moore NC, Owen JJ, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 2.Annunziato F, Romagnani P, Cosmi L, Lazzeri E, Romagnani S. Chemokines and lymphopoiesis in human thymus. Trends Immunol. 2001;22:277–281. doi: 10.1016/s1471-4906(01)01889-0. [DOI] [PubMed] [Google Scholar]
- 3.Bargatze RF, Butcher EC. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J Exp Med. 1993;178:367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beals CR, Wilson CB, Perlmutter RM. A small multigene family encodes Gi signal-transduction proteins. Proc Natl Acad Sci USA. 1987;84:7886–7890. doi: 10.1073/pnas.84.22.7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 6.Chaffin KE, Perlmutter RM. A pertussis toxin-sensitive process controls thymocyte emigration. Eur J Immunol. 1991;21:2565–2573. doi: 10.1002/eji.1830211038. [DOI] [PubMed] [Google Scholar]
- 7.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- 8.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8-triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 10.Gohla A, Klement K, Piekorz RP, Pexa K, vom DS, Spicher K, Dreval V, Haussinger D, Birnbaumer L, Nurnberg B. An obligatory requirement for the heterotrimeric G protein Gi3 in the antiautophagic action of insulin in the liver. Proc Natl Acad Sci USA. 2007;104:3003–3008. doi: 10.1073/pnas.0611434104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamm HE. How activated receptors couple to G proteins. Proc Natl Acad Sci USA. 2001;98:4819–4821. doi: 10.1073/pnas.011099798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang TT, Zong Y, Dalwadi H, Chung C, Miceli MC, Spicher K, Birnbaumer L, Braun J, Aranda R. TCR-mediated hyper-responsiveness of autoimmune Galphai2(−/−) mice is an intrinsic naive CD4(+) T cell disorder selective for the Galphai2 subunit. Int Immunol. 2003;15:1359–1367. doi: 10.1093/intimm/dxg135. [DOI] [PubMed] [Google Scholar]
- 13.Jenkinson EJ, Anderson G, Owen JJ. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J Exp Med. 1992;176:845–853. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaslow HR, Burns DL. Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J. 1992;6:2684–2690. doi: 10.1096/fasebj.6.9.1612292. [DOI] [PubMed] [Google Scholar]
- 16.Kim SY, Ang SL, Bloch DB, Bloch KD, Kawahara Y, Tolman C, Lee R, Seidman JG, Neer EJ. Identification of cDNA encoding an additional alpha subunit of a human GTP-binding protein: expression of three alpha i subtypes in human tissues and cell lines. Proc Natl Acad Sci USA. 1988;85:4153–4157. doi: 10.1073/pnas.85.12.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, Boyd RL, Takahama Y. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172:3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 19.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Saito F, Liu Z, Lei Y, Uehara S, Love P, Lipp M, Kondo S, Manley N, Takahama Y. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 21.Liu YC, Penninger J, Karin M. Immunity by ubiquitylation: a reversible process of modification. Nat Rev Immunol. 2005;5:941–952. doi: 10.1038/nri1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 23.Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Forster R. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200:481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norment AM, Bevan MJ. Role of chemokines in thymocyte development. Semin Immunol. 2000;12:445–455. doi: 10.1006/smim.2000.0261. [DOI] [PubMed] [Google Scholar]
- 25.Petrie HT. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat Rev Immunol. 2003;3:859–866. doi: 10.1038/nri1223. [DOI] [PubMed] [Google Scholar]
- 26.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 27.Porritt HE, Gordon K, Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–962. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi FM, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, So L, Yi L, Ziltener HJ. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 30.Savino W, Mendes-da-Cruz DA, Silva JS, Dardenne M, Cotta-de-Almeida V. Intrathymic T-cell migration: a combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 2002;23:305–313. doi: 10.1016/s1471-4906(02)02224-x. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 32.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci USA. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki G, Sawa H, Kobayashi Y, Nakata Y, Nakagawa K, Uzawa A, Sakiyama H, Kakinuma S, Iwabuchi K, Nagashima K. Pertussis toxin-sensitive signal controls the trafficking of thymocytes across the corticomedullary junction in the thymus. J Immunol. 1999;162:5981–5985. [PubMed] [Google Scholar]
- 34.Thompson BD, Jin Y, Wu KH, Colvin RA, Luster AD, Birnbaumer L, Wu MX. Inhibition of Gai2 activation by Gai3 in CXCR3-mediated signaling. J Biol Chem. 2007:9547–9555. doi: 10.1074/jbc.M610931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 36.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu L, Kincade PW, Shortman K. The CD44 expressed on the earliest intrathymic precursor population functions as a thymus homing molecule but does not bind to hyaluronate. Immunol Lett. 1993;38:69–75. doi: 10.1016/0165-2478(93)90121-h. [DOI] [PubMed] [Google Scholar]
- 38.Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, Malissen B, Carrier A, Naquet P. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–271. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Finegold MJ, Jin Y, Wu MX. Accelerated transition from the double-positive to single-positive thymocytes in G alpha i2-deficient mice. Int Immunol. 2005;17:233–243. doi: 10.1093/intimm/dxh204. [DOI] [PubMed] [Google Scholar]
- 40.Zubkova I, Mostowski H, Zaitseva M. Up-regulation of IL-7, stromal-derived factor-1 alpha, thymus-expressed chemokine, and secondary lymphoid tissue chemokine gene expression in the stromal cells in response to thymocyte depletion: implication for thymus reconstitution. J Immunol. 2005;175:2321–2330. doi: 10.4049/jimmunol.175.4.2321. [DOI] [PubMed] [Google Scholar]