Abstract
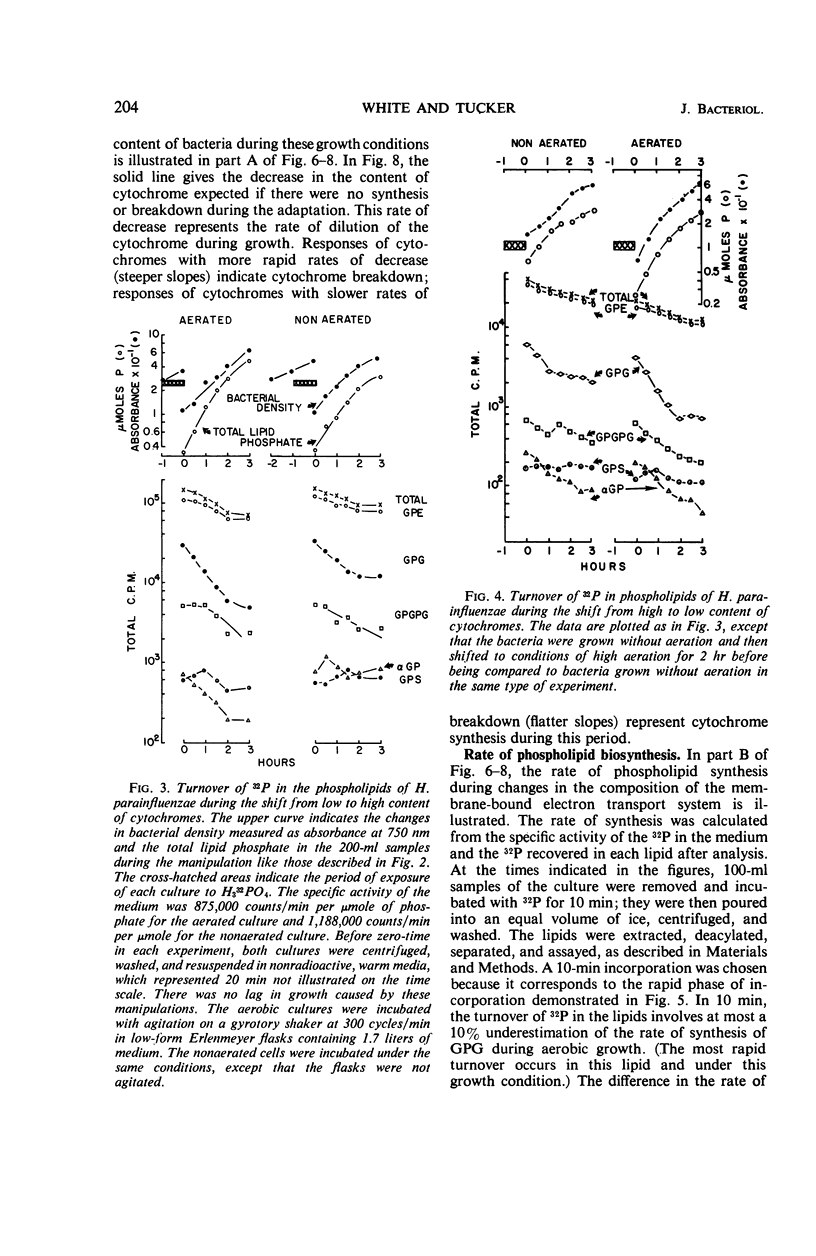
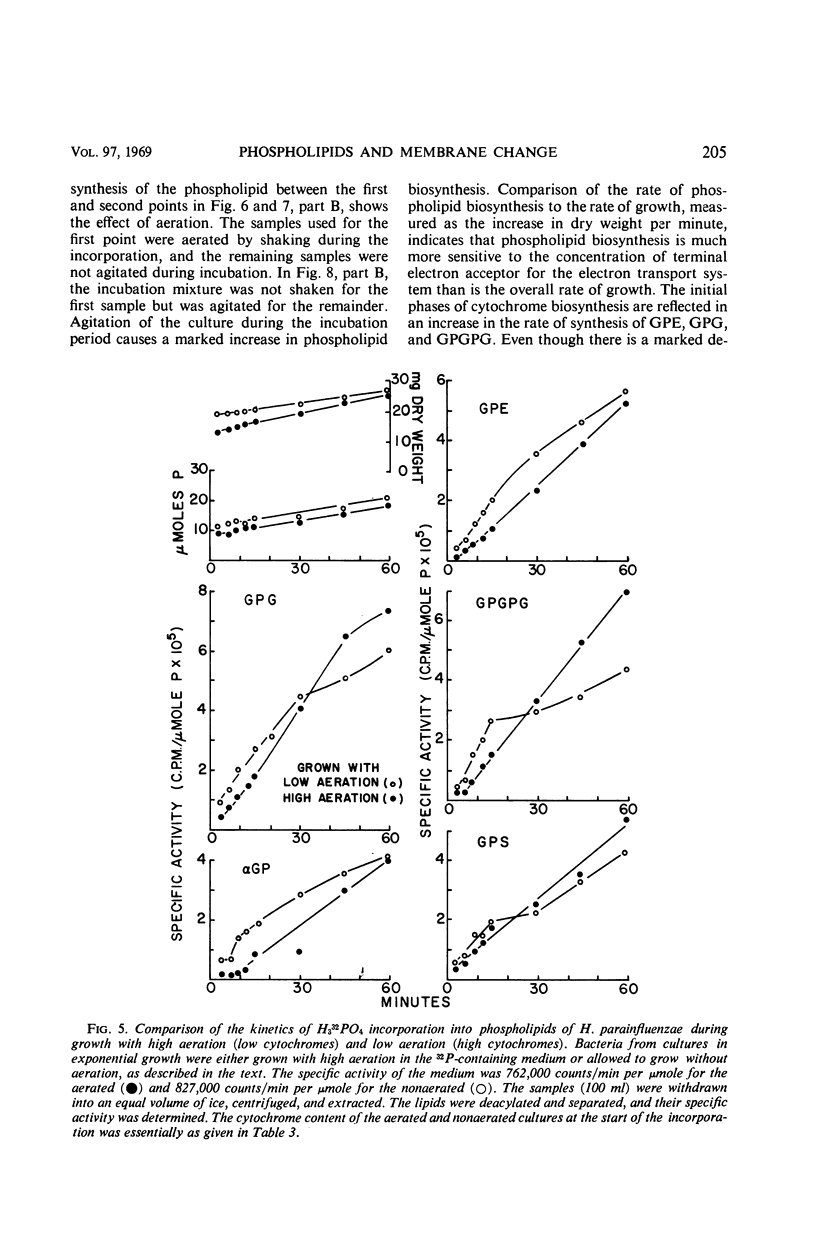
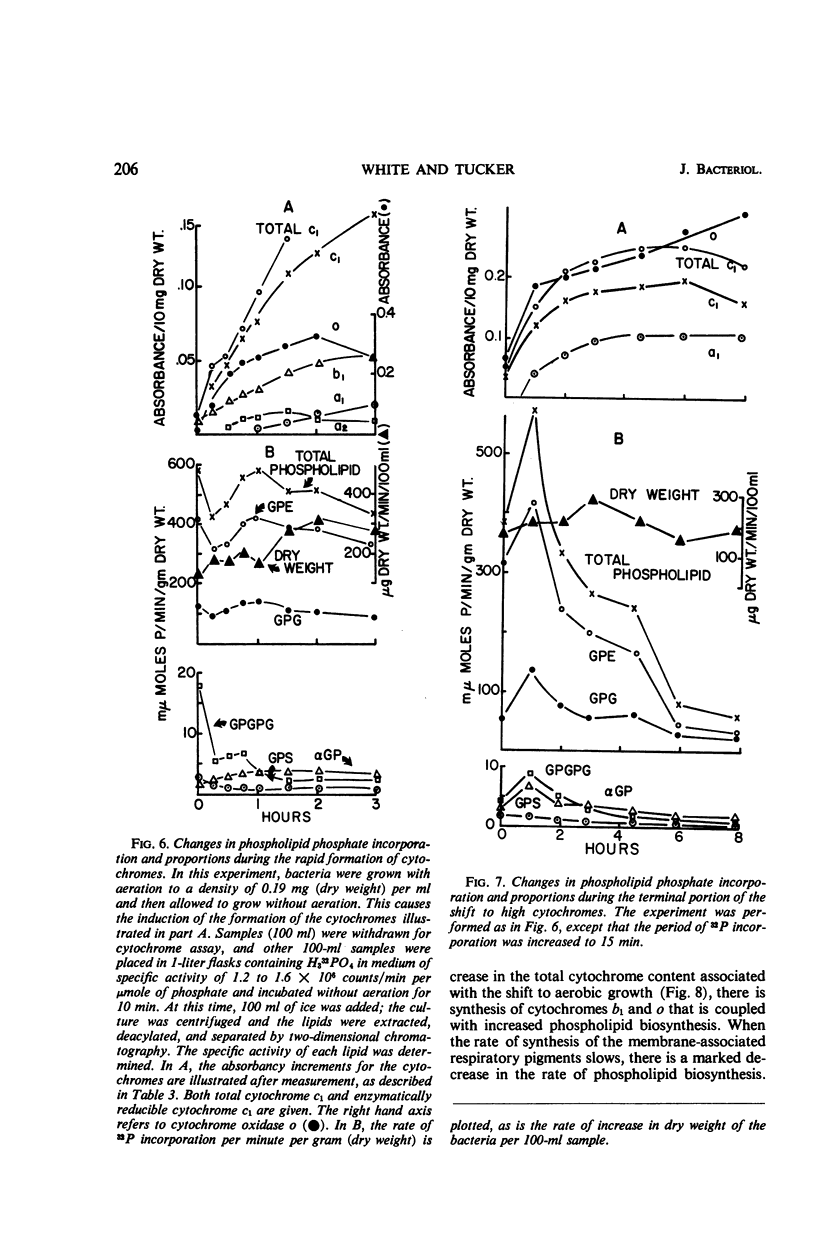
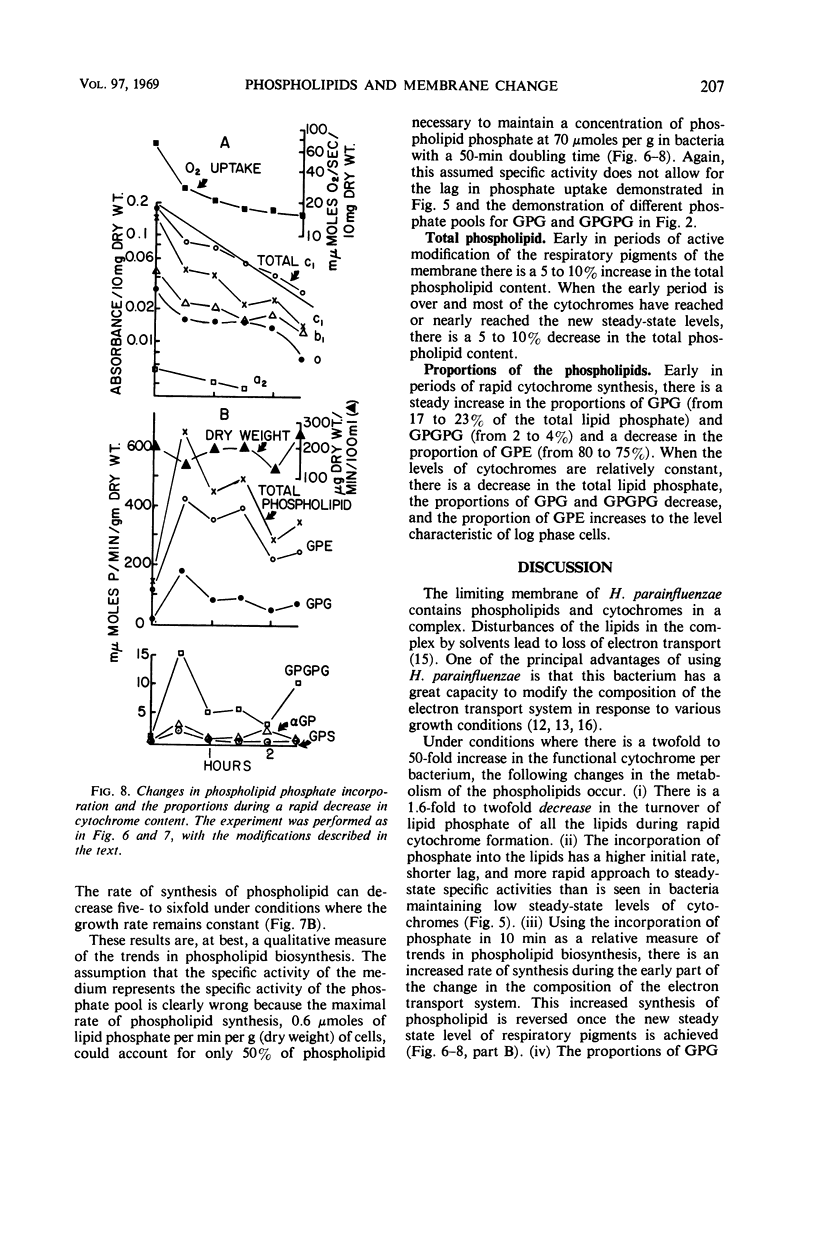
After a transition from high to low oxygen tension, there was a twofold to 50-fold increase in the content of membrane-bound respiratory pigments of Haemophilus parainfluenzae, and there were concurrent changes in the metabolism of the membrane phospholipids: (i) a twofold decrease in the rate of turnover of the phosphate in all the phospholipids; (ii) a shift from simple one-phase, linear incorporation of phosphate into phospholipids to a complex biphasic incorporation of phosphate into phospholipids; and (iii) an increase in the total phospholipids with a slight increase in the proportion of phosphatidylglycerol (PG) and a slight decrease in the proportion of phosphatidylethanolamine (PE). Changes in the rates of incorporation of phosphate into the phospholipids occurred without a change in the rate of bacterial growth. When the compensatory adjustment of the proportions of the respiratory pigments reached a steady state, the total phospholipid, the rate of incorporation of phosphate into phospholipids, and the proportion of PG fell. At steady-state proportions of cytochromes, the proportion of PE and the rate of turnover of the phosphate in the phospholipids increased. All through an incorporation experiment of 1.5 divisions, the specific activity of the phosphate of PG was twice that of phosphatidic acid (PA). The phosphate of PG turned over 1.2 to 1.5 times more rapidly than the phosphate of PA in cells with high and low cytochrome levels. If the PA was an accurate measure of the precursor for the cytidine-5′-diphosphate-diglyceride, which in turn was the precursor of all the lipids, then the results of these experiments suggested that exchange reactions, in addition to synthesis from PA, were involved in phospholipid metabolism. These reactions were more sensitive to changes in oxygen concentration than was the growth rate.
Full text
PDF

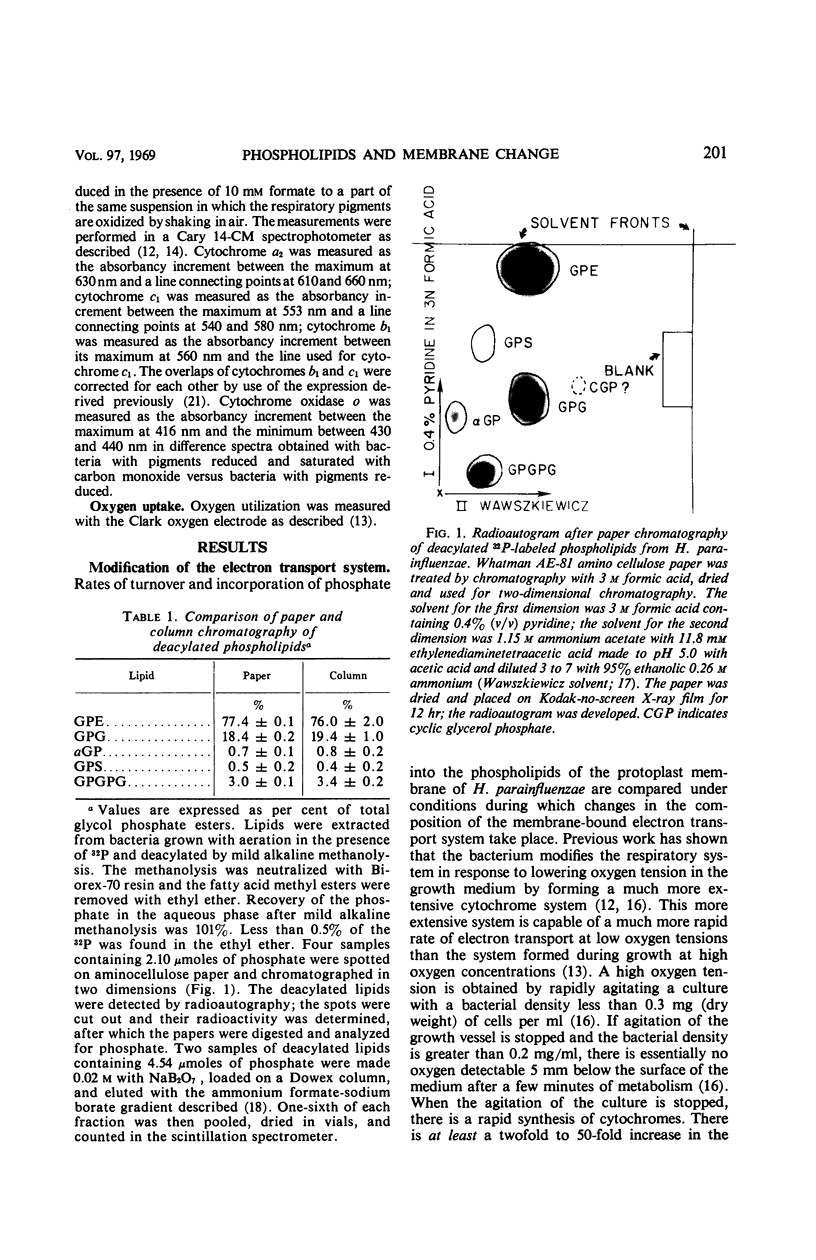
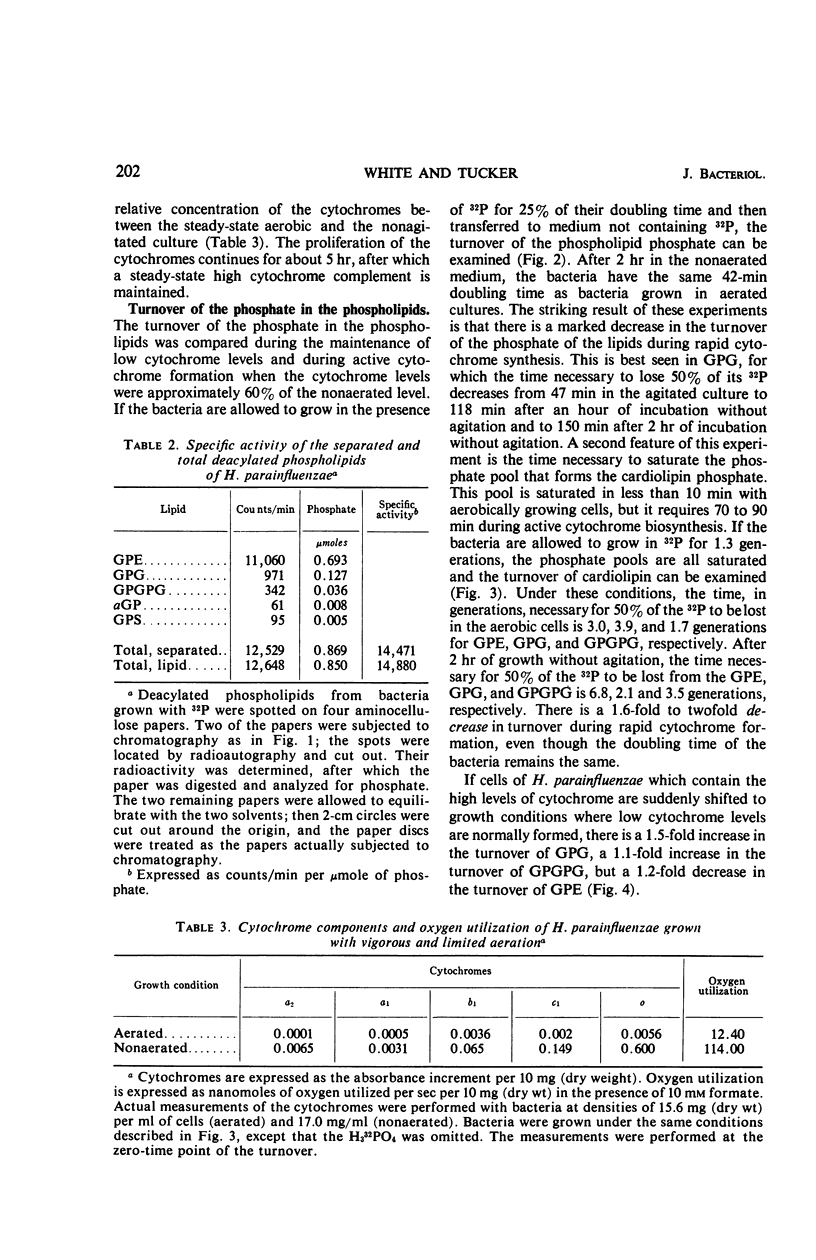
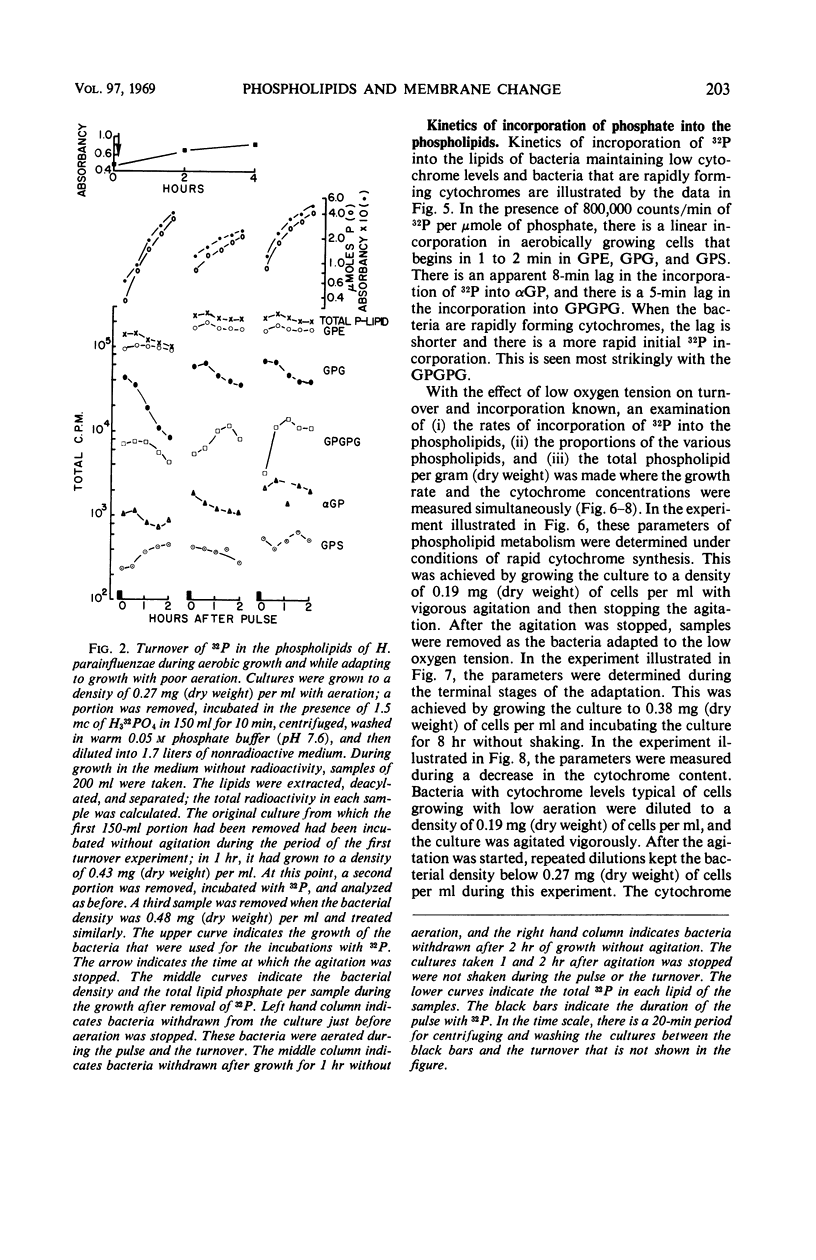


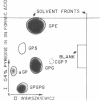
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Biosynthesis of phosphatidyl glycerophosphate in Escherichia coli. J Lipid Res. 1967 Sep;8(5):447–455. [PubMed] [Google Scholar]
- Chang Y. Y., Kennedy E. P. Pathways for the synthesis of glycerophosphatides in Escherichia coli. J Biol Chem. 1967 Feb 10;242(3):516–519. [PubMed] [Google Scholar]
- Frerman F. E., White D. C. Membrane lipid changes during formation of a functional electron transport system in Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1868–1874. doi: 10.1128/jb.94.6.1868-1874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kanemasa Y., Akamatsu Y., Nojima S. Composition and turnover of the phospholipids in Escherichia coli. Biochim Biophys Acta. 1967 Oct 2;144(2):382–390. [PubMed] [Google Scholar]
- LASCELLES J., SZILAGYI J. F. PHOSPHOLIPID SYNTHESIS BY RHODOPSEUDOMONAS SPHEROIDES IN RELATION TO THE FORMATION OF PHOTOSYNTHETIC PIGMENTS. J Gen Microbiol. 1965 Jan;38:55–64. doi: 10.1099/00221287-38-1-55. [DOI] [PubMed] [Google Scholar]
- SMITH L., WHITE D. C. Structure of the respiratory chain system as indicated by studies with Hemophilus parainfluenzae. J Biol Chem. 1962 Apr;237:1337–1341. [PubMed] [Google Scholar]
- Stanacev N. Z., Chang Y. Y., Kennedy E. P. Biosynthesis of cardiolipin in Escherichia coli. J Biol Chem. 1967 Jun 25;242(12):3018–3019. [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. FACTORS AFFECTING THE AFFINITY FOR OXYGEN OF CYTOCHROME OXIDASES IN HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1963 Nov;238:3757–3761. [PubMed] [Google Scholar]
- WHITE D. C., SMITH L. LOCALIZATION OF THE ENZYMES THAT CATALYZE HYDROGEN AND ELECTRON TRANSPORT IN HEMOPHILUS PARAINFLUENZAE AND THE NATURE OF THE RESPIRATORY CHAIN SYSTEM. J Biol Chem. 1964 Nov;239:3956–3963. [PubMed] [Google Scholar]
- WHITE D. C. SYNTHESIS OF 2-DEMETHYL VITAMIN K2 AND THE CYTOCHROME SYSTEM IN HAEMOPHILUS. J Bacteriol. 1965 Feb;89:299–305. doi: 10.1128/jb.89.2.299-305.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. THE FUNCTION OF 2-DEMETHYL VITAMIN K2 IN THE ELECTRON TRANSPORT SYSTEM OF HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1965 Mar;240:1387–1394. [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Effect of glucose on the formation of the membrane-bound electron transport system in Haemophilus parainfluenzae. J Bacteriol. 1967 Feb;93(2):567–573. doi: 10.1128/jb.93.2.567-573.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Fatty acid composition of the complex lipids of Staphylococcus aureus during the formation of the membrane-bound electron transport system. J Bacteriol. 1968 Jun;95(6):2198–2209. doi: 10.1128/jb.95.6.2198-2209.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. A., White D. C. Formation of a functional electron transport system during growth of penicillin-induced spheroplasts of Haemophilus parainfluenzae. J Bacteriol. 1966 Mar;91(3):1356–1362. doi: 10.1128/jb.91.3.1356-1362.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]