Abstract
The notion that maternal personality characteristics influence cognitive development in their children has been grounded in stress moderation theory. Maternal personality traits, such as self-esteem, may buffer maternal stressors or lead to improved maternal-child interactions that directly impact neurodevelopment. This can be extended to suggest that maternal personality may serve to attenuate or exacerbate the effects of other neurotoxicants, although this has not been studied directly. We examined whether mothers’ self-esteem had a direct or main effect on their children's cognitive outcomes. We also explored the modifying effects of maternal self-esteem on the association between exposure to lead and neurodevelopment in these children. Study participants included 379 mother-child pairs from Mexico City. Data included the Coopersmith self-esteem scale in mothers, children's Bayley's Scale of Infant Development (BSID) scores, and sociodemographic information. Linear regression was used to model the relationship between maternal self-esteem and the Bayley's Mental Development Index (MDI) and Psychomotor Development Index (PDI) scores at age 24 months using regression models stratified by levels of maternal self-esteem. In adjusted models, each point increase in maternal self-esteem was associated with children having 0.2 higher score on the Bayley's MDI (p=0.04). Similar results were observed using the PDI outcome. Moreover, there was evidence that maternal self-esteem attenuated the negative effects of lead exposure, although the interaction fell short of conventional levels of statistical significance.
Keywords: child, cognition, lead, neurotoxicology, mother-child relations
Introduction
The possibility that social context may modify the toxicity of lead has been previously considered (Bellinger, 1995; Bellinger, 2000), but little research has been conducted in human populations. The social environment appears to impact neurodevelopment (Francis et al., 2002) and might also influence the response to neurotoxic chemicals. Experimental research shows that an enriched social environment will mitigate the neurotoxicity of lead in rats when it precedes lead exposure (Schneider et al., 2001). Likewise, an enriched social and stimulating environment can reverse the neurotoxic effects of lead on memory function when the social enrichment follows lead exposure (Guilarte et al., 2003).
Children living with lower socio-economic status are more likely to be exposed to lead and some evidence suggests that the adverse effects of lead may be more pronounced in lower compared to higher socio-economic groups (Bellinger et al., 1989; Tong et al., 2000; Winneke and Kraemer, 1984). Some other previous work supports the concept that social context modifies the effects of chemical neurotoxins. For example, material hardship has been demonstrated to modify the neurotoxic effects of tobacco smoke in children (Rauh et al., 2004). There is also evidence that the adverse affects of PCBs on children are modified by breastfeeding (Jacobson and Jacobson, 2003). This differential vulnerability to PCBs in children who were breastfed versus formula fed appears to be more likely due to social characteristics than to the nutrients associated with breastfeeding (Vreugdenhil et al., 2002).
Socially disadvantaged environments increase the risk of exposure to chronic stress and chronic stress is toxic to the developing brain (McEwen, 2003). The effect of stress on the brain varies among individuals, suggesting there may also be potential psychosocial factors that safeguard against stress. For example, higher self-esteem has been inversely correlated with perceived stress (Abel, 1996; Kreger, 1995) and may be a buffer to the potentially toxic effects of the neuroendocrine responses associated with chronic stress (Creswell et al., 2005). The same negative life events may cause more stress in people with lower self-esteem than people with higher self-esteem (Brown and Dutton, 1995). Self-esteem may also correlate with parent child interactions. Low parental self-esteem is related to increased risk of authoritative parenting styles (Aunola et al., 1999; Lutenbacher and Hall, 1998). High self-esteem in mothers could act as a buffer in an environment of increased stress allowing a mother to maintain her ability to effectively parent the child, thereby enriching the child's family environment.
Given this background, our goals for this study were to determine if maternal self-esteem predicted children's neurodevelopmental outcomes and to explore whether maternal self-esteem modified the effects of lead on these outcomes.
Methods
Study participants included 379 mother-child pairs from three cohorts from the Harvard-Mexico Project on Fetal Lead Exposure, Risks and Intervention Strategies (FLERIS). These cohorts consisted of 247 mother-child pairs with MDI and PDI measured between 1996 to 2001, and 132 mother-child pairs measured in 2004 to 2005. All three cohorts used virtually identical inclusion criteria, methods, and sampling frameworks and were recruited from one of the three maternity hospitals in Mexico City. Women who attended the National Institute of Perinatology for the 24 month developmental evaluation of their children were asked to participate. Of theses mother-infant pairs, 309 had complete data on blood lead, self-esteem, Bayley's scales, and covariates. The main difference was that Cohort 1 was recruited at the time of delivery and Cohorts 2 & 3 during pregnancy. The study cohort was recruited from three maternity hospitals in Mexico City that serve a low to moderate income populations. A month after delivery (±5 days) each mother-infant pair attended the research center to be evaluated. Participating mother-infant pairs were subsequently assessed and interviewed at later time points including the ones used in this analysis at 24, 30 and 36 months. Full details are described elsewhere (Gomaa et al., 2002; Tellez-Rojo et al., 2004) Exclusion criteria included medical conditions that could cause low birth weight, living in a household outside the metropolitan area, intention not to breastfeed, prematurity or an Apgar score of 6 or less (at 5 minutes), a condition requiring treatment in neonatal intensive care unit, birthweight <2000 g, or serious birth defects, a physical diagnosis of multiple fetuses, preeclampsia, psychiatric, kidney or cardiac diseases, gestational diabetes, history of repeated urinary infections, family or personal history of kidney stone formation, seizure disorder requiring daily medications, ingestion of corticosteroids or blood pressure >140 mmHg systolic or >90 mmHg diastolic, single parent households, and factors that could interfere with calcium metabolism. The three cohorts differed also in that Cohort 1 was a randomized controlled trial of calcium supplementation given to women during lactation, Cohort 2 was purely an observational study, and Cohort 3 was a randomized controlled trial of calcium supplementation to women during pregnancy. Otherwise, the baseline measurements and methods to make those measurements were identical.
For Cohort 1, within the first month of delivery, eligible participants were interviewed to gather information about health status and social and demographic characteristics. For Cohorts 2 and 3 these demographic data were collected during pregnancy. For all three cohorts when the index child was 24 months of age, self-esteem was measured using a Spanish version of the Coopersmith Self-Esteem Inventory (Adult Short Form), a 25-item scale with good reliability and validity (Blascovich and Tomaka, 1991; Lara et al., 1993). Cognitive outcomes were measured with the Spanish version of the Bayley Scale of Infant Development II, administered at 24, 30, and 36 months. Lead exposure was assessed using blood lead when children were 24 months of age. Covariates included child gender, maternal IQ, and maternal education, parity, smoking, and alcohol consumption. Maternal IQ was measured using the Wechsler Adult Intelligence Scale (WAIS) as a continuous variable. Maternal education was used as a continuous variable, indicating number of years of schooling completed. Smoking use was classified as never or ever. Parity was classified as number of previous pregnancies that resulted in a live birth, not counting stillbirths or abortions. Alcohol was measured in grams per day. In cohort 1 and 2 this measurement was taken at 1 month postpartum and corresponded to alcohol in the last year. In cohort three, the measurement was taken in the 3rd trimester and referred to the previous month.
Using linear regression we modeled the relationship between mother's self-esteem and children's neurobehavioral test scores. Analyses examined maternal self-esteem in relation to Bayley's Mental Development Index (MDI) and Psychomotor Development Index (PDI) in children. The cohorts spanned over 10 years time and the MDI and PDI were conducted by different personnel in each of the 3 cohorts. Therefore, in order to account for inter-examiner variation cohort was adjusted for in the analysis. Associations were also modeled adjusting for potential confounders including child gender, maternal IQ (WAIS), maternal education, parity, alcohol consumption, and smoking. Both self-esteem and blood lead levels were measured on continuous scales therefore, in order to simplify the interpretation of this interaction, we chose to categorize self-esteem and examine the effect of blood lead within strata of self-esteem. We were able to take advantage of measures at multiple time points to gain power by including MDI and PDI scores at 24, 30, and 36 months. This was done using proc mixed in SAS, a mixed model regression method to consider the effect of self-esteem on cognition over time, while accounting for the correlation between cognition measured in the same individuals across exams, baseline cognition, and also controlling for potential confounders.
Because we were uncertain of the most appropriate cut-off, we conducted a sensitivity analysis to test the interaction between lead (used as a continuous variable) and self-esteem categorized in two different ways: by the highest versus lowest tertiles and quartiles. We assumed that the potential to mitigate the adverse effects of lead exposure would be the greatest among the mothers with the very highest self-esteem. Since this study provided the first test of this hypothesis, we chose to focus on the highest quartile group since we expected it might have the greatest likelihood of detecting effects. All models and cutpoints for self-esteem gave similar results. Based on examination of this analysis, we chose the analysis of the association between blood lead and child MDI and PDI scores in children with mothers scoring in the highest quartile of self-esteem versus children whose mothers were in the lowers three quartiles of self-esteem. In order to see if there was a graded relationship between the effects of lead on children's cognition by level of self-esteem, we also estimated associations separately within each quartile of self-esteem. Finally, we plotted smoothed curves using the lowess smoothing function in STATA to visually explore the relationship between children's Bayley's MDI scores and blood lead (both measured at 24 months).
Results
Of the 422 mother-child pairs invited to participate, 31 of 278 did not participate from Cohorts 1 and 2. From Cohort 3, twelve out of 144 eligible mothers declined participation. According to the examiners, the main reason for not answering the inventory was lack of time.
The average blood lead level for children at age 24 months was 6.4 μg/dl (Table 1). Data on the Coopersmith Self-Esteem Scale was complete for 379 participants. Complete data on the Bayley scale MDI/PDI, lead, self-esteem and other covariates were available on 309 mother-child pairs with at least one Bayley scale assessment. The majority of the missing data was from blood lead measurements. MDI and PDI scores were available from 350, 185 and 225 children at 24, 30 and 36 months, respectively. Average scores on the Bayley mental development and psychomotor development indices at 24 months were 88 (sd 13.5) and 92 (sd 10.9), respectively. Mothers' average score on the WAIS was 88 (sd 13.3). Our sample included 57% boys and 43% girls. On average, the women in our sample had completed ten years of education. The average number of births was 1.8. Sixty-four percent of the women in the study reported having ever smoked. The average alcohol consumption per day was 0.3 grams during a period that corresponded to at least part of the pregnancy.
Table 1.
Descriptive statistics for maternal self-esteem, Bayley's Mental Development Index and Psychomotor Development Index scores and other sociodemographic variables
| No. (%) | Mean (sd) | Range | N | |
|---|---|---|---|---|
| Coopersmith self-esteem (24 mo) | 17.2 (5.0) | 1-25 | 379 | |
| Highest quartile | 75 (19.8) | 23.1(1.0) | ||
| Lowest three quartiles | 304 (80.2) | 15.7(4.5) | ||
| Child blood lead (24 mo) μg/dL | 6.4 (4.3) | 0.8-25.8 | 319 | |
|
Mental Development Index (24 mo) |
88.2 (13.5) | 58-128 | 350 | |
|
Psychomotor Development Index (24 mo) |
92.0 (10.9) | 61-128 | 350 | |
| Child Gender | ||||
| Male | 216 (57.0) | 379 | ||
| Female | 163 (43.0) | |||
| Mother's IQ (WAIS) | 87.7 (13.3) | 52-135 | 372 | |
| Mother's Education | 10.2 (3.0) | 1-18 | 374 | |
| Parity | 1.4 (1.4) | 0-7 | 362 | |
| Maternal Smoking | ||||
| Ever | 232 (64.1) | 362 | ||
| Never | 130 (35.9) | |||
| Alcohol (grams) | 0.3 (1.4) | 0-13 | 354 | |
In Table 2, we display a model showing the relationships of self-esteem, blood lead, gender, maternal IQ, maternal education, parity, alcohol consumption and smoking to MDI and PDI scores. In models adjusting for all covariates an increase of one standard deviation (5 units) change on the mother's Coopersmith was associated with a 2.0 point increase in children's MDI score (p=0.04). Female gender was related to a 3.0 point higher MDI score compared with male gender (p=<0.01). Each one unit increase in mother's IQ was associated with a 0.1 increase in MDI score (p<0.01). Using unadjusted models, we also plotted the relationship between Bayley's MDI in relation to blood lead stratified by maternal self-esteem (Figure 1). We hypothesized that maternal self-esteem may be on the causal pathway of lead exposure since mothers with low self-esteem may be less likely to take measures to prevent or monitor their lead exposure. However, the association between maternal self-esteem and MDI score changed negligibly when child's blood lead was not included (data not shown). Somewhat similar results were observed for the fully adjusted model using the PDI as an outcome. Each one standard deviation increase in maternal self-esteem was marginally associated with 1.5 unit higher PDI score (p=0.1). There were no notable or statistically significant associations between child gender, mother's IQ, maternal education, parity, smoking, or alcohol consumption and the PDI.
Table 2.
Model of main effects of lead, self-esteem and covariates on Bayley's MDI and PDI using repeated measures at 24, 30 and 36
| Mental Development Index β (SE) |
p-value | Psychomotor Development Index β (SE) |
p-value | |
|---|---|---|---|---|
| Coopersmith | ||||
| self-esteem (24 mo) | 0.24 (0.12) | 0.04 | 0.18 (0.12) | 0.13 |
| Child blood lead | ||||
| (24 mo) | −0.18 (0.14) | 0.19 | −0.14 (0.14) | 0.32 |
| Child Gender | ||||
| Female | 3.01 (1.10) | <0.01 | 1.48 (1.10) | 0.18 |
| Male | reference | reference | ||
| Mother's IQ | ||||
| (WAIS) | 0.14 (0.05) | 0.01 | 0.04 (0.05) | 0.45 |
| Mother's Education | 0.04 (0.27) | 0.87 | −0.30 (0.27) | 0.27 |
| Parity | −0.68 (0.51) | 0.19 | −0.62 (0.52) | 0.23 |
| Maternal Smoking | ||||
| Never | −1.41 (1.15) | 0.22 | −2.2 (1.16) | 0.06 |
| Ever | reference | reference | ||
| Alcohol (grams) | 0.13 (0.38) | 0.74 | 0.63 (0.38) | 0.10 |
Models are adjusted for cohort. Analyses included 607 observations on 309 subjects.
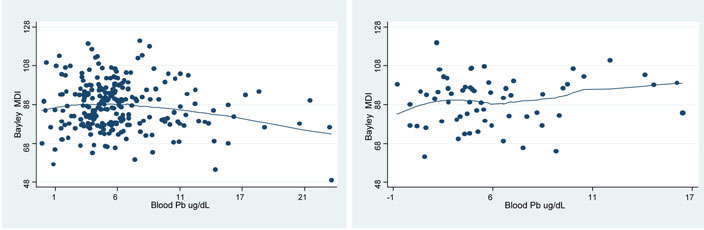
Figure 1.
Adjusted smoothed curves of Bayley's MDI at 24 months in relation to blood lead. Comparison of the lower four quartiles of maternal self-esteem (left) with the highest quartile of maternal self-esteem (right)
In Table 3 we display childhood blood lead and MDI and PDI scales stratified by the Coopersmith selfesteem score. We found that for children with mothers with self-esteem in the lower three quartiles, a one μg/dL increase in blood lead was associated with a 0.3 lower score on the MDI (p=0.04), compared to a 0.4 higher score for children with mothers with high self-esteem (p=0.4). This pattern also was similar that for our child psychomotor skill outcome. Although not statistically significant, in children of mothers with lower self-esteem each unit increase in blood lead was associated with an average a 0.3 lower score on the PDI (p=0.1), while a μg/dL increase in blood lead in children of mothers with the highest self-esteem was associated with a 0.5 point higher PDI score (p=0.2).
Table 3.
Relationship between childhood blood lead and Mental Development Index and Psychomotor Development Index scales using repeated measures at 24, 30 and 36 stratified by Coopersmith self-esteem score
| Mental Development Index |
Psychomotor Development Index |
|||||
|---|---|---|---|---|---|---|
|
β (SE) p-value |
p-value for interaction |
β (SE) p-value |
p-value for interaction |
|||
|
Lowest three quartiles self-esteem |
Highest quartile self-esteem |
Lowest three quartiles self-esteem |
Highest quartile self-esteem |
|||
| Child blood | −0.31 (0.15) | 0.36 (0.44) | 0.11 | −0.25 (0.15) | 0.49 (0.40) | 0.21 |
| lead | p=0.04 | p=0.42 | p=0.10 | p=0.23 | ||
| (24 mo) | ||||||
| μg/dL | ||||||
Models are adjusted for cohort, child gender, mothers IQ, and mother's educational attainment, parity, smoking, alcohol consumption, and self-esteem as a continuous variable.
To test the significance of these differences, we used interaction terms in models with all subjects. In sensitivity analyses testing the interaction between maternal self-esteem and lead levels estimated at different cutpoints for self-esteem (tertiles and quartiles), the interaction neared statistical significance for both MDI (p=0.11) and PDI (p=0.21) when using quartiles. The effects were in the same direction (i.e. higher self-esteem was protective of lead exposure) but were not statistically significant at the other cutpoints. Figure 1 displays smoothed curves representing the relationship of child blood lead and Bayley's MDI stratified by maternal self-esteem by quartiles.
In the analyses that were stratified separately into each quartile of maternal self-esteem, we found that for each of the 3 lower quartiles of self-esteem child blood lead was negatively related to MDI score, while the coefficients were in the positive direction for children of mothers scoring in the highest quartile of self-esteem (Table 4). Regarding the PDI measure, the association between child blood lead level and PDI was negatively related in children of mothers scoring in the 2nd to lowest quartile of self esteem (p<0.01). This is consistent with our hypothesis that exposure to lead would have the most severe negative effects on development in children with mothers in the lowest self-esteem. The estimated coefficient for the effects of lead on the PDI in the children with mothers in lowest quartile of self-esteem was not in the direction we expected, but was not statistically significant. Only one of the associations between exposure to lead and developmental scores in any of the eight strata of self-esteem reached statistical significance.
Table 4.
Relationship between childhood blood lead and Mental Development Index and Psychomotor Development Index scales using repeated measures at 24, 30 and 36 stratified by quartiles of the Coopersmith self-esteem score
| Mental Development Index β (SE) p-value Quartile of maternal self-esteem |
Psychomotor Development Index β (SE) p-value Quartile of maternal self-esteem |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1st (low) | 2nd | 3rd | 4th (high) | 1st (low) | 2nd | 3rd | 4th (high) | |
| n=145 | n=148 | n=178 | n=136 | n=146 | n=147 | n=178 | n=135 | |
|
Child blood lead |
−0.17(0.25) | −0.40(0.25) | −0.35(0.31) | 0.37(0.44) | 0.22(0.26) | −0.82(0.23) | −0.12(0.33) | 0.50(0.40) |
|
(24 mo) μg/dL |
p=0.50 | p=0.12 | p=0.25 | p=0.40 | p=0.41 | p<0.01 | p=0.71 | p=0.22 |
Models are adjusted for cohort, child gender, mothers IQ, and mother's educational attainment, parity, smoking, and alcohol consumption.
Discussion
This study shows an association between maternal self-esteem and children's cognitive development, whereby increased levels of maternal self-esteem are associated with better neurodevelopmental tests scores. Furthermore, this effect was independent of child sex, blood lead levels, maternal IQ and years of education. The finding that this effect is independent of maternal education and IQ suggests that self-esteem is not merely a marker of mothers who are better educated. To place the size of this effect into context, after adjusting for covariates, an increase of one standard deviation on the Coopersmith's self-esteem score in mothers was associated with a two point increase in children's MDI and PDI Bayley scores. In addition, our exploratory interaction analysis suggested the potential relevance of maternal self-esteem as a modifier of cognitive abilities in lead exposed children. Specifically, the negative effects of exposure to lead on the MDI score appear to be more pronounced in children of mothers in the three lowest quartiles of self-esteem compared to those with self-esteem in the highest quartile.
There are several potential mechanisms which may explain our findings. Self-esteem may work via behavioral pathways, biological pathways or both. With respect to behavioral pathways, higher self-esteem is associated with a variety of positive health practices (Mitchell et al., 2005; Torres and Fernandez, 1995; Yarcheski et al., 2004), suggesting it is likely also to be associated with other positive mother-child interactions, such as more educational interactions, improved dietary practices, or other caregiving practices that may lead to children's improved cognitive abilities. As such, self-esteem may be a marker of parenting qualities not captured by other covariates. We speculate that there may be other reasons why higher maternal self-esteem could be beneficial for lead exposed children, e.g. mothers with higher self-esteem may be less depressed, communicate a more positive attitude to their children, and provide a higher level of intellectual stimulation and/or emotional support. Strong evidence exists to show that depressed mothers have poorer interactions with their children (Cooper and Murray, 1998; Newport et al., 2002). Indeed our results demonstrate positive effects of self-esteem independent of factors such as maternal IQ or years of education. Since we do not have other indicators of socioeconomic status (e.g. income data), our study does not provide information about the relationship between income and self-esteem.
Besides behavioral pathways involving parenting, there are other potential mechanisms for our observed association between self-esteem and neurodevelopment. We hypothesize that self-esteem could be a buffer to lessen the stress response, a notion grounded in stress moderation theory (Cohen and Rodriquez, 1995). Because low maternal self-esteem is correlated with maternal stress (Aunola et al., 1999), it may serve as a marker of a social environment which is also stressful for the child (Caldji et al., 2000; Essex et al., 2002). Self-esteem may lessen a mother's perception of stress or enhance her ability to deal with it, i.e. high self-esteem may directly help her to protect herself from stressors (Longmore and DeMaris, 1997; Spencer et al., 1993). Animal behavioral studies have confirmed the adverse effects of both prenatal and post-natal chronic stress on memory and learning (Aleksandrov et al., 2001; Frisone et al., 2002; Vallee et al., 1999; Zaharia et al., 1996). In humans, chronic stress in parents has been associated with lower child development scores, suggesting that maternal stress is a marker of child stress and therefore a risk factor for predicting neurodevelopmental outcomes. For example, maternal anxiety during pregnancy and postnatally, have been associated with a 1.5- to 2-fold increase in risk for behavioral/emotional problems in children at 4 years of age (O'Connor et al., 2002a; O'Connor et al., 2002b). In addition, Badr Zahr (2001) reported that parental measures of perceived stress predicted lower 24-month Bayley MDI scores in a cohort of low-birth weight Latino children (Badr Zahr, 2001). The positive effects of maternal self-esteem noted in our study may well be due to self-esteem buffering the stress response in mothers and decreasing the stress levels experienced by the child. Indeed, higher self-esteem has been noted to attenuate the biological response to stress towards more adaptive neuroendocrine rhythms (Creswell et al., 2005). It is likely that these pathways are not mutually exclusive and the most likely explanation for our findings is a combination of the behavioral and biological benefits of higher esteem.
We believe our exploratory analysis of stress-lead interactions is particularly intriguing and may suggest that higher maternal self-esteem may mitigate the effects of toxic neurochemicals such as lead in children. The modifying effect of self-esteem on the association of lead and MDI scores suggest some support for interactions documented in animal studies (Guilarte et al., 2003; Schneider et al., 2001), although our sample size is too small to measure a stable estimate of this interaction. This effect is consistent with the concept that social environment modifies the neurotoxicity of lead. Previous work on this topic has been conducted mainly in animals, and to our knowledge this is the first study to address the effect of social environment on lead toxicity in humans. These studies include experiments in rats suggesting that social engagement can lessen the effects of lead in abilities of spatial-memory (Schneider et al., 2001)and that exposure to stimulating environments even following lead exposure can recuperate some memory function (Guilarte et al., 2003).
One of the principal strengths of this study is the novelty of our study question. Little prior research exists to explore the influence of maternal self-esteem on child development or the effects of maternal psychosocial resources on lead exposed children. To our knowledge, no prior studies have examined the relationship between maternal self-esteem and mental development in children. Most research to evaluate lead-stress interactions has been done in laboratory based studies in animals (Cory-Slechta et al., 2004). Studying interactions between the social environment and neurotoxins may be more pertinent to human experience since they more closely resemble what commonly occurs outside of the laboratory (Cory-Slechta et al., 2004). The prospective nature of our data collection, with self-esteem measurements preceding the children's cognitive tests in two of our cohorts that were combined for use in this study (at 30 and 36 months), is another strength of this study.
Our population did not include women of very high (or very low) socioeconomic status. Likewise there was almost no variability in ethnicity in our population, suggesting that cultural factors do not confound our results. Restriction of our sample to lower middle class families of Mexican heritage enhances the internal validity of our study, since confounding by these factors is essentially controlled. However, we cannot comment on the external validity of our study with respect to culture and income. Nonetheless, we believe maternal self-esteem is an important marker of the social environment in which a child develops and should be considered as a potential predictor of cognition/behavior in neurodevelopmental studies, either as a covariate or a modifier of other exposures. In addition, our analysis was restricted to a sample with fairly low lead exposures (μ=6.4, s.d=4.3, with 75% of the children with levels below 8.1μg/dL) preventing generalizability of these findings to populations in which social factors such as maternal self-esteem may be more or less important.
One of the main study limitations is the absence of a stress biomarker such as salivary cortisol to provide information as to how maternal self-esteem could alter the effects of lead on children's mental development through specific pathways (i.e. whether the effect is via mediation of stress effects). Another major limitation of the study is the relatively small number of participants evaluated in our exploratory analysis. Tests of interaction tend to require sample sizes that are substantially higher than when examining main effects and the interactions we report have relatively low power in our sample. Thus, the borderline significant interaction effects seen in these analyses may be related to the relatively small sample size and unstable effect estimates. Concerning data collection, we had consistent differences in interrater reliability on the MDI/PDI across the three waves of Bayley's Scale of Infant Development data collection, as different psychologists evaluated the children in each cohort. We have addressed this by adjusting for the cohort effect in the analysis, although it is still possible that interexaminer differences may have introduced error that was not completely controlled by adjusting models for examiners. We were also limited to the measurement of lead and self-esteem available in our data, which as a pilot data, was only a subset of the larger cohorts. Other unmeasured time points (such as early pregnancy) may represent critical developmental windows in which the proposed main effects (or interactions) are more prominent. Likewise, because we were restricted to variables in our data base, the role of other factors related to self-esteem could not be evaluated.
While the interaction between self-esteem and blood lead is considered preliminary, we believe this analysis provides justification for further study of social context and chemical poisoning. Guilarte et al demonstrated that social environment can mitigate lead poisoning in animals (Guilarte et al., 2003). Likewise, although it is speculative, it may be that social interventions can mitigate the effects of lead in humans. There is uncertain but burgeoning evidence from interventions that self-esteem and/or other features of the social environment may be somewhat modifiable (Chen et al., 2006; Ekeland et al., 2005; Knapen et al., 2005), leaving open the possibility that one day behavioral interventions directed at parents might reduce lead toxicity in children.
In summary, we observed that increased maternal self-esteem predicted higher mental developmental scores in children at two years of age independent of socioeconomic status. An exploratory analysis provides suggestive but inconclusive evidence for effect modification between lower levels of maternal self-esteem and the effects of lead exposure in children. These findings open up an array of questions outside the scope of this study regarding the nature of this relationship and mechanisms that may be important. These topics and the issue of potential effect-modification by maternal self-esteem and/or stress need to be further explored and confirmed in studies designed specifically to address these important questions.
Acknowledgments
We would like to thank the American British Cowdray Hospital for hosting this study. We are also grateful to Hongshu Guan and Maritsa Solano González for assistance with data management. This study was supported by U.S. National Institute of Environmental Health Sciences (NIEHS), NIH grant number P42-ES05947 Superfund Basic Research Program, NIEHS P01ES012874, NIEHS RO1-013744, NIEHS RO1 ES014930, NIEHS Center Grant 2 P30-ES 00002, NIEHS grant number K23ES000381, and NIEHS RO1 ES 007821. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH or EPA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel MH. Self-esteem moderator or mediator between perceived stress and expectancy of success. Psychol Rep. 1996;79:635–41. doi: 10.2466/pr0.1996.79.2.635. [DOI] [PubMed] [Google Scholar]
- Aleksandrov AA, Polyakova ON, Batuev AS. The effects of prenatal stress on learning in rats in a Morris maze. Neurosci Behav Physiol. 2001;31:71–4. doi: 10.1023/a:1026682415860. [DOI] [PubMed] [Google Scholar]
- Aunola K, Nurmi JE, Onatsu-Arvilommi T, Pulkkinen L. The role of parents' self-esteem, mastery-orientation and social background in their parenting styles. Scand J Psychol. 1999;40:307–17. doi: 10.1111/1467-9450.404131. [DOI] [PubMed] [Google Scholar]
- Badr Zahr LK. Quantitative and qualitative predictors of development for low-birth weight infants of Latino background. Appl Nurs Res. 2001;14:125–35. doi: 10.1053/apnr.2001.24411. [DOI] [PubMed] [Google Scholar]
- Bellinger D, Leviton A, Waternaux C. Lead, IQ and social class. Int J Epidemiol. 1989;18:180–5. doi: 10.1093/ije/18.1.180. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Interpreting the literature on lead and child development: the neglected role of the “experimental system”. Neurotoxicol Teratol. 1995;17:201–12. doi: 10.1016/0892-0362(94)00081-n. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicol Teratol. 2000;22:133–40. doi: 10.1016/s0892-0362(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Tomaka J. Measures of self-esteem. In: Robinson PR, Shaver LS, Wrightsman LS, editors. Measures of personality and social psychological attitudes. Vol. 1. Academic Press; San Diego, CA: 1991. [Google Scholar]
- Brown JD, Dutton KA. The thrill of victory, the complexity of defeat: self-esteem and people's emotional reactions to success and failure. J Pers Soc Psychol. 1995;68:712–22. doi: 10.1037//0022-3514.68.4.712. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–74. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Chen TH, Lu RB, Chang AJ, Chu DM, Chou KR. The evaluation of cognitive-behavioral group therapy on patient depression and self-esteem. Arch Psychiatr Nurs. 2006;20:3–11. doi: 10.1016/j.apnu.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Cohen S, Rodriquez MS. Pathways linking affective disturbances and physical disorders. Health Psychol. 1995;14:374–80. doi: 10.1037//0278-6133.14.5.374. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Murray L. Postnatal depression. BMJ. 1998;316:1884–6. doi: 10.1136/bmj.316.7148.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112:717–30. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Welch WT, Taylor SE, Sherman DK, Gruenewald TL, Mann T. Affirmation of personal values buffers neuroendocrine and psychological stress responses. Psychol Sci. 2005;16:846–51. doi: 10.1111/j.1467-9280.2005.01624.x. [DOI] [PubMed] [Google Scholar]
- Ekeland E, Heian F, Hagen KB. Can exercise improve self esteem in children and young people? A systematic review of randomised controlled trials. Br J Sports Med. 2005;39:792–8. doi: 10.1136/bjsm.2004.017707. discussion 92-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiatry. 2002;52:776–84. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–3. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisone DF, Frye CA, Zimmerberg B. Social isolation stress during the third week of life has agedependent effects on spatial learning in rats. Behav Brain Res. 2002;128:153–60. doi: 10.1016/s0166-4328(01)00315-1. [DOI] [PubMed] [Google Scholar]
- Gomaa A, Hu H, Bellinger D, Schwartz J, Tsaih SW, Gonzalez-Cossio T, Schnaas L, Peterson K, Aro A, Hernandez-Avila M. Maternal bone lead as an independent risk factor for fetal neurotoxicity: a prospective study. Pediatrics. 2002;110:110–8. doi: 10.1542/peds.110.1.110. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann Neurol. 2003;53:50–6. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143:780–8. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Knapen J, Van de Vliet P, Van Coppenolle H, David A, Peuskens J, Pieters G, Knapen K. Comparison of changes in physical self-concept, global self-esteem, depression and anxiety following two different psychomotor therapy programs in nonpsychotic psychiatric inpatients. Psychother Psychosom. 2005;74:353–61. doi: 10.1159/000087782. [DOI] [PubMed] [Google Scholar]
- Kreger DW. Self-esteem, stress, and depression among graduate students. Psychol Rep. 1995;76:345–6. doi: 10.2466/pr0.1995.76.1.345. [DOI] [PubMed] [Google Scholar]
- Lara MA, Verduzco MA, Acevedo M, Cortes J. Validez y confiabilidad del inventário de autoestima de Coopersmith para adultos, en una población mexicana. Rev Latinoamericana Psicologia. 1993;25:247–55. [Google Scholar]
- Longmore MA, DeMaris A. Perceived inequity and depression in intimate relationships: The moderating effect of self-esteem. Social Psychology Quarterly. 1997;60:172–84. [Google Scholar]
- Lutenbacher M, Hall LA. The effects of maternal psychosocial factors on parenting attitudes of lowincome, single mothers with young children. Nurs Res. 1998;47:25–34. doi: 10.1097/00006199-199801000-00006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–54. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- Mitchell DK, Adams SK, Murdock KK. Associations among risk factors, individual resources, and indices of school-related asthma morbidity in urban, school-aged children: a pilot study. J Sch Health. 2005;75:375–83. doi: 10.1111/j.1746-1561.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Wilcox MM, Stowe ZN. Maternal depression: a child's first adverse life event. Semin Clin Neuropsychiatry. 2002;7:113–9. doi: 10.1053/scnp.2002.31789. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Glover V. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adolesc Psychiatry. 2002a;41:1470–7. doi: 10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002b;180:502–8. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, Diaz D, Camann D, Perera FP. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26:373–85. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Lee MH, Anderson DW, Zuck L, Lidsky TI. Enriched environment during development is protective against lead-induced neurotoxicity. Brain Res. 2001;896:48–55. doi: 10.1016/s0006-8993(00)03249-2. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Josephs RA, Steele CM. Low self-esteem: The uphill struggle for self-integrity. In: Baumeister RF, editor. Self-esteem: The puzzle of low self-regard. Plenum; New York: 1993. [Google Scholar]
- Tellez-Rojo MM, Hernandez-Avila M, Lamadrid-Figueroa H, Smith D, Hernandez-Cadena L, Mercado A, Aro A, Schwartz J, Hu H. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am J Epidemiol. 2004;160:668–78. doi: 10.1093/aje/kwh271. [DOI] [PubMed] [Google Scholar]
- Tong S, McMichael AJ, Baghurst PA. Interactions between environmental lead exposure and sociodemographic factors on cognitive development. Arch Environ Health. 2000;55:330–5. doi: 10.1080/00039890009604025. [DOI] [PubMed] [Google Scholar]
- Torres R, Fernandez F. Self-esteem and value of health as determinants of adolescent health behavior. J Adolesc Health. 1995;16:60–3. doi: 10.1016/1054-139X(94)00045-G. [DOI] [PubMed] [Google Scholar]
- Vallee M, MacCari S, Dellu F, Simon H, Le Moal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur J Neurosci. 1999;11:2906–16. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil HJ, Lanting CI, Mulder PG, Boersma ER, Weisglas-Kuperus N. Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J Pediatr. 2002;140:48–56. doi: 10.1067/mpd.2002.119625. [DOI] [PubMed] [Google Scholar]
- Winneke G, Kraemer U. Neuropsychological effects of lead in children: interactions with social background variables. Neuropsychobiology. 1984;11:195–202. doi: 10.1159/000118077. [DOI] [PubMed] [Google Scholar]
- Yarcheski A, Mahon NE, Yarcheski TJ, Cannella BL. A meta-analysis of predictors of positive health practices. J Nurs Scholarsh. 2004;36:102–8. doi: 10.1111/j.1547-5069.2004.04021.x. [DOI] [PubMed] [Google Scholar]
- Zaharia MD, Kulczycki J, Shanks N, Meaney MJ, Anisman H. The effects of early postnatal stimulation on Morris water-maze acquisition in adult mice: genetic and maternal factors. Psychopharmacology (Berl) 1996;128:227–39. doi: 10.1007/s002130050130. [DOI] [PubMed] [Google Scholar]