Abstract
HOX gene expression determines the differential developmental identity of the Müllerian duct. DES and several environmental xenoestrogens disrupt the development of the female reproductive tract by altering HOX gene expression, leading to structural and functional defects.
The effects of diethylstilbestrol (DES) on development of the female reproductive tract is well recognized, however the molecular mechanisms that lead to DES related anomalies is still poorly characterized. Women exposed to DES in utero frequently have alterations in Müllerian structures. In the fetus the Müllerian duct is initially a uniform undifferentiated axial structure that gives rise to the fallopian tubes, uterus, cervix and upper vagina. The molecular mechanism by which differential positional identity is assigned to this axis involves HOX genes. (1,2) HOX genes are essential mediators of axial development; they are highly evolutionarily conserved transcription factors that are expressed in a temporally and spatially linear manner. (3) The HOX genes are located in four separate genomic clusters. Their linear organization in each cluster precisely parallels their expression; those HOX genes at the 3′ end of each cluster are expressed earlier in development and in anterior locations, while the more 5′ genes are expressed later and in more posterior locations. This unique organization allows for genetic information to instruct spatial patterning during embryogenesis. The particular HOX gene or combination of HOX genes expressed in an organized linear fashion result in differential axial identity.
The developing female reproductive tract is patterned by the differential expression of HOX genes in the Müllerian duct. (1,2) HOXA9 is expressed at the anterior end and drives developmental identity toward fallopian tube or oviduct. HOXA10 is expressed in the mid-portion of the duct and leads to uterine identity. HOXA11 is expressed just posterior to HOXA10 and results in lower uterine segment identity as well as development of the cervix. Finally HOXA13 is expressed at the distal end of the Müllerian duct which gives rise to the upper vagina. This ‘HOX code’ for the developing Müllerian duct is leads to the appropriate development of the female internal genital tract. Mutation of either HOXA10 or HOXA11, the two genes that give identity to the developing uterus, result in uterine factor infertility in mice. (4,5) Embryos do not implant in the HOXA10 or HOXA11 knock-out; even normal wild-type embryos fail to implant. The embryos from either the HOXA10 or HOXA11 knock out will implant in pseudopregnant wild-type females, demonstrating that the embryos are capable of implantation and development; the defect is in the uterus. These effects are likely due to failed regulation of the targets of HOXA10 transcriptional regulation; these genes include β3 Integrin and EMX2 (6,7) In addition, in both the HOXA10 and HOXA11 knock-out, there is a transformation of the distal end of the uterine horns toward the morphologic and histologic appearance of the oviduct.
The normal patterning of the Müllerian duct can be disrupted by exposure to the synthetic estrogen, diethylstilbestrol (DES). (8) We have previously shown that in utero DES exposure in mice leads to alterations in the normal pattern of HOX gene expression. The typical linear distribution is preserved, however the expression domain is shifted posteriorly. HOXA9, typically expressed in the fallopian tubes is expressed in the uterus. HOXA10 is expressed in the lower uterus, while HOXA11 expression is greatly diminished throughout the uterus. Each of these genes is expressed at higher levels in the vagina. This altered expression leads to altered positional identity. A common finding in women exposed to DES in utero is vaginal adenosis, where glandular tissue typical of mucosal surfaces of the endocervix or endometrium is found in the vagina. This displaced developmental identity is likely the result of the changes in the spatial expression of the HOX genes that lead to this identity. The consequent increase in adverse pregnancy outcome in DES exposed women is likely the result of this altered developmental programming. HOXA10, expression which is necessary for normal uterine development and subsequent embryo implantation, is decreased in the uterus of DES exposed women. This likely contributes to the limited ability to successfully carry a pregnancy.
Humans are exposed to a wide variety of chemicals that have estrogenic properties and the potential to alter estrogen responsive genes. We investigated the ability of several estrogen-like endocrine disruptors (xenoestrogens) to alter the expression of essential developmental genes such as the HOX genes. (9) Methoxychlor (MXC) is a pesticide and a xenoestrogen. MXC alters HOX gene expression in a manner similar to DES. (10) Specifically the HOX gene responsible for normal uterine development and fertility, HOXA10, is permanently repressed in the uterus of mice exposed to MXC in utero. This effect is mediated through the HOXA10 estrogen response element (ERE) in a dose dependent fashion. MXC has been previously associated with reproductive defects in mice, likely mediated through this molecular mechanism (11)
The response to all xenoestrogens is not always the same. Bisphenol A (BPA) is a xenoestrogen and a common component of polycarbonate plastics, epoxies used in food storage, and dental sealants. It has been previously associated with adverse reproductive outcomes in both animal models and humans. (12, 13) When administered to mice in utero the females demonstrate increased HOXA10 expression in the adult. (14) The expression persists long after exposure and is the result of altered developmental programming induced by the xenoestrogen. Although the developmental consequences of excess HOXA10 exposure in the adult are not known, the consequences of BPA on reproductive success is well know. It is likely that the permanently modified expression of HOXA10 contributes to the decline in reproductive potential. Alterations in the normally precise temporal regulation of HOXA10, either increased or decreased, may have implications for reproductive success. Despite its opposite effect on HOX gene expression in vivo, BPA behaves similarly to MXC in vitro, stimulating the HOXA10 ERE (14). In Vitro testing does not reflect the unexpected effect that these xenoestrogens have on developmental programming.
Estrogenic compounds exhibit profound and lasting effects on essential developmental genes in the reproductive tract. These changes are likely to influence reproductive competence. Humans are constantly simultaneously exposed to a wide variety of xenoestrogens. The effect of this bulk exposure is still unknown. Screening for an effect on essential development gene such as HOX genes may give insight to the effect of these compounds or mixtures on developmental programming. The identification of these agents, combinations and vulnerable periods in development deserves high priority for future investigation.
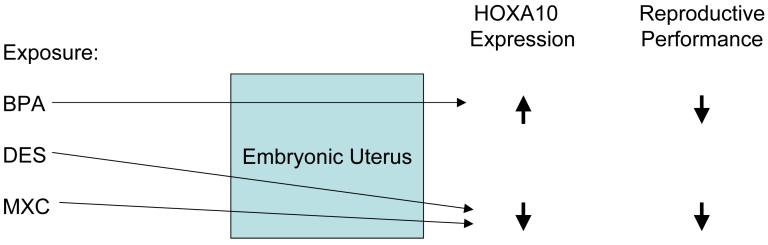
Figure 1.
Exposure to various xenoestrogens alters HOXA10 gene expression in the developing reproductive tract. These exposures lead to permanent alteration of gene expression in the adult. BPA, bisphenol A; DES, diethystilbestrol: MXC, methoxychlor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor HS, Vanden Heuvel GB, Igarashi P. A Conserved Hox Axis in the Mouse and Human Female Reproductive System: Late Establishment and Persistent Adult Expression of the HOXA Cluster Genes. Biol Reprod. 1997;57:1338–1345. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- 2.Du H, Taylor HS. Molecular regulation of Müllerian development by HOX genes. Ann NY Acad Sci. 2004;1034:152–165. doi: 10.1196/annals.1335.018. [DOI] [PubMed] [Google Scholar]
- 3.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 4.Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10 deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh-li HM, Witte DP, Weinstein M, et al. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121:1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- 6.Daftary G, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of Beta-3 Integrin Subunit Gene Expression by HOXA10 in Endometrial Cells. Mol Endocrinol. 2002;16:571–579. doi: 10.1210/mend.16.3.0792. [DOI] [PubMed] [Google Scholar]
- 7.Troy PJ, Daftary G, Taylor HS. Transcriptional repression of peri-implantation of EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol. 2003;23:1–13. doi: 10.1128/MCB.23.1.1-13.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block K, Kardana A, Igarashi P, Taylor HS. In Utero Diethylstilbestrol (DES), Exposure Alters Hox Gene Expression in the Developing Müllerian System. FASEB J. 2000;14:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- 9.Daftary G, Taylor HS. Endocrine regulation of Hox genes. Endocrine Reviews. 2006;27:331–335. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- 10.Fei X, Chung H, Taylor HS. Methoxychlor disrupts uterine Hoxa10 gene expression. Endocrinolgy. 2005;146:3445–3451. doi: 10.1210/en.2005-0341. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Lee HC, Chiba S, Yonezawa T, Nishihara M. Effects of methoxychlor exposure during perinatal period on reproductive function after maturation in rats. J Reprod Dev. 2004;50:455–61. doi: 10.1262/jrd.50.455. [DOI] [PubMed] [Google Scholar]
- 12.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–51. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20:2325–9. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 14.Smith C, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J. 2007;21:239–246. doi: 10.1096/fj.06-6635com. [DOI] [PubMed] [Google Scholar]