Abstract
We previously demonstrated that hybrid retrotransposons composed of the yeast Ty1 element and the reverse transcriptase (RT) of HIV-1 are active in the yeast Saccharomyces cerevisiae. The RT activity of these hybrid Ty1/HIV-1 (his3AI/AIDS RT; HART) elements can be monitored by using a simple genetic assay. HART element reverse transcription depends on both the polymerase and RNase H domains of HIV-1 RT. Here we demonstrate that the HART assay is sensitive to inhibitors of HIV-1 RT. (−)-(S)-8-Chloro-4,5,6,7-tetrahydro-5-methyl-6-(3-methyl-2-butenyl)imidazo[4,5,1-jk][1,4]-benzodiazepin-2(1H)-thione monohydrochloride (8 Cl-TIBO), a well characterized non-nucleoside RT inhibitor (NNRTI) of HIV-1 RT, blocks propagation of HART elements. HART elements that express NNRTI-resistant RT variants of HIV-1 are insensitive to 8 Cl-TIBO, demonstrating the specificity of inhibition in this assay. HART elements carrying NNRTI-resistant variants of HIV-1 RT can be used to identify compounds that are active against drug-resistant viruses.
Reverse transcriptase (RT) is essential for the replication of retroviruses and retroelements. RT copies single-stranded viral RNA into double-stranded DNA that is subsequently inserted into the host genome. The RTs of HIVs are the focus of extensive in vivo, biochemical, and structural studies. Drug therapies directed against HIV RT are in clinical use. However, because drug-resistant viruses arise in patients treated with HIV RT inhibitors, it is important to develop new anti-HIV compounds, and in particular to develop compounds that are active against resistant RTs. Rapid, simple, and efficient screens are an integral part of such a drug development program.
The yeast Saccharomyces cerevisiae has endogenous retrotransposons (Ty elements) that transpose through RNA intermediates (1), and their replication depends on an element-encoded RT. In addition to its dependence on RT, Ty1 shares other characteristics with retroviruses including protease processing of precursor polypeptides (2) and integrase-mediated insertion of a DNA copy of the Ty1 genome into the host genome (3). Hybrids between Ty1 and other retroelements have been used to demonstrate RT activity of human long interspersed nuclear elements (LINEs) (4) and LINE-like elements from a trypanosomatid (5). Hybrid elements make it possible to study the RTs of diverse retroelements in a simple model organism, as we demonstrated for an element that contains HIV-1 RT (6).
In these hybrid Ty1/HIV-1 [his3AI (artificial intron)/AIDS RT; HART] elements, the RT coding region of Ty1 was replaced by the RT coding region from HIV-1 (6). This substitution relied on identification of the protease cleavage site between integrase and Ty1 RT (Fig. 1B) (7). HIV-1 RT is composed of two subunits, p66 and p51 (see refs. 8 and 9). DNA encoding the p66 subunit of HIV-1 RT (10, 11) was inserted in place of the Ty1 RT coding region to create HART elements (6). Reverse transcription of these HART elements occurs at high frequency and is monitored by an RT-dependent genetic assay that involves the production of histidine prototrophs (12).
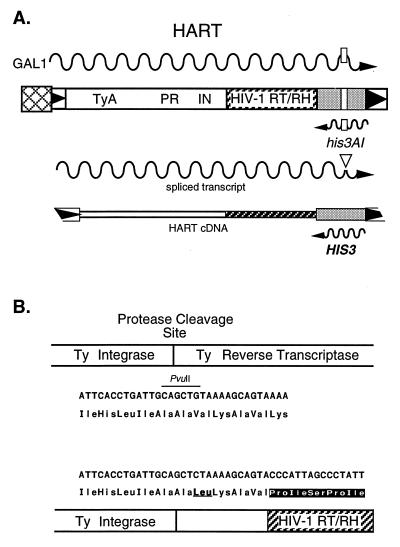
Figure 1.
Hybrid Ty1/HIV-1 RT/RH elements. (A) The RT/RH domain of HIV-1 was used to replace Ty1 RT/RH, resulting in HART (his3AI AIDS Reverse Transcriptase) elements. The inducible galactose promoter-driven hybrid HIV/Ty element marked with his3AI, HART, is shown. his3AI serves as an indicator of passage through an RNA intermediate and of reverse transcription. Expression results in full-length element RNA (wavy line) carrying an antisense copy of his3AI at the 3′ end of the transcript. An artificial intron (open rectangele) interrupts the his3 coding region and is flanked by splice donor and splice acceptor sites such that RNA splicing (▿) of the intron can occur only on the antisense transcript (the sense his3AI transcript is interrupted by a “backward” intron). Splicing, followed by reverse transcription, yields a cDNA copy of the hybrid element carrying a functional HIS3 gene. Integrase (IN)-mediated insertion or cDNA (homologous) recombination of this Ty-HIS3 cDNA results in histidine prototrophy that is scored by genetic selection. Hybrid elements carrying wild-type HIV-1 RT (HART) and the NNRTI-resistant variants HART-L100I and HART-Y181C, along with Ty, can all be assayed for RT activity with this genetic selection. (B) The nucleotide and amino acid sequences of the Ty/HIV-1 RT junction and the Ty protease (PR) cleavage site are shown.
In contrast to the reverse transcription of Ty1 RNA by Ty1 RT, HART element reverse transcription generates a substrate that cannot be inserted into the genome by Ty1 integrase. The priming mechanism for the initiation of HART reverse transcription is unknown; it is unlikely that HIV-1 RT recognizes the Ty1 primer binding site and polypurine-tract sequences required to generate correct Ty1 ends. Insertion of HART element DNA requires RAD52 (6), a gene required for recombination in yeast, which suggests that insertion occurs via homologous recombination between HART cDNA and resident Ty1 elements.
HART element reverse transcription is monitored in a yeast strain (spt3) that does not express endogenous Ty elements, so that the only source of RT is the hybrid elements. The polymerase and RNase H domains of HIV-1 RT are absolutely required for HART element reverse transcription (6). Active-site mutations in both the polymerase (D185E) and RNase H (E478Q) domains result in a 10,000-fold loss of activity. Consequently, this system can be used to monitor both the polymerase and the RNase H activities of HIV-1 RT. As described below, the HART assay can also be used to screen for compounds that inhibit HIV-1 RT.
Considerable effort has been invested in the identification and development of compounds that inhibit the replication of HIV-1. Most HIV-1 RT inhibitors can be divided into two classes, nucleoside analog RT inhibitors (NRTIs) and non-nucleoside RT inhibitors (NNRTIs). NRTIs such as AZT (3′-azido-3′-deoxythymidine) and ddI (dideoxyinosine) inhibit reverse transcription by a chain-termination mechanism; when they are added to a growing DNA chain they block further DNA synthesis. NRTIs, although effective inhibitors of HIV-1, are not highly specific for HIV-1 RT and can inhibit cellular polymerases, resulting in cytotoxicity. NNRTIs show promise because they are specific for HIV-1 RT and as a consequence are less cytotoxic. Two NNRTIs have been approved for clinical use and as therapeutic agents (13). NNRTIs, although chemically diverse, consist of aromatic moieties and bind to a hydrophobic pocket in HIV-1 RT (14–20). It has been proposed that NNRTIs inhibit the nucleotide transfer step of reverse transcription (21, 22) by distorting the alignment of the primer–template and the active site of HIV-1 RT.
The TIBO family of NNRTIs (23) are effective inhibitors of wild-type HIV-1 RT and block viral propagation. The clinical usefulness of NNRTIs, like all HIV-1 inhibitors, is compromised by the ability of HIV-1 to develop drug resistance. Viral propagation in the presence of NNRTIs selects for NNRTI-resistant HIV-1 variants (24–27) that carry changes in the amino acids that make up the NNRTI-binding site. These amino acid changes alter interactions between the inhibitor and the amino acid side chains or restrict access to the binding site (28). Because all of the NNRTIs bind to the same site, there is in some cases considerable cross-resistance to other NNRTIs. Because each NNRTI binds to the pocket in a slightly different way, resistant variants display various levels of polymerase activity in the presence of particular inhibitors (14–18, 29). The development of more effective HIV-1 RT inhibitors will involve identifying compounds that are active against both wild-type and NNRTI-resistant strains of HIV-1.
The HART assay (6) provides a simple alternative approach to the identification and evaluation of HIV-1 inhibitors. Here we demonstrate that a known HIV-1 RT inhibitor (−)-(S)-8-chloro-4,5,6,7-tetrahydro-5-methyl-6-(3-methyl-2-butenyl)imidazo[4,5,1-jk][1,4]-benzodiazepin-2(1H)-thione monohydrochloride (8 Cl-TIBO) specifically inhibits HIV-1 reverse transcription in the HART assay system. We also show that NNRTI-resistant RT variants retain their activity in the presence of 8 Cl-TIBO. The activity of wild-type and variant RTs and their response to NNRTI compounds can be quantitated. We studied the NNRTI thiazolobenzimidazole (TBZ; 1-(2′, 6′-difluorophenyl)-1H,3H-thiazolo[3,4-a]benzimidazole) (30, 31) and showed that it inhibits both wild-type and an NNRTI-resistant HIV-1 RT (L100I).
MATERIALS AND METHODS
Yeast Strains.
The yeast strain BDG1251 (MATα ura3–167 trp1-GB spt3–101 his3Δ200) is the host for HART elements in which reverse transcription is monitored. The spt3 mutation blocks expression of endogenous Ty1 elements. The his3-Δ200 mutation deletes the entire HIS3 gene.
Plasmids.
Nuvec08 (galactose-regulated Ty-his3AI) was constructed by modifying pGTy-H3 mhis3AI (12): the long terminal repeat-URA3 interval was shortened, the NcoI and SmaI sites in URA3 were removed, and the ClaI site between the 3′ long terminal repeat and the end of his3AI was removed. HART-21 was constructed by replacing the RT domain of Ty1 with that of HIV-1 RT in the plasmid Nuvec08 (Fig. 1). The non-nucleoside-resistant HIV-1 RT variants, L100I and Y181C, were placed in HART-21 by conventional cloning using a SmaI site in HIV-1 [between HIV-1 RT codons 14 and 15 (10)] and the ClaI site between the 3′ end of HIV-1 RT and his3AI, resulting in the plasmids HART-L100I and HART-Y181C. The nucleic acid and amino acid sequences of the Ty1/HIV fusions at the Ty1 protease cleavage site between Ty1 integrase and Ty1 RT are shown in Fig. 1C.
Induction of Reverse Transcription on Plates.
Strains carrying Nuvec08 or HART plasmids were maintained by growth on SC − URA (synthetic complete media missing uracil) + glucose plates (32). Expression of Ty1 and hybrid elements was induced by replica plating or patching to SC − URA + galactose [1% DMSO (dimethyl sulfoxide)]. These plates were then replica plated onto SC − HIS + glucose (1% DMSO) to select for histidine prototrophs. Induction and selection of reverse transcription were done at 30°C to minimize endogenous Ty activity.
Liquid Inductions.
Strains carrying either Nuvec08 or HART plasmids were grown in SC − URA + glucose (1% DMSO) to saturation. Saturated cultures (20–50 μl, ≈106 cells) were transferred to 1 ml of SC − URA + galactose (1% DMSO) and grown for 6 hr. Two hundred microliters was plated directly onto SC−HIS (1% DMSO) plates. Five microliters of culture was transferred into 5 ml of water and 50–100 μl was plated on yeast extract/peptone/dextrose (YEPD) plates to determine cell titer. All incubation steps were performed at 30°C. Ty1 and HART element reverse transcription was quantitated by determining the frequency of histidine prototrophy in the presence and absence of inhibitors. Ty1 and HART element frequencies were determined by dividing the number of histidine prototrophs by the total viable cell count. The frequencies in the absence of inhibitor and at each inhibitor concentrations are the average of at least 12 replicates.
Inhibitors.
The inhibitor 8 Cl-TIBO (R091767) was provided by Janssen Research Foundation. The inhibitor TBZ (NSC625487) was synthesized and provided by T. H. Roth and C. J. Michejda. RT inhibitors were dissolved in DMSO to give 20 mM stock solutions and added to liquid media and plates such that all media contained 1% DMSO. When testing the effect of the inhibitors on reverse transcription, inhibitors were present both during induction (growth on galactose) and during selection (growth in the absence of histidine).
RESULTS
We previously showed that hybrid Ty1/HIV-1 retroelements are active in yeast and that the activity of HIV-1 RT can be monitored by using a simple genetic assay (6). The assay is based on the expression of an indicator gene his3AI (Fig. 1) that requires transcription, RNA splicing to remove the artificial intron, and reverse transcription to generate an intact cDNA copy of the HIS3 gene (12). Insertion of this HIS3 cDNA into HART plasmids or into the yeast genome is detected by genetic selection for histidine prototrophy. HART elements marked with his3AI (Fig. 1) have the RT-coding region of Ty1 replaced by sequences encoding HIV-1 RT. The HART elements were designed so that Ty1 protease can cleave HIV-1 RT from the Ty1/HIV precursor polypeptide, yielding an active form of HIV-1 RT (p66/p66). This HIV-1 RT carries out reverse transcription of the hybrid elements, resulting in histidine prototrophy.
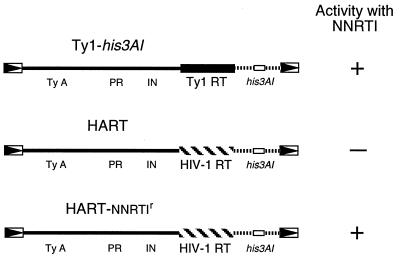
To establish that a compound is an inhibitor of a specific RT, in this case HIV-1 RT, requires that other RTs, cellular polymerases, and general cellular functions are unaffected. Specificity was demonstrated by using isogenic strains that differ only in the RTs present in the plasmid-borne retroelements (Fig. 2). Ty1-his3AI and HART elements were expressed in a yeast strain carrying a spt3 mutation (33) in which endogenous Ty1s are not expressed, but galactose induction of elements under the control of the Gal1 promoter is efficient (34). The Ty1-his3AI element serves as a positive control to show that the growth, induction of transcription, reverse transcription, and splicing needed to generate histidine prototrophs are unaffected by a test compound.
Figure 2.
Scheme demonstrating that inhibition of HIV-1 RT can be monitored in yeast. To demonstrate that the HART assay is an appropriate means to screen for inhibitors of HIV-1 RT in yeast, the HART assay was carried out with a previously characterized NNRTI (8 Cl-TIBO) and controls for the specificity of inhibition. The rationale for that experiment is shown. Specific inhibitors of HIV-1 RT should not inhibit the activity of Ty elements (Ty-his3AI) because reverse transcription is carried out by Ty RT. HART elements should be inhibited by 8 Cl-TIBO. HIV-1 RT variants (L100I and Y181C) are resistant to 8 Cl-TIBO, and HART elements carrying them should be active.
The NNRTI 8 Cl-TIBO Inhibits HART Element Reverse Transcription.
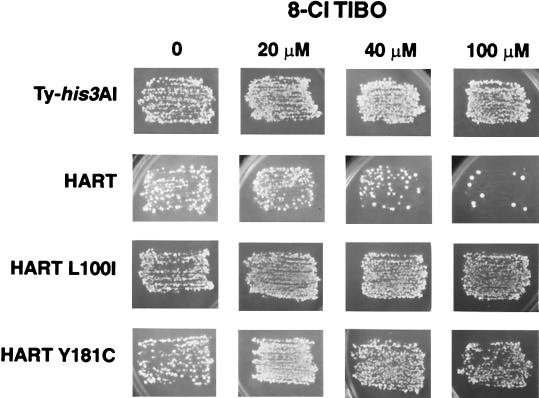
The NNRTI 8 Cl-TIBO was used to show that, in the context of the Ty1/HIV-1 hybrid in yeast, HIV-1 RT is sensitive to a well characterized NNRTI. Ty-his3AI and HART elements were induced by growth on media containing galactose and then replica-plated onto media lacking histidine to select RT-mediated events. Both induction and selection of RT-mediated events were carried out in the presence of various concentrations of 8 Cl-TIBO. The results shown in Fig. 3 demonstrate that the HART assay can be used to monitor the drug-dependent inhibition of HIV-1 RT. In the presence of 8 Cl-TIBO, Ty1 reverse transcription and other cellular processes are unaffected; histidine prototrophs are efficiently generated with or without the inhibitor. In contrast, HART element reverse transcription is inhibited in the presence of 8 Cl-TIBO. Inhibition is dose-dependent and results in a dramatic decrease in the number of histidine prototrophs.
Figure 3.
HIV-1 reverse transcription is inhibited by 8 Cl-TIBO in yeast. Inhibition of HIV-1 RT activity in yeast was tested by using the RT inhibitor 8 Cl-TIBO and controls for the specificity of inhibition. Yeast patches carrying Ty (Ty-his3AI) or hybrid retroelements (HART, HART-L100I, and HART-Y181C) were replica-plated onto galactose plates containing no or various concentrations of 8 Cl-TIBO, to induce expression of the elements. These plates were then replica-plated to media lacking histidine, containing no or various concentrations of 8 Cl-TIBO, to select for RT-mediated events.
The efficiency of reverse transcription in the HART assay is measured by using a genetic selection and classical plating techniques. Reverse transcription can be measured quantitatively by using a protocol based on short inductions in liquid followed by titering of histidine prototrophs. When this protocol is used the frequency of Ty1 reverse transcription is similar to previous studies and HART element reverse transcription is efficient. This analysis establishes a baseline for the RT activity of each element and demonstrates that the assay can be used to quantitate the activity of HIV-1 variants (Table 1).
Table 1.
HIV-1 RT activity and inhibition of HIV-1 RT can be quantitatively assayed in yeast
| Element | Relative frequency of histidine prototrophy with indicated inhibitor
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No inhibitor | 8 Cl-TIBO
|
TBZ
|
|||||||||||
| 0 | 5 μm | 10 μm | 20 μm | 40 μm | 80 μm | 0 | 5 μm | 10 μm | 20 μm | 40 μm | 80 μm | ||
| Ty | 2.9 (±1.9) × 10−2 | 1.0 | 1.06 | 0.85 | 1.24 | 0.92 | 1.28 | 1.0 | 0.88 | 1.01 | 0.90 | 0.98 | 0.97 |
| HART | 1.1 (±0.8) × 10−3 | 1.0 | 0.35 | 0.25 | 0.11 | 0.05 | 0.02 | 1.0 | 0.30 | 0.25 | 0.14 | 0.04 | 0.01 |
| L100I | 4.4 (±3.2) × 10−4 | 1.0 | 0.93 | 1.13 | 0.98 | 0.86 | 0.55 | 1.0 | 0.71 | 0.64 | 0.25 | 0.23 | 0.12 |
| Y181C | 1.4 (±0.8) × 10−4 | 1.0 | 0.88 | 1.0 | 1.03 | 0.90 | 0.58 | 1.0 | 0.88 | 1.0 | 1.03 | 0.90 | 0.58 |
The frequency of RT-mediated histidine prototrophy was determined for Ty and hybrid Ty/HIV elements after brief induction of expression. The frequency of RT-mediated events is shown in the “No inhibitor” column. The frequency of RT-mediated histidine prototrophy was determined for Ty and hybrid Ty/HIV elements in the presence and absence of 8 Cl-TIBO and TBZ. The frequencies for each element-inhibitor combination are expressed relative to the frequency of the element in the absence of inhibtor (no inhibtor = 1.0).
Quantitation of reverse transcription with the HART assay makes it possible not only to monitor the activity of an RT, but also to measure the extent of inhibition by specific compounds. The extent of HIV-1 reverse transcription inhibition by 8 Cl-TIBO was quantitated by performing the assay using several concentrations of that drug (Table 1). As suggested by the plate assay in Fig. 3, 8 Cl-TIBO does not inhibit Ty1 reverse transcription or the generation of histidine prototrophs at any of the concentrations we tested. In contrast, HART elements are inhibited in a dose-dependent manner by 8 Cl-TIBO with 50% inhibition of HIV-1 reverse transcription occurring at 3–4 μM.
HART Elements Carrying NNRTI-Resistant HIV-1 RTs Are Resistant to 8 Cl-TIBO.
Propagation of HIV-1 in the presence of NNRTIs selects for resistant variants with alterations in the RT domain (15). The HIV-1 RT variants L100I and Y181C were selected during virus propagation as resistant to 8 Cl-TIBO in tissue culture, are found in HIV-1-infected individuals, and result in cross resistance to multiple NNRTIs (25–27). We constructed Ty1/HIV-1 elements carrying these drug-resistant variants, designated HART-L100I and HART-Y181C. HART-L100I and HART-Y181C elements are resistant to inhibition by 8 Cl-TIBO (Fig. 3). These elements form histidine prototrophs even at inhibitor concentrations of at least 80 μM. The resistance of these elements to 8 Cl-TIBO further demonstrates that the target of inhibition in the wild-type HART element is HIV-1 RT and not some cellular function (such as recombination) required by HART elements but not the Ty-his3AI retrotransposon.
It has been suggested that HIV-1 carrying NNRTI-resistant mutants replicates less efficiently than the wild-type virus (35, 36). We measured the production of histidine prototrophs by using HART elements carrying the 8 Cl-TIBO-resistant variants (Table 1). In the absence of inhibitor, HART-L100I activity is reduced 2–3-fold compared with HART, and HART-Y181C activity is reduced 8-fold, suggesting that these variant RTs reverse-transcribe element RNA less efficiently than wild-type HIV-1 RT. The response of HART elements carrying the NNRTI-resistant variants of HIV-1 RT to 8 Cl-TIBO is shown in Table 1. As expected from the behavior of HIV-1 viruses carrying these mutations, HART-L100I and HART-Y181C are at least 20-fold more resistant to 8-Cl TIBO than HART. In this assay, 50% inhibition requires more than 80 μM 8 Cl-TIBO, a level at which wild-type HIV-1 RT is effectively inhibited. Thus, both the extent of inhibition by an NNRTI and the drug resistance by variant HIV-1 RTs can be quantitatively evaluated in yeast.
HART Elements Can Detect Compounds Active Against NNRTI-Resistant RTs.
Having established that the HART assay responds appropriately to one well characterized inhibitor, we used the HART system to look at an additional HIV-1 RT inhibitor. TBZ (30, 31) is a potent NNRTI active against HIV-1 (37). TBZ has been used as the lead compound for a family of NNRTIs modeled to fit the NNRTI-binding pocket (38). TBZ inhibits both wild-type HIV-1 RT and surprisingly,the NNRTI-resistant variant HART-L100I (Table 1). TBZ inhibits HART in a dose-dependent manner with 50% inhibition at ≈3–4 μM. TBZ inhibits HART-L100I with 50% inhibition at ≈12 μM. HART-Y181C is not inhibited by TBZ, nor is Ty1, demonstrating that the action of TBZ is specific for HIV-1 RT. In contrast to our results, resistance to TBZ is not apparent when a L100I variant of HIV-1/111B was tested in a cytopathic killing assay (39). The observation that TBZ inhibits a NNRTI-resistant variant shows that the HART system can be used to identify compounds that inhibit both wild-type and drug-resistant HIV-1 RT variants.
DISCUSSION
The RT of HIV is an important target for antiviral drug development. Our experiments demonstrate that hybrid elements composed of the yeast retrotransposon Ty1 and the RT of HIV-1 are useful tools for the identification of anti-HIV-1 RT drugs. We previously described a simple genetic assay that depends on the RT and RNase H activities of these HART elements (6). Here we demonstrate that HIV-1 reverse transcription is sensitive to the NNRTI 8 Cl-TIBO in yeast. TBZ, another NNRTI, blocks the RT activity of both wild-type and a NNRTI-resistant HIV-1 RT. This safe and simple assay can augment biochemical approaches to the analysis of RT and can be used by laboratories without the facilities to propagate live virus.
Comparison to Other Assays.
Several approaches have been used to identify therapeutics against HIV-1 RT. Anti-RT drugs that block viral replication have been identified in a variety of tissue culture systems (37, 40, 41). RT inhibitors have also been identified by biochemical screens for compounds that block activity on RNA and/or DNA substrates (42). Kim and Loeb (43) developed a plasmid-replication assay that depends on the DNA polymerase activity of HIV-1 RT and showed that the activity is sensitive to nucleoside analogs (44). Structural determinations of HIV-1 RT (45–48) and of RT complexed with inhibitors (14, 16–18, 20, 49, 50) have been used to design novel anti-RT drugs (28, 38).
To demonstrate that specific inhibitors of HIV-1 reverse transcription can also be identified by using the HART assay, we constructed a set of target retro-elements that differ only in the RT-coding region present. HART elements undergo efficient protease cleavage to generate the p66 subunit of HIV-1 RT, which presumably forms a p66/p66 homodimer (D.N. & S. Moore, unpublished data). HIV-1 protease is not present in these elements, and there is no evidence that any p51 subunit is made. The homodimeric form of HIV-1 RT p66/p66 is active, carrying out both RNA-dependent and DNA-dependent polymerization (10, 51–53); thus, it is not surprising that a p66/p66 homodimer can carry out reverse transcription in the HART assay.
HART elements generate RT-mediated events at an easily scorable frequency. The RT activity of naturally occurring and drug-induced HIV-1 RT variants can be differentiated and characterized over a 100-fold range with the HART assay. The activity of HART elements increases with longer induction periods and with short inductions is 5–10% of that observed previously with longer inductions (Table 1; ref. 6). Induction for 6 hr provides sufficient signal for the quantitation of reverse transcription and yields reproducible results.
The HART assay is sensitive to inhibitors of HIV-1 RT. HART element reverse transcription is inhibited in the presence of 8 Cl-TIBO and TBZ with 50% inhibition of HIV-1 RT activity at 3–4 μM. Both 8 Cl-TIBO (23) and TBZ (37) are reported to inhibit HIV-1 replication using at 1–10% of the concentrations used in other in vivo assays. The HART assay differs from in vivo HIV-1 replication assays in that it is a “single-pass” assay. That is, histidine prototrophy, the reverse transcription indicator, can be generated by a single round of reverse transcription. Standard HIV replication assays involve multiple rounds of viral replication. Consequently, inhibition in each round of reverse transcription is compounded in multiple cycles of viral replication. A single-pass assay is expected to require higher concentrations of inhibitor to achieve comparable levels of inhibition. Inefficient uptake and the effects of metabolism on inhibitor levels may also increase the amount of inhibitor needed in the HART assay. Although the assay requires higher drug concentrations, it can be used to monitor RT inhibition at micromolar concentrations of drug.
Differences in RT activity can be monitored with the HART assay. The activities of the NNRTI-resistant RT elements HART-L100I and HART-Y181C are reduced 3- and 8-fold respectively in comparison to wild-type HIV-1 RT (Table 1). The reduction in RT activity for HART-L100I is consistent with biochemical RT assays in which L100I has 40% of the activity of wild-type HIV-1 RT (29). In contrast to our results, a biochemical analysis of the RNA- and DNA-dependent polymerase activies of the Y181C variant on artificial templates (54) indicate that it has catalytic properties similar to wild-type RT. Although viruses carrying this RT variant can be propagated (55–57), it is possible that it is less processive than wild-type RT. The HART assay requires sufficient reverse transcription to generate a HIS3 cDNA substrate for recombination that results in histidine prototrophy. An RT with low processivity may be deficient in generating the cDNA substrate required for the HART assay.
The demonstration that TBZ inhibits HART and HART-L100I suggests that the assay can be used to identify novel NNRTIs and NNRTIs that are effective against both wild-type and drug-resistant RTs. TBZ inhibits HART-L100I at concentrations at which Ty1 and HART-Y181C are unaffected. This inhibition is dose-dependent, with 50% inhibition at ≈12 μM TBZ. Analysis of TBZ activity against virus containing the L100I mutation in a viral propagation assay did not reveal an activity against this drug-resistant variant (37, 39). Although it is possible that the p66/p66 homodimer present in the HART assay interacts differently with TBZ than does the p66/p51 heterodimer present in HIV-1, there is no indication that other NNRTIs interact differently with p66/66 versus p66/p51. One of the strengths of the HART assay is the ease of running multiple replicates; hence, it may detect subtle differences in activity more readily than conventional in vivo HIV-1 replication assays. Compounds like TBZ are candidates for therapeutics that inhibit wild-type HIV-1 and also suppress the emergence of drug-resistant variants (42).
We view the HART assay as a complement to biochemical and viral propagation assays for monitoring drug sensitivities. The strength of this assay lies in the ease and speed with which it can be performed and the absence of a requirement for the biocontainment necessary for live-virus studies. We recognize that some potential HIV-1 RT inhibitors will be missed because of permeability problems with yeast, differences in the metabolism of compounds in yeast and humans, or aspects of reverse transcription (e.g., priming) that are not components of this assay. We have, however, had initial success using this assay as a primary screen of new NNRTIs (D.N. & C. Michejda, unpublished data).
Other Uses of the HART Assay.
HART elements carrying a library of variant HIV-1 RT sequences would be helpful in identifying mutations that confer drug resistance. Such libraries are easy to construct in yeast because of the efficiency of DNA gap repair. Cotransformation of linearized HART plasmids carrying a partial deletion of HIV-1 RT DNA sequences and RT-domain DNA results in the regeneration of plasmid-borne HART elements by homologous recombination. RT-domain DNA can be obtained by PCR of virus present in any viral pool, including patient blood. The activity profile and inhibitor-resistance profile of the RTs present in the original pool can be established by looking at the frequency of histidine prototrophy in the presence and absence of inhibitor. Starting with a mixed pool of HIV-1 RT domains, we have constructed HART RT libraries that are representative of the starting RTs (D.V.N., unpublished data). This approach can also be used to mutagenize the RT domain of HIV-1 and generate a pool of HART variants that can be analyzed for RT activity, the fidelity of reverse transcription, and the generation of novel drug-resistant variants.
The role of RNase H in reverse transcription is well established, and this requirement is relatively unexplored as a target for inhibition of HIV-1 reverse transcription. The HART assay offers an attractive system for screening potential RNase H inhibitors because activity is also dependent on RNase H (6) and inhibition of HIV-1 RNase H will result in a loss of HART activity. Just as it has for RT polymerase inhibitors, the HART assay can be used to look for RNase H inhibitors, to mutagenize the RNase H domain, and to characterize RNase H variants.
In summary, the ability to characterize the activity of exogenous RTs in yeast facilitates the analysis of HIV-1 reverse transcription in an in vivo assay. The HART assay responds appropriately to established inhibitors, can be used to look for novel RT inhibitors, and can be used to characterize the RTs present in a viral population.
Acknowledgments
Thanks to Mary Ellen Palko for support and discussion. This research was sponsored by the National Cancer Institute, under contract with the Applied BioSciences Laboratories.
ABBREVIATIONS
- RT
reverse transcriptase
- HART
his3AI AIDS RT
- 8 Cl-TIBO
(−)-(S)-8-chloro-4,5,6,7-tetrahydro-5-methyl-6-(3-methyl-2-butenyl)imidazo[4,5,1-jk][1,4]-benzodiazepin-2(1H)-thione monohydrochloride
- TBZ
thiazolobenzimidazole
- NRTI
nucleoside RT inhibitor
- NNRTI
non-nucleoside RT inhibitor
- DMSO
dimethyl sulfoxide
References
- 1.Boeke J D, Garfinkel D J, Styles C A, Fink G R. Cell. 1985;40:491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- 2.Garfinkel D J, Hedge A M, Youngren S D, Copeland T D. J Virol. 1991;65:4573–4581. doi: 10.1128/jvi.65.9.4573-4581.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eichinger D J, Boeke J D. Cell. 1988;54:955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- 4.Dombroski B A, Feng Q, Mathias S L, Sassaman D M, Scott A F, Kazazian H J, Boeke J D. Mol Cell Biol. 1994;14:4485–4492. doi: 10.1128/mcb.14.7.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathias S L, Scott A F, Kazazian H J, Boeke J D, Gabriel A. Science. 1991;254:1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- 6.Nissley D V, Garfinkel D J, Strathern J N. Nature (London) 1996;380:30. doi: 10.1038/380030a0. [DOI] [PubMed] [Google Scholar]
- 7.Moore S P, Garfinkel D J. Proc Natl Acad Sci USA. 1994;91:1843–1847. doi: 10.1073/pnas.91.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skalka A M, Goff S P, editors. Reverse Transcriptase. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. [PubMed] [Google Scholar]
- 9.Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. [PubMed] [Google Scholar]
- 10.Hizi A, McGill C, Hughes S H. Proc Natl Acad Sci USA. 1988;85:1218–1222. doi: 10.1073/pnas.85.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyer P L, Ferris A L, Hughes S H. J Virol. 1992;66:1031–1039. doi: 10.1128/jvi.66.2.1031-1039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curcio M J, Garfinkel D J. Proc Natl Acad Sci USA. 1991;88:936–940. doi: 10.1073/pnas.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinchington D, Minshull C, Drummond C. Int Antiviral News. 1996;4:132–144. [Google Scholar]
- 14.Kohlstaedt L A, Wang J, Friedman J M, Rice P A, Steitz T A. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 15.Tantillo C, Ding J, Jacobo-Molina A, Nanni R G, Boyer P L, Hughes S H, Pauwels R, Andries K, Janssen P A, Arnold E. J Mol Biol. 1994;243:369–387. doi: 10.1006/jmbi.1994.1665. [DOI] [PubMed] [Google Scholar]
- 16.Smerdon S J, Jager J, Wang J, Kohlstaedt L A, Chirino A J, Friedman J M, Rice P A, Steitz T A. Proc Natl Acad Sci USA. 1994;91:3911–3915. doi: 10.1073/pnas.91.9.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Das K, Tantillo C, Zhang W, Clark A D, Jr, Jessen S, Lu X, Hsiou Y, Jacobo-Molina A, Andries K, et al. Structure. 1995;3:365–379. doi: 10.1016/s0969-2126(01)00168-x. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Das K, Moereels H, Koymans L, Andries K, Janssen P A, Hughes S H, Arnold E. Nat Struct Biol. 1995;2:407–415. doi: 10.1038/nsb0595-407. [DOI] [PubMed] [Google Scholar]
- 19.Ren J, Esnouf R, Garman E, Somers D, Ross C, Kirby I, Keeling J, Darby G, Jones Y, Stuart D, et al. Nat Struct Biol. 1995;2:293–302. doi: 10.1038/nsb0495-293. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins A L, Ren J, Esnouf R M, Willcox B E, Jones E Y, Ross C, Miyasaka T, Walker R T, Tanaka H, Stammers D K, et al. J Med Chem. 1996;39:1589–1600. doi: 10.1021/jm960056x. [DOI] [PubMed] [Google Scholar]
- 21.Rittinger K, Divita G, Goody R S. Proc Natl Acad Sci USA. 1995;92:8046–8049. doi: 10.1073/pnas.92.17.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spence R A, Kati W M, Anderson K S, Johnson K A. Science. 1995;267:988–993. doi: 10.1126/science.7532321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pauwels R, Andries K, Desmyter J, Schols D, Kukla M J, Breslin H J, Raeymaeckers A, Van Gelder J, Woestenborghs R, Heykants J, et al. Nature (London) 1990;343:470–474. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- 24.Balzarini J, Karlsson A, Perez-Perez M J, Vrang L, Walbers J, Zhang H, Oberg B, Vandamme A M, Camarasa M J, De Clercq E. Virology. 1993;192:246–253. doi: 10.1006/viro.1993.1027. [DOI] [PubMed] [Google Scholar]
- 25.de Vreese K, Debyser Z, Vandamme A M, Pauwels R, Desmyter J, de Clercq E, Anne J. Virology. 1992;188:900–904. doi: 10.1016/0042-6822(92)90550-9. [DOI] [PubMed] [Google Scholar]
- 26.Mellors J W, Im G J, Tramontano E, Winkler S R, Medina D J, Dutschman G E, Bazmi H Z, Piras G, Gonzalez C J, Cheng Y C. Mol Pharmacol. 1993;43:11–16. [PubMed] [Google Scholar]
- 27.Schinazi R F, Larder B A, Mellors J W. Int Antiviral News. 1997;5:129–141. [Google Scholar]
- 28.Arnold E, Das K, Ding J, Yadav P N, Hsiou Y, Boyer P L, Hughes S H. Drug Des Discov. 1996;13:29–47. [PubMed] [Google Scholar]
- 29.Boyer P L, Currens M J, McMahon J B, Boyd M R, Hughes S H. J Virol. 1993;67:2412–2420. doi: 10.1128/jvi.67.4.2412-2420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chimirri A, Grasso S, Monforte A M, Monforte P, Zappala M. Farmaco. 1991;46:817–823. [PubMed] [Google Scholar]
- 31.Chimirri A, Grasso S, Monforte A M, Monforte P, Zappala M. Farmaco. 1991;46:925–933. [PubMed] [Google Scholar]
- 32.Rose M D, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. [Google Scholar]
- 33.Winston F, Durbin K J, Fink G R. Cell. 1984;39:675–682. doi: 10.1016/0092-8674(84)90474-4. [DOI] [PubMed] [Google Scholar]
- 34.Boeke J D, Styles C A, Fink G R. Mol Cell Biol. 1986;6:3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coffin J M. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 36.Rayner M M, Cordova B, Jackson D A. Virology. 1997;236:85–94. doi: 10.1006/viro.1997.8620. [DOI] [PubMed] [Google Scholar]
- 37.Buckheit R W, Jr, Hollingshead M G, Germany-Decker J, White E L, McMahon J B, Allen L B, Ross L J, Decker W D, Westbrook L, Shannon W M, et al. Antiviral Res. 1993;21:247–265. doi: 10.1016/0166-3542(93)90031-d. [DOI] [PubMed] [Google Scholar]
- 38.Kroeger Smith M B, Rouzer C A, Taneyhill L A, Smith N A, Hughes S H, Boyer P L, Janssen P A, Moereels H, Koymans L, Arnold E, et al. Protein Sci. 1995;4:2203–2222. doi: 10.1002/pro.5560041026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, S. S., Fliakas-Boltz, V., Bader, J. P. & Buckheit, R. W., Jr. (1995) Leukemia 9, Suppl. 1, S75–S85. [PubMed]
- 40.Nara P L, Fischinger P J. Nature (London) 1988;332:469–470. doi: 10.1038/332469a0. [DOI] [PubMed] [Google Scholar]
- 41.Weislow O S, Kiser R, Fine D L, Bader J, Shoemaker R H, Boyd M R. J Natl Cancer Inst. 1989;81:577–586. doi: 10.1093/jnci/81.8.577. [DOI] [PubMed] [Google Scholar]
- 42.Roth T, Morningstar M L, Boyer P L, Hughes S H, Buckheit R W, Jr, Michejda C J. J Med Chem. 1997;40:4199–4207. doi: 10.1021/jm970096g. [DOI] [PubMed] [Google Scholar]
- 43.Kim B, Loeb L A. Proc Natl Acad Sci USA. 1995;92:684–688. doi: 10.1073/pnas.92.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim B, Loeb L A. J Virol. 1995;69:6563–6566. doi: 10.1128/jvi.69.10.6563-6566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsiou Y, Ding J, Das K, Clark A D, Jr, Hughes S H, Arnold E. Structure (London) 1996;4:853–860. doi: 10.1016/s0969-2126(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 46.Jacobo-Molina A, Ding J, Nanni R G, Clark A D, Jr, Lu X, Tantillo C, Williams R L, Kamer G, Ferris A L, Clark P, et al. Proc Natl Acad Sci USA. 1993;90:6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stammers D K, Somers D O, Ross C K, Kirby I, Ray P H, Wilson J E, Norman M, Ren J S, Esnouf R M, Garman E F, et al. J Mol Biol. 1994;242:586–588. doi: 10.1006/jmbi.1994.1604. [DOI] [PubMed] [Google Scholar]
- 48.Rodgers D W, Gamblin S J, Harris B A, Ray S, Culp J S, Hellmig B, Woolf D J, Debouck C, Harrison S C. Proc Natl Acad Sci USA. 1995;92:1222–1226. doi: 10.1073/pnas.92.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das K, Ding J, Hsiou Y, Clark A D, Jr, Moereels H, Koymans L, Andries K, Pauwels R, Janssen P A, Boyer P L, et al. J Mol Biol. 1996;264:1085–1100. doi: 10.1006/jmbi.1996.0698. [DOI] [PubMed] [Google Scholar]
- 50.Ren J, Esnouf R, Hopkins A, Ross C, Jones Y, Stammers D, Stuart D. Structure (London) 1995;3:915–926. doi: 10.1016/S0969-2126(01)00226-X. [DOI] [PubMed] [Google Scholar]
- 51.Hizi A, Barber A, Hughes S H. Virology. 1989;170:326–329. doi: 10.1016/0042-6822(89)90389-9. [DOI] [PubMed] [Google Scholar]
- 52.Larder B, Purifoy D, Powell K, Darby G. EMBO J. 1987;6:3133–3137. doi: 10.1002/j.1460-2075.1987.tb02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andreola M L, Nevinsky G A, Barr P J, Sarih-Cottin L, Bordier B, Fournier M, Litvak S, Tarrago-Litvak L. J Biol Chem. 1992;267:19356–19362. [PubMed] [Google Scholar]
- 54.Debyser Z, De Vreese K, Knops-Gerrits P P, Baekelandt V, Bhikhabhai R, Strandberg B, Pauwels R, Anne J, Desmyter J, De Clercq E. Mol Pharmacol. 1993;43:521–526. [PubMed] [Google Scholar]
- 55.Nunberg J H, Schleif W A, Boots E J, O’Brien J A, Quintero J C, Hoffman J M, Emini E A, Goldman M E. J Virol. 1991;65:4887–4892. doi: 10.1128/jvi.65.9.4887-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larder B A. Antimicrob Agents Chemother. 1992;36:2664–2669. doi: 10.1128/aac.36.12.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spence R A, Anderson K S, Johnson K A. Biochemistry. 1996;35:1054–1063. doi: 10.1021/bi952058+. [DOI] [PubMed] [Google Scholar]