Abstract
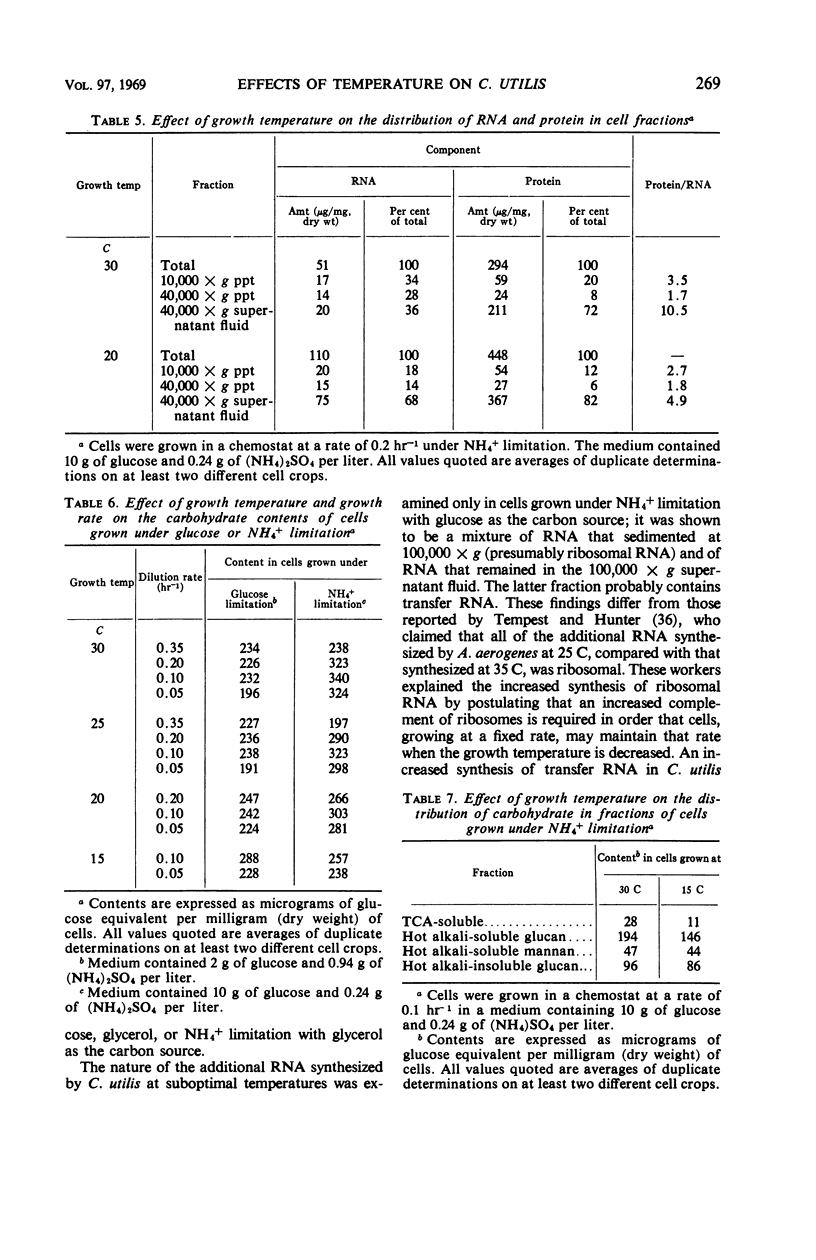
Candida utilis NCYC 321 was grown in steady-state culture in a chemostat under glucose limitation or NH4+ limitation at temperatures of 30, 25, 20, and 15 C and at dilution rates (equal to growth rates) in the range of 0.35 to 0.05 hr−1. Deoxyribonucleic acid contents of cells grown under the various conditions remained approximately constant, but the contents of several other cell components varied. Over the range of 30 to 15 C, the greatest differences were in the ribonucleic acid (RNA) and protein contents of cells grown under NH4+ limitation, which increased as the temperature was decreased. The contents of other components, particularly adenosine triphosphate in cells grown under glucose limitation, varied more when the cells were grown at different rates at a fixed temperature. Cells grown at a fixed rate under NH4+ limitation increased in volume as the temperature was decreased below 30 C. The increase in volume was closely correlated with increases in the proportions of RNA and protein in the dry weight of cells. Cells grown under glucose limitation showed much smaller increases in volume; these increases were poorly correlated with the increased RNA content and hardly at all with the increased protein content. Increases in volume with a decrease in growth temperature from 30 to 20 C were also demonstrated in cells grown under phosphate limitation and to a much smaller extent in cells grown under glycerol limitation. The increased RNA synthesized at low temperatures by cells grown under NH4+ limitation was found almost exclusively in the 40,000 × g supernatant fluid, but only about 40% of it sedimented at 100,000 × g. Cells grown at a fixed rate under NH4+ limitation synthesized less total carbohydrate when the temperature was decreased from 30 to 15 C. This decrease was mainly in the trichloroacetic acid-soluble fraction (probably trehalose) and in the intracellular hot alkali-soluble glucan (probably glycogen). Cells grown at a fixed rate under glucose limitation showed a small increase in carbohydrate content as the temperature was decreased from 30 to 15 C.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki S., Sotos J. F., Rearick P. D. Rapid determination of picomole quantities of ATP with a liquid scintillation counter. Anal Biochem. 1966 Feb;14(2):261–264. doi: 10.1016/0003-2697(66)90135-7. [DOI] [PubMed] [Google Scholar]
- BUCHER T., REDETZKI H. Eine spezifische photometrische Bestimmung von Athylalkohol auf fermentativen Wege. Klin Wochenschr. 1951 Sep 15;29(35-36):615–616. doi: 10.1007/BF01485653. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Hough J. S. Elongation of yeast cells in continuous culture. Nature. 1965 May 15;206(985):676–678. doi: 10.1038/206676a0. [DOI] [PubMed] [Google Scholar]
- Dawson P. S. The intracellular amino acid pool of Candida utilis during growth in batch and continuous flow cultures. Biochim Biophys Acta. 1965 Nov 15;111(1):51–66. doi: 10.1016/0304-4165(65)90472-1. [DOI] [PubMed] [Google Scholar]
- Dean A. C., Rogers P. L. The cell size and macromolecular composition of Aerobacter aerogenes in various systems of continuous culture. Biochim Biophys Acta. 1967 Oct 9;148(1):267–279. doi: 10.1016/0304-4165(67)90302-9. [DOI] [PubMed] [Google Scholar]
- Eaton N. R. Intracellular distribution and characterization of yeast RNA. Biochim Biophys Acta. 1966 Dec 21;129(3):511–518. doi: 10.1016/0005-2787(66)90066-9. [DOI] [PubMed] [Google Scholar]
- Farrell J., Rose A. Temperature effects on microorganisms. Annu Rev Microbiol. 1967;21:101–120. doi: 10.1146/annurev.mi.21.100167.000533. [DOI] [PubMed] [Google Scholar]
- GHOSH A., CHARALAMPOUS F., SISON Y., BORER R. Metabolic function of myo-inositol. I. Cytological and chemical alterations in yeast resulting from inositol deficiency. J Biol Chem. 1960 Sep;235:2522–2528. [PubMed] [Google Scholar]
- Harder W., Veldkamp H. A continuous culture study of an obligately psychrophilic Pseudomonas species. Arch Mikrobiol. 1967;59(1):123–130. doi: 10.1007/BF00406323. [DOI] [PubMed] [Google Scholar]
- Herbert D., Phipps P. J., Tempest D. W. The chemostat: design and instrumentation. Lab Pract. 1965 Oct;14(10):1150–1161. [PubMed] [Google Scholar]
- KJELDGAARD N. O. The kinetics of ribonucleic acid- and protein formation in Salmonella typhimurium during the transition between different states of balance growth. Biochim Biophys Acta. 1961 Apr 29;49:64–76. doi: 10.1016/0006-3002(61)90870-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leick V. Ratios between contents of DNA, RNA and protein in different micro-organisms as a function of maximal growth rate. Nature. 1968 Mar 23;217(5134):1153–1155. doi: 10.1038/2171153a0. [DOI] [PubMed] [Google Scholar]
- MITCHISON J. M. The growth of single cells. II. Saccharomyces cerevisiae. Exp Cell Res. 1958 Aug;15(1):214–221. doi: 10.1016/0014-4827(58)90077-6. [DOI] [PubMed] [Google Scholar]
- Markham R. A steam distillation apparatus suitable for micro-Kjeldahl analysis. Biochem J. 1942 Dec;36(10-12):790–791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effect of growth rate and substrate limitation on the composition and structure of the cell wall of Saccharomyces cerevisiae. Biochem J. 1967 Oct;105(1):189–203. doi: 10.1042/bj1050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEELY W. B. Dextran: structure and synthesis. Adv Carbohydr Chem. 1960;15:341–369. doi: 10.1016/s0096-5332(08)60191-5. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- ROSE A. H., EVISON L. M. STUDIES ON THE BIOCHEMICAL BASIS OF THE MINIMUM TEMPERATURES FOR GROWTH OF CERTAIN PSYCHROPHILIC AND MESOPHILIC MICRO-ORGANISMS. J Gen Microbiol. 1965 Jan;38:131–141. doi: 10.1099/00221287-38-1-131. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SYKES J., TEMPEST D. W. THE EFFECT OF MAGNESIUM AND OF CARBON LIMITATION ON THE MACROMOLECULAR ORGANISATION AND METABOLIC ACTIVITY OF PSEUDOMONAS SP., STRAIN C-IB. Biochim Biophys Acta. 1965 May 11;103:93–108. doi: 10.1016/0005-2787(65)90543-5. [DOI] [PubMed] [Google Scholar]
- Skinner C. E., Fletcher D. W. A REVIEW OF THE GENUS CANDIDA. Bacteriol Rev. 1960 Dec;24(4):397–416. doi: 10.1128/br.24.4.397-416.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. 7. Yeast carbohydrate fractions. Separation from nucleic acid, analysis, and behaviour during anaerobic fermentation. Biochem J. 1956 May;63(1):23–33. doi: 10.1042/bj0630023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Hunter J. R. The influence of temperature and pH value on the macro-molecular composition of magnesium-limited and glycerol-limited Aerobacter aerogenes growing in a chemostat. J Gen Microbiol. 1965 Nov;41(2):267–273. doi: 10.1099/00221287-41-2-267. [DOI] [PubMed] [Google Scholar]
- Uffen R. L., Canale-Parola E. Temperature-dependent pigment production by Bacillus cereus var. alesti. Can J Microbiol. 1966 Jun;12(3):590–593. doi: 10.1139/m66-084. [DOI] [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. The nature of the fluctuating ribonucleic acid in Escherichia coli. Biochem J. 1957 Feb;65(2):321–331. doi: 10.1042/bj0650321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. P., GOLDSCHMIDT M. E., GOTT C. L. INHIBITION BY TEMPERATURE OF THE TERMINAL STEP IN BIOSYNTHESIS OF PRODIGIOSIN. Biochem Biophys Res Commun. 1965 Apr 9;19:177–181. doi: 10.1016/0006-291x(65)90500-0. [DOI] [PubMed] [Google Scholar]



